Abstract
Substitution of a hydrogen atom with its heavy isotope deuterium entails the addition of one neutron to a molecule. Despite being a subtle change, this structural modification, known as deuteration, may improve the pharmacokinetic and/or toxicity profile of drugs, potentially translating into improvements in efficacy and safety compared with the non-deuterated counterparts. Initially, efforts to exploit this potential primarily led to the development of deuterated analogues of marketed drugs through a ‘deuterium switch’ approach, such as deutetrabenazine, which became the first deuterated drug to receive FDA approval in 2017. In the past few years, the focus has shifted to applying deuteration in novel drug discovery, and the FDA approved the pioneering de novo deuterated drug deucravacitinib in 2022. In this Review, we highlight key milestones in the field of deuteration in drug discovery and development, emphasizing recent and instructive medicinal chemistry programmes and discussing the opportunities and hurdles for drug developers, as well as the questions that remain to be addressed.
Similar content being viewed by others
Introduction
Medicinal chemists use a wide range of approaches to optimize the efficacy and safety of small-molecule compounds in drug discovery and development. Among them is bioisosterism, whereby one substructure is replaced with another to improve one or more properties of the original compound while retaining its biological activity1. For example, the replacement of hydrogen with deuterium — arguably the smallest possible chemical change — can have a substantial impact on various drug characteristics. Deuterium incorporation was initially believed to merely increase the metabolic stability of a compound, but it has become evident that the effect of this modification can go well beyond simple pharmacokinetic (PK) improvements to have a major impact on drug efficacy and safety.
The incorporation of deuterium in drug compounds began in the early 1960s, when two independent works on d2-tyramine2 and d3-morphine3 were published, but only a few studies followed up on this topic over subsequent years. However, in the past two decades, deuteration has increasingly been used to potentially improve the PK profile of marketed drugs — an approach named ‘deuterium switch’, in analogy with the term chiral switch4. This strategy has attracted commercial interest, with a handful of companies having deuteration as their core technology and several licensing deals, mergers and acquisitions occurring in the field5. Such investments led to the development of the first deuterated drug, deutetrabenazine, which was approved by the FDA in 2017. This deuterated analogue showed a far superior PK profile compared with undeuterated tetrabenazine, a vesicular monoamine transporter 2 inhibitor approved in 2008 for the treatment of chorea associated with Huntington disease6, which allowed a significant reduction in dose and dosing frequency.
The pioneering experience with deutetrabenazine paved the way for other deuterium switches. In 2021, donafenib, the result of a deuterium switch on the multi-kinase inhibitor sorafenib, was approved in China for unresectable hepatocellular carcinoma and is currently being developed for the treatment of various cancers7. In particular, donafenib provided better PK properties, higher efficacy and less-frequent adverse effects when compared head-to-head with sorafenib in clinical studies. During the same year, the oral remdesivir derivative VV116 was approved for emergency treatment of patients with coronavirus disease 2019 (COVID-19) in Uzbekistan8. In this case, deuterium incorporation combined with prodrug design allowed the development of an orally bioavailable antiviral agent with the same mechanism of action as remdesivir.
Overall, the clinical relevance of a potential deuterium switch approach is underscored by the existence of at least 15 compounds under clinical investigation. However, the use of deuterium has recently expanded beyond the improvement of already marketed drugs to become an integral part of the drug discovery process, in which it is often used at early stages to overcome PK shortcomings. Deucravacitinib, an allosteric tyrosine kinase 2 (TYK2) inhibitor approved for psoriasis in September 2022, is the first example of a novel deuterated FDA-approved drug. Here, deuterium incorporation avoids the formation of a non-selective metabolite and preserves the exquisite specificity of the parent drug for TYK2 over the other enzymes belonging to the Janus kinase (JAK) family9. At present, four novel deuterated compounds are under clinical investigation: BMS-986322 and BMS-986202 (ref. 10), follow-up candidates of deucravacitinib; deucrictibant, a bradykinin B2 receptor antagonist for hereditary angioedema attacks11; and VX-984, a DNA-protein kinase inhibitor for radiosensitization in oncology12.
In this Review, we start by providing a brief account of deuterium properties along with the rationale for its use in drug development. We then elaborate on the different effects that deuterium can exert on bioactive molecules. Given that the use of deuterium in drug discovery has already been reviewed elsewhere13,14,15,16,17, and that a comprehensive catalogue of examples is beyond the scope of this study, we focus on the most instructive examples that showcase the multifaceted potential of deuterium in drug research and development (R&D), mainly analysing primary peer-reviewed studies published between 2020 and 2022. We further highlight the progress made in the field by reporting successful examples of both deuterium switches and de novo deuterated compounds, either on the market or in the pipeline. Finally, we share our perspectives on the challenges faced by drug developers when translating deuterated drugs from in vitro settings to the market.
Deuterium and rationale for drug deuteration
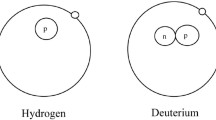
Hydrogen, the most abundant element in the universe, has three isotopes that exist naturally: protium (1H, hereinafter abbreviated as H, which has one proton and one electron, with a 99.9844% abundance), deuterium (2H, hereinafter abbreviated as D, bearing an added neutron, with a 0.0156% abundance)18 and tritium (3H, hereinafter abbreviated as T, bearing two added neutrons, present in traces) (Fig. 1). As D and T can be easily detected by mass spectrometry and radioactivity measurements, respectively, they are extensively used in life sciences to identify, monitor and understand biological and chemical processes19,20. Whereas T is a radioactive isotope, albeit with a long half-life (t½), D is stable and can be handled without any special requirements. As such, with the advent of ultrasensitive mass spectrometry, D is complementing, or even replacing, T in many biomedical areas.
a, Comparison between protium (H) and deuterium (D), showing the difference in atomic mass between the two species. b, The different extent of activation energy for C–H and C–D cleavage steps. c, A representation of the deuterium kinetic isotope effect (DKIE) for cytochrome P450 (CYP)-mediated oxidative metabolism. d, Ranking of the main CYP-mediated oxidative transformations in terms of sensitivity to deuteration and approximate DKIE values.
Produced by nucleosynthesis during the Big Bang, D was first discovered and named by Harold Urey in 1932 (ref. 21). Even though this heavy isotope is made in stars, it can also be found in small amounts on Earth (120–150 ppm), with slight variations between the marine and non-marine worlds. Of note, the recent discovery that bone collagen from Scotland’s grey seals has the highest level of D ever found in biogenic samples has extended the upper limit of its natural abundance to 310 ppm. Even more surprisingly, this enrichment is only related to the amino acid proline and its derivative hydroxyproline, a phenomenon that is still poorly understood22.
Prompted by reassuring data on D safety in humans that has emerged over the years23,24, the popularity of this isotope in drug discovery and development has increased owing not only to its safety as a tracer in metabolism studies, in which the administration of the D-labelled drug helps to track the fate of a molecule through the body and excreta, but also to the fact that it can be incorporated in pharmacologically active compounds as a bioisostere.
Arguably, the H to D substitution is one of the most conservative examples of bioisosterism. The two isotopes are very similar in terms of physicochemical properties, with D displaying a smaller molar volume (by 0.140 cm3 mol−1 per atom), a lower lipophilicity (ΔlogPoct −0.006), slightly altered pKa values (higher or lower depending on the specific compound) and C–D bonds being shorter than C–H bonds by 0.005 Å. The only substantial difference is that D has a twofold larger mass than H (Fig. 1a). As a result, the C–D bond is associated with a reduced vibrational stretching frequency compared with the C–H bond, a lower ground-state energy and, in turn, a greater activation energy for cleavage (Fig. 1b). It follows that the C–D bond is more stable than the C–H bond (with a difference of 1.2–1.5 kcal mol−1)25, and its cleavage occurs in a slower fashion. Such a difference in reactivity is quantified by the so-called deuterium kinetic isotope effect (DKIE), which is expressed as a ratio of the rate constants (kH/kD)26,27: the higher the DKIE value, the slower the C–D bond breaking rate compared with that of C–H. A primary isotope effect occurs when the rate of a C–H cleavage is measured and compared with the rate of the same C–D cleavage, with typical values between 1 and 5. In specific cases, DKIEs might be <1 (inverse DKIE) or >5 (large DKIE), with a theoretical upper limit of 9 (ref. 28). Secondary isotope effects from adjacent atoms can affect the cleavage rate as well, although to a lesser extent. However, given that in certain contexts DKIEs accumulate and isotopic substitutions influence cleavage even from a long distance in the molecule (that is, distal DKIE)29, DKIEs might reach significant values and slow down the rate of bond cleavage, with a profound impact on pure chemical as well as enzyme-catalysed processes, and can therefore be capitalized upon to improve drug profiles from different perspectives (Fig. 2).
A key use of deuteration is to reduce metabolism at vulnerable sites on the drug molecule, often called soft spots. This can improve the pharmacokinetic properties of the compound, resulting in lower and/or less-frequent dosing, as with deutetrabenazine. It could also decrease the formation of non-selective drug metabolites and thereby improve drug selectivity, as with deucravacitinib. These and other examples are described in depth in the main text.
Possible benefits of deuterium incorporation in drugs
Influence on oxidative metabolism
It is estimated that more than half of the marketed drugs are metabolized by enzymes in the cytochrome P450 (CYP) family30. Unfortunately, oxidative metabolism might be associated with several drawbacks: formation of unstable, reactive, non-selective and toxic intermediates and/or metabolites; occurrence of inter-patient variability owing to genetic polymorphisms; and saturation, induction or inhibition of the isozyme, leading to potentially harmful drug–drug interactions. Therefore, the replacement of H with D, based on the DKIE, is a strategy particularly pursued by drug developers to attenuate CYP-mediated metabolism and its inherent disadvantages31,32,33 (Fig. 1c). Importantly, to observe a significant DKIE and an impact of D on oxidative metabolism rate, the catalytic cycle must include a C–D cleavage step that should be, at least partially, rate limiting. Moreover, the magnitude of DKIE depends on the route of metabolism, the enzyme involved and the specific substrate, and it cannot be predicted a priori. Yet, as a general rule, O-dealkylation is the reaction that is most sensitive to deuterium incorporation, followed by amide N-dealkylation, and oxidation of alkyl groups. Conversely, amine N-dealkylation is the least sensitive transformation, and aryl hydroxylation is not influenced by H to D substitution as it does not involve a direct abstraction step during enzyme catalysis33 (Fig. 1d).
The primary effect of the H to D switch is the improvement of the PK performance, mainly at the level of t½, area under the curve (AUC) and maximum concentration (Cmax). This may result in a lower dose or dose frequency required to achieve adequate efficacious drug exposure in patients. Nevertheless, caution must be exercised, as deuteration at one site might divert the compound to other biotransformation routes and increase the metabolism at other sites, a phenomenon termed metabolic switching or metabolic shunting. Metabolic switching is a more appropriate term when a collateral and undesired metabolic pathway is increased (for example, oxidation of an alternative site)34. As CYPs usually oxidize substrates at multiple sites through alternative pathways, a decrease in metabolite formation rate following deuteration of oxidizable soft spots may increase the oxidation rate at one or more alternative undeuterated soft spots. In contrast, metabolic shunting is the preferred expression when a metabolic pathway that leads to desirable and non-toxic metabolites is increased (for example, glucuronidation). In both scenarios, the result is that the metabolic pattern is modified upon deuteration, giving rise to different levels of metabolites, while there is no significant change in the overall extent of metabolism.
The changes in metabolic pathways are not always predictable and therefore are measured by in vitro and/or in vivo metabolism studies. For example, caffeine deuteration at one methyl group at a time attenuates the oxidation at the deuterated position, whereas it induces significant metabolic switching at the non-deuterated soft spots34,35. Only per-deuteration of all the three methyl groups (d9-caffeine) (Table 1) can effectively reduce N-dealkylation reactions and improve the PK profile36,37, as judged by a fourfold higher exposure to d9-caffeine and a fivefold to tenfold reduced exposure to downstream metabolites relative to unlabelled caffeine in a phase I trial38. Even though the use of d9-caffeine in food and beverages seems unlikely, the fact that this deuterated compound requires lower doses and dosing frequency, while ensuring less exposure to downstream metabolites, makes it an alternative option to caffeine in the clinic, where it could be used to treat apnoea in premature infants or, in combination with analgesic agents, to manage pain. Interestingly, following oral administration in rats, more gradual absorption was observed (Cmax at 2 h for d9-caffeine versus 1 h for caffeine)39, suggesting that, even though the difference in lipophilicity between H and D is not significant, when multiple D atoms are present, the variation might add up and result in slower absorption. Lastly, a study from 1987 reported a significant increase in d9-caffeine binding to human serum albumin compared with that of caffeine (46% versus 27%, respectively), suggesting that this feature, which is often overlooked, should also be taken into consideration when developing deuterated drugs40.
In the case of enzalutamide, an androgen receptor inhibitor approved for the treatment of castration-resistant prostate cancer, no metabolic switching occurs, and deuteration at the amide N-methyl group (deutenzalutamide, d3-enzalutamide; Tables 1 and 2) is sufficient to afford a superior analogue, at least from a PK perspective. Enzalutamide undergoes two different reactions: hydrolysis leading to M1, a non-active carboxylic acid metabolite, and N-demethylation mediated by CYP2C8 and CYP3A4/5 leading to M2, an active metabolite, which is thought to increase the risk of seizures. Notably, the observation that M1 was mainly formed by hydrolysis of M2 rather than enzalutamide itself led to the conclusion that deuteration reduced the production of both M1 and M2 in the absence of a metabolic switch in favour of M1. These improved PK parameters in preclinical studies translated into a stronger antitumour activity in xenograft models41. As expected, in phase I trials the steady-state administration of 80 mg of deutenzalutamide achieved the same plasma concentration as 160 mg of enzalutamide42. However, only future head-to-head trials enrolling a higher number of patients will provide conclusive evidence in terms of reduced risk of epilepsy and better safety profile.
Improved PK and no occurrence of metabolic switching events were also reported with the deuteration of ivacaftor, the first drug approved for cystic fibrosis. Ivacaftor has two tert-butyl moieties, but its oxidative metabolism is mainly focused on only one, whereby one of the three methyl groups undergoes CYP3A4-mediated hydroxylation to an alcohol and further oxidation to a carboxylic acid. D was incorporated at the soft tert-butyl moiety, affording deutivacaftor (d9-ivacaftor; Tables 1 and 2). No significant metabolic switching occurred on the other tert-butyl moiety, as demonstrated by similar PK parameters obtained when deutivacaftor and d18-ivacaftor, containing two per-deuterated tert-butyl groups, were assessed in a single-dose crossover study conducted by Concert Pharmaceuticals in healthy human volunteers (t½ 15.9 h; AUC0–inf 3,812 h ng ml−1 versus t½ 16.4 h; AUC0–inf 3,196 h ng ml−1)43. Additional clinical studies assessing the efficacy of deutivacaftor versus ivacaftor, both given at a single dose of 150 mg, showed that deutivacaftor could be administered once daily instead of twice per day as needed for ivacaftor. Deutivacaftor is currently in phase III clinical trials as part of a triple combination therapy43.
Improving the PK profile of a drug through deuteration may also decrease the amount of a co-administered booster (that is, a substance with the function of increasing the bioavailability of the co-administered drug) required to achieve efficacious exposure. Dextromethorphan is an active ingredient in many over-the-counter antitussive medications, and quinidine is an antimalarial and antiarrhythmic agent. In 2010, a fixed-dose combination of these two compounds (AVP-923: dextromethorphan 20 mg and quinidine 10 mg) became the first FDA-approved treatment for pseudobulbar affect44. The reason for devising this combination was that dextromethorphan undergoes extensive first-pass O-demethylation mediated by CYP2D6, whereas quinidine acts as a booster as it reversibly inhibits CYP2D6, thereby increasing dextromethorphan t½ and brain penetration. Conversely, quinidine administration may increase the risk of QT prolongation, torsades de pointe and drug–drug interactions. Hence, D was incorporated in dextromethorphan at both the methoxy group and the tertiary amine to give rise to d6-dextromethorphan. In a phase I trial, a fixed-dose combination of this deuterated compound and quinidine, termed AVP-786 (Fig. 2 and Tables 1 and 2), was able to replicate the steady-state plasma levels of AVP-923 using a lower dose of quinidine (4.5 mg)45. Two years later, the FDA granted fast-track designation to AVP-786 for the treatment of agitation in patients with Alzheimer disease, for which no approved therapy is currently available46. From a regulatory standpoint, the FDA allowed the use of data available on AVP-923 based on a previous phase II study of efficacy for the same disorder to support the investigational new drug (IND) application and regulatory filings for AVP-786. Consequently, from 2015 on, AVP-786 has been assessed only in phase III trials without the need for a phase II study. Nevertheless, the clinical trial data on the efficacy for the treatment of agitation in Alzheimer disease shared so far have shown inconsistent results, which await further clarification47. AVP-786 is also being investigated in clinical trials for the treatment of other disorders that affect the central nervous system.
Deuteration might also decrease the formation of non-selective metabolites and provide a more desirable safety profile. Osimertinib is a standard-of-care therapy for previously untreated advanced EGFR mutation-positive non-small-cell lung cancer48. Although this drug results in strong clinical responses, the metabolite resulting from N-demethylation has reduced selectivity for mutant EGFR compared with wild-type EGFR, which might be responsible for the adverse effects of the drug49. Deuterium was therefore incorporated at both the terminal CH2 of the acrylamide moiety, the key site for covalent binding to the cysteine of EGFR, and the N-methyl of the indole50. The mesylate of the resulting d5-compound, named dosimertinib (Table 1), showed a similar metabolic stability but a reduced generation of the N-demethylated metabolite after oral dosing in rats (AUC 67.3 nM h versus 2.64 nM h). Subsequent in vivo efficacy studies revealed that dosimertinib showed comparable antitumour activity to its non-deuterated counterpart, but did not cause any significant body weight loss, suggesting that the alterations in metabolism might result in a better safety profile. This preclinical observation is being further tested in ongoing clinical trials in China (CXHL2000060 and CXHL2000061).
Significant DKIE values are associated not only with CYP-mediated reactions, as mentioned earlier, but also with transformations catalysed by enzymes other than cytochromes, including alcohol and aldehyde dehydrogenases51, monoamine oxidases2,52, lysyl oxidase53, vascular adhesion protein 1 (VAP1)54 and aldehyde oxidase (AO)55. As highlighted in Box 1, AO is gaining increasing interest among drug developers as some advanced clinical candidates have recently failed owing to metabolism mediated by this oxidase. In the case of JNJ38877605, an inhibitor of MET kinase, a phase I study was terminated owing to the renal toxicity caused by the AO-catalysed formation of quinoline-2-one and species-specific insoluble metabolites56. Incorporation of D at the 2-position of the quinoline ring (d1-JNJ38877605; Table 1) proved to be a successful strategy in reducing the formation of nephrotoxic metabolites in monkeys, both in vitro, using liver S9 fraction assay, and in vivo, after oral administration. In the latter study, d1-JNJ38877605 treatment led to a 1.88- and 1.56-fold increase in AUC and Cmax, respectively, and to decreased levels of the renal toxic metabolites when compared with its undeuterated counterpart. On the other hand, incorporation of fluorine at the same position, which is a common strategy in medicinal chemistry to block metabolic soft spots, proved to be unfeasible as it impaired an essential hydrogen bond interaction between the quinoline nitrogen atom and a key methionine residue in the enzyme57, indicating that D might be a better option when no change in steric hindrance is tolerated. Based on these promising results, d1-JNJ38877605 will be advanced by DeuterOncology into phase I trials as a potential lung cancer treatment5.
Influence on the interconversion between stereoisomers
Not only is deuterium able to affect metabolic processes, it also influences pure chemical events involving a C–H(D) cleavage step, such as enantiomerization or epimerization, a phenomenon quite important in the context of chiral switches (Box 2). Incorporation of D at the stereocentre through the deuterium-enabled chiral switch (DECS) approach can decrease the rate of atom abstraction and stabilize interconverting stereoisomers. Over the years, DECS has been used as a means to stabilize, isolate, characterize and also administer the preferred stereoisomer, thereby improving the therapeutic promise of configurationally unstable drugs58. DECS was first validated on telaprevir, an inhibitor of hepatitis C protease59, and subsequently applied to the immunomodulatory drugs thalidomide (Box 2), lenalidomide and avadomide. DRX-164 (Table 1), the D-stabilized (S)-enantiomer of avadomide, proved to be 20-fold more potent than the (R)-enantiomer in inhibiting tumour necrosis factor (TNF) production in human peripheral blood monocytes after lipopolysaccharide stimulation58,60. Based on greater efficacy in a myeloma xenograft model compared with avadomide, DRX-164 has been recently selected as a clinical candidate for the treatment of haematological cancers and solid tumours.
DECS can also be used to perform a thorough characterization of the two stereoisomers and to decipher their activities, as in the case of pioglitazone, an antidiabetic drug, sold as racemate, recommended for the off-label treatment of nonalcoholic steatohepatitis (NASH). Although both its enantiomers equally inhibited the mitochondrial pyruvate carrier, a mechanism responsible for the therapeutic effect, only the d1-(S)-enantiomer displayed agonism for peroxisome proliferator-activated receptor-γ (PPARγ), an off-target effect associated with adverse events, such as weight gain, oedema and bone loss. Conversely, d1-(R)-pioglitazone (PXL065; Fig. 2 and Tables 1 and 2) showed no activity on PPARγ and, therefore, no weight gain in mice. In a subsequent phase Ia study, PXL065 treatment resulted in preferential exposure to the (R)-enantiomer compared with a higher dose of racemic pioglitazone. As it was well tolerated, PXL065 was advanced into a phase II trial for NASH61. Owing to the lack of PPARγ agonism and side effects, PXL065 has also been assessed in preclinical models of X-linked adrenoleukodystrophy, a rare neurometabolic disease61,62, and the evaluation of its clinical efficacy in patients with X-linked adrenoleukodystrophy is planned to begin in 2023.
Finally, the deuteration approach has been exploited for the discovery of antivirals against COVID-19 (ref. 63) and in particular novel inhibitors of the main protease (Mpro), given the success of paxlovid, an oral combination of the Mpro inhibitor nirmatrelvir and the PK booster ritonavir that is authorized for emergency use. In this context, α-ketoamides were generated as epimeric mixtures through the Ugi multicomponent reaction. However, in vitro biological evaluation of the pure epimers showed that the (R)-epimers had a higher inhibitory potency than the (S)-epimers. Notably, the d1-(R)-compound Y180 (Table 1) showed low conversion rate from (R)- to (S)-epimers in vivo when orally administered in rats, as well as excellent oral bioavailability in mice and dogs. Oral treatment with Y180 ameliorated the virus-induced tissue damage in both nasal turbinate and lung of transgenic humanized ACE2 mice infected with the Alpha/B.1.1.7 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant. Lastly, the antiviral activity of the Y180–ritonavir combination against the Omicron variant was more potent than that of nirmatrelvir–ritonavir in the same transgenic mouse model64.
Influence on mechanisms of action: RT001 as a case study
Deuterium has been incorporated in nutritional agents to slow down processes such as dimerization or lipid peroxidation, and thereby provide novel pharmacological activities. This approach has led to the generation of deuterated dietary compounds such as d3-vitamin A (ALK001; Table 2), which is in clinical trials for ophthalmological disorders65,66,67, and d2-linoleic acid ethyl ester (RT001; Fig. 2 and Tables 1 and 2), which is in clinical trials for several neurodegenerative diseases.
Linoleic acid, one of the most abundant dietary polyunsaturated fatty acids (PUFAs), is highly susceptible to reactive oxygen species (ROS)-driven oxidation owing to its labile bis-allylic position, where hydrogen abstraction yields a radical that can trigger a self-propagating PUFA oxidation chain reaction and initiate lipid peroxidation. Deuteration at this soft position led to the generation of RT001, a compound more resistant to oxidative damage, which is being tested in several neurodegenerative diseases in which lipid peroxidation has a crucial pathophysiological role. On the basis of satisfactory PK and safety data in humans68, RT001 has been advanced into clinical trials for the treatment of Friedrich ataxia69, infantile neuroaxonal dystrophy, amyotrophic lateral sclerosis and, more recently, progressive supranuclear palsy70.
Influence on drug–target interactions: an open question
The H to D switch is not thought to influence the biochemical potency or the selectivity profile of a molecule given that electronic properties, shape, size and steric flexibility of the deuterated analogue are very similar to those of its undeuterated counterpart. This is consistent with all the examples described above, in which deuterated and undeuterated versions had overlapping in vitro activities.
However, a few recent reports have questioned this common belief — derived from unrelated and different perspectives — and have argued that D might actually be able to modulate drug–target interactions. For instance, a recent study by Ben Abu et al.71 showing that humans are able to distinguish the taste of heavy water (D2O) from that of normal water (H2O), with D2O having a distinct sweeter taste than H2O, has seemingly put an end to a long-standing controversy dating back to the 1930s with a paper by Urey and Failla72. In the same study, it was shown that D2O but not H2O treatment activated TAS1R2/TAS1R3, the main receptor for sweet taste, when transiently expressed in human cell lines. Interestingly, computational simulations showed that exposure to D2O versus H2O promoted a slight attenuation of the structural fluctuations of most receptor residues, particularly those directly exposed to the aqueous environment, with the whole protein becoming more rigid. Even though the specific molecular mechanism underlying the activation of TAS1R2/TAS1R3 by D2O remains to be fully established, this work clearly demonstrates that surrounding heavy water molecules can trigger TAS1R2/TAS1R3 signalling so that D2O tastes sweet to humans.
Another example is related to the management of opioid dependence through immunopharmacotherapy. The rationale behind this approach is that an antibody harbouring a hapten structurally similar to the target drug can generate an immune response against an opioid molecule, thereby reducing its concentration at the site of action and minimizing its pharmacodynamic effects. This has drawn increasing interest in the development of heroin vaccines bearing deuterated heroin-like haptens. Indeed, by postulating that D might influence the binding to B cell receptors, thus modulating B cell recognition, Janda and colleagues73 have recently compared the host immune response to heroin vaccines harbouring a non-deuterated hapten with that of their d6-analogue (Fig. 2 and Table 1). Specifically, both haptens were coupled to the highly immunogenic T cell-dependent antigen keyhole limpet haemocyanin (KLH) as a carrier to generate the corresponding conjugates (HAc and HdAc, respectively). Notably, when these molecules were compared in an inclusive vaccine study in mice, HdAc-vaccinated animals produced higher antibody titres than those observed in the HAc groups. A surface plasmon resonance experiment also showed tighter binding affinity for heroin for HdAc (half-maximal inhibitory concentration (IC50) 136 nM) versus HAc (IC50 >2 µM). As a result, the HdAc vaccine displayed a greater efficacy in two murine behavioural models (that is, hot plate antinociception and tail-flick assays) than HAc. The authors ruled out that their results may have been due to differences in terms of stability towards aqueous hydrolysis, consistent with the fact that hydrolytic transformations are devoid of DKIE. However, the exact mechanism responsible for the boost in the immune response by the HdAc vaccine remains to be determined.
Deuterium switches approved so far
Over the past two decades, several companies have invested in deuterium switches, attracting significant commercial interest, with total transactions estimated to have exceeded US$7 billion dollars5,74. These efforts led to FDA approval of deutetrabenazine and the development of two new drug candidates, donafenib and VV116, which have so far received approval in China and Uzbekistan, respectively. On a wave of enthusiasm following the deutetrabenazine approval, at least 15 more candidates are currently being evaluated in clinical trials (Table 2), six of which are in phase III and might obtain approval in the near future.
Deutetrabenazine, the first FDA-approved deuterated drug
As mentioned above, deuterium substitution is particularly effective in reducing O-dealkylation by CYP isozymes. This characteristic has been exploited to synthesize deutetrabenazine (d6-tetrabenazine; Fig. 2 and Tables 1 and 2), the first deuterium switch-derived drug to be granted marketing approval75. Its undeuterated counterpart, tetrabenazine, an inhibitor of vesicular monoamine transporter 2 (VMAT2), was first discovered in the 1960s as an antipsychotic agent76. However, later on it proved to be far more valuable for the treatment of involuntary movement disorders, which led to its FDA approval for chorea associated with Huntington disease in 2008. Unfortunately, it soon became apparent that tetrabenazine administration reduced serotonin levels, which could worsen depression and suicidal ideation in certain psychiatric patients. This resulted in the FDA issuing a black box warning, restricting its use in patients who were actively suicidal or suffered from untreated depression. Another disadvantage of this drug is that it is metabolized through reduction by carbonyl reductase to an active metabolite and subsequent CYP2D6-mediated O-dealkylation to an inactive O-desmethyl metabolite, which often leads to subtherapeutic drug levels requiring increased dosing frequency (that is, three times daily).
Thus, seeking to improve dose regimen and adherence to treatment, Auspex Pharmaceuticals developed a deuterated analogue of tetrabenazine, d6-tetrabenazine, that, thanks to the incorporation of D at the two soft spots, made the active metabolite more resistant to O-dealkylation by CYP2D6. A phase I crossover study to assess the PK of deutetrabenazine versus tetrabenazine in healthy volunteers showed increased exposure to the active metabolites77,78. As a result, deutetrabenazine could be administered twice a day at a lower dose than tetrabenazine. In 2015, Teva Pharmaceuticals acquired the rights from Auspex Pharmaceuticals, and deutetrabenazine was approved by the FDA for the treatment of both chorea in 2017 (ref. 79) and tardive dyskinesia the following year6. Conversely, trials evaluating both fixed80 and flexible81 doses of this heavy drug for the treatment of Tourette syndrome did not meet the primary end point. Currently, deutetrabenazine is being evaluated in phase III studies for the treatment of involuntary movements in patients with cerebral palsy.
Although deuterium incorporation can significantly improve the dosing regimen of tetrabenazine, it is still not clear whether its improved PK profile results in better efficacy and/or safety than that of the undeuterated compound. Over the years, a cluster of placebo-controlled studies evaluating the efficacy and safety of deutetrabenazine in patients affected by Huntington disease82,83,84 and tardive dyskinesia85,86,87 and subsequent retrospective analyses88,89 have shown how the improved dosing regimen is more effective in attenuating chorea and mitigating some undesirable side effects, mainly of a neuropsychiatric nature. Nonetheless, data in this regard are still conflicting and inconclusive90. Hence, there is a clear need for a direct head-to-head clinical trial to rigorously evaluate the long-term safety and efficacy of deutetrabenazine versus tetrabenazine and its added clinical value91. Until then, caution is advised when administering deutetrabenazine, which should not be given to patients with active suicidality, untreated depression or hepatic impairment or to patients taking monoamine oxidase inhibitors, reserpine or tetrabenazine.
From a regulatory standpoint, deutetrabenazine represented a major milestone in the advancement of deuterium switches and established a clearer framework in the deuterium switch field. Indeed, it received the new chemical entity (NCE) exclusivity, a status that provides the licence holder with a 5-year protection from market competition. Subsequently, it obtained approval via the 505(b)(2) pathway, which allows the applicant to leverage safety and efficacy data already collected for approval of the undeuterated counterpart even when “some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference”92. Although 505(b)(2) applications generally consist of modifications of previously approved drug products that do not change the chemical structure of the active species (that is, new dosage forms, routes of administration and prodrugs)93, the deutetrabenazine case has proved that deuteration may also be a viable bridging approach, which is now being pursued for at least two other deuterated drugs, deutenzalutamide and PXL065.
Donafenib for hepatocellular carcinoma
Sorafenib is a multi-kinase inhibitor approved for the treatment of unresectable or metastatic liver carcinoma and advanced renal carcinoma. Despite its misleading name, donafenib (d3-sorafenib; Fig. 2 and Tables 1 and 2) is the result of a deuterium switch, whereby three D atoms are incorporated at the secondary amide on the pyridine ring of sorafenib. Curiously, a clear rationale for the deuteration of sorafenib cannot be found in the literature, probably owing to the fact that the main circulating metabolite of sorafenib is the equally potent pyridine N-oxide. Developed by Suzhou Zelgen Biopharmaceuticals, donafenib was approved by the National Medical Products Administration of China in 2021 for the first-line treatment of patients with unresectable hepatocellular carcinoma94 who had not previously received systemic treatment7, and it is being evaluated, alone or in combination, for the treatment of other cancers.
Following encouraging phase I trials95,96, donafenib was compared with sorafenib in a phase II/III trial. Although drug absorption and exposure were lower on day 1 in patients with hepatocellular carcinoma who received twice-daily oral donafenib 200 mg than in those who received twice-daily sorafenib 400 mg, PK values at steady state (day 14) were slightly higher in donafenib-treated versus sorafenib-treated patients (Cmax 6.6 μg ml−1 versus 5.0 μg ml−1; mean AUC 45.4 h μg ml−1 versus 38.1 h μg ml−1). Moreover, the authors reported that median overall survival was longer in the donafenib group than the sorafenib group (that is, 12.1 versus 10.3 months; hazard ratio 0.831), and that the incidence of adverse events (AEs) was lower (25% versus 36% for AEs, which led to treatment suspension and dose reduction; 38% versus 50% for drug-related AEs grade ≥3)97,98. This study represents the first head-to-head comparison to show a statistically significant superiority of the deuterated drug compared with the non-deuterated compound in terms of PK, efficacy and safety. Nevertheless, it should be pointed out that these results were obtained in a study population that consisted for the most part of Chinese patients and therefore cannot be generalized to other populations. Finally, results from an analysis have suggested that donafenib is unlikely to be cost-effective at its current price, which is much higher than that of generic sorafenib99.
VV116 for COVID-19
Remdesivir, a broad-spectrum antiviral agent originally developed by Gilead Sciences as a potential agent for Ebola virus infection, was repurposed for COVID-19 and approved by the FDA in 2020 for emergency use100. It is a monophosphoramidate prodrug of GS-441524 (ref. 101), an inhibitor of the RNA-dependent RNA polymerase of coronaviruses that requires intravenous administration owing to extensive oxidation and ring opening reactions involving its adenine-mimicking pyrrolotriazine moiety102. In an effort to circumvent these PK limitations, the soft spot of GS-441524 was deuterated to reduce degradation of the ring, the hydroxyl groups of the ribose fragment were esterified to afford a prodrug and improve the oral bioavailability, and the hydrobromide salt was prepared to increase water solubility, yielding VV116 (Fig. 2 and Tables 1 and 2). In preclinical investigations, VV116 showed optimal oral bioavailability as well as wide distribution of the active metabolite in tissues targeted by SARS-CoV-2. In a mouse model of SARS-CoV-2 infection, VV116 was more effective than molnupiravir, an oral RNA-dependent RNA polymerase inhibitor approved for emergency use, decreasing the virus titres below the detection limit103.
On the basis of encouraging results on safety, tolerability and PK obtained in phase I studies, VV116 has advanced into international and registrational clinical trials in patients infected with SARS-CoV-2 (ref. 8). So far, its efficacy seems promising104, but no direct comparison with other drugs for COVID-19 has been performed, except for a study comparing VV116 with paxlovid, for which results have not yet been disclosed. In the meantime, VV116 has been approved for emergency use as an oral treatment for COVID-19 in Uzbekistan. Remarkably, VV116 has been recently found to be highly effective against respiratory syncytial virus. Specifically, 25 mg kg−1 of VV116 in mice had a comparable antiviral effect to 100 mg kg−1 of ribavirin, an off-label drug used to treat this infection105.
Deuterium in de novo drug discovery
Deucravacitinib is the first approved drug incorporating D without having an undeuterated counterpart on the market. Other de novo deuterated candidates that have reached clinical trials include two follow-up compounds for deucravacitinib (BMS-986202 (ref. 10) (Fig. 3) and BMS-986322), deucrictibant11 and VX-984 (ref. 12). Besides these advanced candidates, other examples can be found in the literature in which D is used at an early stage of the R&D process to overcome PK hurdles, in addition to potentially improving other drug properties. These include inhibitors of the MDM2–p53 protein–protein interaction for cancer106, MNK1/2 protein degraders for triple-negative breast cancer107, Mpro inhibitors for COVID-19 (refs. 108,109), memantine analogues for Alzheimer disease110, dual TYK2/JAK1 inhibitors for autoimmune diseases111,112 and PET ligands for Huntington disease113.
Starting from hit compound 1, an extensive chemical refinement led to the discovery of deucravacitinib, the first clinical pseudokinase inhibitor endowed with selectivity for tyrosine kinase 2 (TYK2) over other isoforms. First, a ‘magic’ methylation of the primary amide of compound 1 to give compound 2 boosted selectivity for TYK2 over other isoforms. Adoption of the deuterium switch approach increased the metabolic stability of compound 4, reducing the formation of non-selective metabolites such as compound 3. Subsequent late-stage optimizations afforded deucravacitinib. A parallel medicinal chemistry campaign led to the discovery of the back-up compound, BMS-986202.
Deuterated JAK inhibitors for autoimmune and inflammatory disorders
The JAK family consists of four non-receptor tyrosine kinases (JAK1–3 and TYK2), which are involved in the pathogenesis of many immune-mediated diseases. Their inhibition is considered a treatment option for multiple autoimmune and chronic inflammatory conditions114,115. JAK kinases have two domains, a catalytically active JAK homology 1 (JH1) domain and a regulatory domain JAK homology 2 (JH2, also known as a pseudokinase domain), proximal to each other. Although the former domain shows high sequence homology among the different JAKs, the latter domain has much greater differences in the binding pockets. Therefore, JAK inhibitors that are JH1 competitive inhibitors have moderate selectivity and show an increased risk of serious side effects (for example, infections, heart-related events, malignancies and vascular events), with narrow therapeutic margins. For this reason, they are currently under the lens of regulatory agencies, and a black box warning has been issued for JAK inhibitors that are approved for the treatment of chronic inflammatory conditions116. Hence, interest has recently focused on targeting TYK2 (refs. 117,118), an isoform that mediates signalling of IL-12, IL-23 and type I interferons, sustaining both signalling and functional responses of immune cells119. A special and leading place in this area is held by deucravacitinib120 (Figs. 2 and 3 and Table 3), a first-in-class pseudokinase inhibitor that binds to the JH2 regulatory domain via a highly specific allosteric mechanism, resulting in exquisite selectivity for TYK2 over other isoforms.
Deucravacitinib is the result of an elegant and extensive medicinal chemistry campaign started at Bristol-Myers Squibb with the discovery of the hit compound 1 (Fig. 3) by high-throughput screening. Both potency and selectivity of the TYK2 inhibitor were improved, thanks to iterative cycles of chemical refinement, and resulted in the potent and specific TYK2 inhibitor 2 (TYK2 JH2 IC50 0.9 nM; TYK2/JAK1/JAK2 JH1 IC50 >50 μM; Fig. 3). Unfortunately, the compound underwent an N-dealkylation reaction, forming metabolite 3 that showed a significant drop in selectivity (TYK2 IC50 42 nM; JAK1 IC50 43 nM; JAK2 IC50 18 nM; Fig. 3). Indeed, the presence of the methylamide has a pivotal role in conferring specificity for TYK2 as it fits a unique ‘alanine pocket’, whereas it is not tolerated by other kinases with larger residues. Incorporation of three D atoms shunted the metabolism away from the methylamide, avoided the formation of the non-selective metabolite and led to the discovery of compound 4 (Fig. 3), which is equally potent and selective but more stable than 2 in terms of oxidative metabolism of the N-methylamide. A late-stage optimization eventually led to deucravacitinib, a potent and oral inhibitor of the TYK2 pseudokinase domain (TYK2 JH2 IC50 0.2 nM; Ki 0.02 nM), displaying high selectivity over other kinases (>10,000 nM)9, with the only exception being JAK1 (JAK1 JH2 IC50 1 nM; Ki 0.33 nM). The in vitro selectivity for TYK2 over other isoforms is supported by clinical data demonstrating that deucravacitinib given at therapeutic doses does not cause any meaningful treatment-related laboratory abnormalities usually seen with JAK1/2/3 inhibitors (for example, decrease in haemoglobin or neutrophil number, or altered platelet homeostasis)121.
On the basis of preclinical data in multiple models of autoimmune and chronic inflammatory diseases122, deucravacitinib progressed into clinical development. A phase I trial indicated that the compound was able to attenuate multiple signalling and functional responses related to autoimmunity in healthy volunteers122,123. In a subsequent randomized, double-blind, placebo-controlled phase II trial, the therapeutic benefit of deucravacitinib in resolving moderate-to-severe psoriasis was clear, despite the limitations of the study in terms of sample size and duration124. These data correlated with the pharmacodynamic changes in biomarkers of both the IL-23 and type I interferon pathways observed in skin biopsy samples of patients with moderate-to-severe psoriasis121. Patients with greater improvement in psoriasis-related clinical signs and symptoms also reported substantial amelioration of quality-of-life outcomes125. From a safety perspective, deucravacitinib was generally well tolerated126. Given the chronic nature of psoriasis, larger trials over longer durations were needed to understand the risk associated with the compound and to validate the maintenance of response. Thus, deucravacitinib underwent a pivotal phase III trial for the treatment of moderate-to severe plaque psoriasis (POETYK PSO-1), which was conducted during the COVID-19 pandemic in 2020 (ref. 127). Efficacy and safety were evaluated in patients treated for 1 year with a single daily dose of deucravacitinib (6 mg) compared with placebo and apremilast (30 mg). The results revealed that deucravacitinib was superior to placebo and apremilast across multiple end points, and that it was also well tolerated, with AEs being predominantly of mild to moderate severity. A slightly increased rate of non-serious viral infections, particularly those due to herpes zoster reactivation, was observed. Notably, this AE has also been documented with other JAK inhibitors, independently of differences in JAK selectivity128. Additionally, deucravacitinib has recently completed the phase III POETYK PSO-2 trial and a 2-year POETYK PSO long-term extension trial. On the basis of these results, it has received FDA and EMA approval for the treatment of adults with moderate-to severe plaque psoriasis129, and it is under evaluation in clinical trials for multiple immune-mediated diseases (that is psoriatic arthritis130, inflammatory bowel diseases and systemic lupus erythematosus131).
Overall, deucravacitinib is expected to be a game changer in the JAK field thanks to its mechanism of action, which makes this drug highly specific for TYK2. However, recent reports on serious AEs associated with pan-JAK inhibitors call for a thorough follow-up, given that it is still not clear whether the reported events are associated with broad activity against multiple JAK isoforms.
Deuterium incorporation has also been used for another JAK inhibitor, ruxolitinib, a JAK1 and JAK2 inhibitor approved for the oral treatment of myelofibrosis, polycythemia vera and graft-versus-host disease, and for the topical treatment of atopic dermatitis. The deuterated analogue deuruxolitinib (d8-ruxolitinib; Tables 1 and 2) has been recently developed132, which avoids extensive oxidative metabolism around the cyclopentyl ring and provides more sustained JAK inhibition. A phase III trial of this compound for the oral treatment of alopecia areata has recently concluded, and if approved, it will become the second FDA-approved drug for alopecia aerata after baricitinib. Sun Pharma has recently announced that it would acquire the deuruxolitinib developer, Concert Pharmaceuticals, for US$576 million5.
Deucrictibant for hereditary angioedema
Hereditary angioedema (HAE) is a rare disease characterized by recurrent episodes of localized oedema of subcutaneous and submucosal tissues. As these episodes are mainly caused by the release of the nonapeptide bradykinin (BK), bradykinin B2 receptor (B2R) antagonists are effective in treating patients with HAE. Icatibant, a synthetic peptidomimetic B2R antagonist consisting of ten amino acids that is administered subcutaneously, is approved for the treatment of acute HAE episodes133. However, given its short t½, icatibant requires multiple injections, thus reducing treatment adherence.
With the aim of improving patient adherence, deucrictibant (Tables 1 and 3) is being developed by Pharvaris as an oral on-demand treatment134. It has improved intrinsic clearance characteristics compared with the corresponding undeuterated compound, as judged by in vitro microsomal stability assay, even though the full rationale for its deuteration is not clear. Moreover, when orally administered in cynomolgus monkeys after BK intravenous injection, this deuterated drug showed a faster onset of action and prolonged efficacy compared with icatibant11. On the basis of the results from early clinical studies in healthy subjects showing a good safety profile and oral bioavailability11, deucrictibant has been taken into phase II and III trials for the acute treatment and prophylaxis of oedema attacks in patients with HAE.
VX-984 for radiosensitization in oncology
VX-984 (Tables 1 and 3) is an inhibitor of DNA-dependent protein kinases (DNA-PKs). The compound bears two D atoms on the pyrimidine ring and is one of the few examples of DKIE applied to mitigate AO metabolism55, although no data have been published in this regard. DNA-PKs are enzymes that repair double-strand breaks (DSBs) in DNA using non-homologous end joining (NHEJ) and have a role in the resistance of tumour cells to chemo- and radiotherapy. Therefore, pharmacological inhibitors of DNA-PKs are able to prevent DSB repair and enhance the cytotoxicity caused by chemotherapeutic treatment or ionizing radiation. In this context, VX-984 proved to act as a radiosensitizer in both in vitro and in vivo assays, with a greater therapeutic specificity for transformed compared with normal target cells12,135. VX-984 was licensed to Merck KGaA from Vertex Pharmaceuticals in 2017 and has completed a phase I trial, but no information on the compound has been posted on ClinicalTrials.gov since 2019.
Opportunities and challenges for deuteration
Although the use of D in drug discovery offers many advantages, it also has several shortcomings. The SWOT analysis shown in Fig. 4 summarizes the main strengths and weaknesses, along with opportunities and threats, of the deuteration approach, and the outlook for it is further discussed below.
The ability to increase the resistance of a molecule to bond cleavage without significantly altering its steric hindrance or electronic properties is the greatest advantage of deuterium incorporation, which clearly makes this approach stand out from other metabolic blockers, such as halides136. Moreover, in some instances D might represent a safer bioisostere compared with fluorine, based on recent reports raising awareness of instability and metabolism issues around fluorinated drugs that may produce species that are dangerous to both human health and the environment (for example, fluoride or fluoroacetate)137,138.
The impact of D atoms on absorption, distribution, metabolism, excretion and toxicity (ADMET) parameters goes beyond metabolism. Although the difference in lipophilicity between H and D is negligible, when multiple D atoms are present the additive effect might lead to significant differences in terms of plasma protein binding (for example, as with d9-caffeine)40, extent and/or rate of absorption39 and P-glycoprotein efflux, all effects that are still underexplored and warrant future investigations. In this regard, a recent report showing a lower brain-to-plasma ratio of d9-methadone than that of its unlabelled counterpart upon intravenous injection in mice suggests that the reduction in lipophilicity due to the introduction of nine D atoms is sufficient to reduce the passage of deuterated molecules across the blood–brain barrier139.
Other benefits of deuteration that are emerging — but are still not completely understood — include a potential effect of D on both drug–target interactions (as discussed above) and time-dependent inhibition of CYP enzymes140,141,142,143. Inhibition of cytochromes is undesirable in drug R&D as it might lead to drug–drug interactions in patients undergoing multiple therapies. Very recently, a few reports have highlighted that deuterium incorporation might reduce the time-dependent inhibition of CYP enzymes, although the reason behind this effect is still not clear (for example, BMS-986144140,VU6032952141 and d3-clopidogrel142,144).
Deuteration is a challenging technique limited to defined and well-known pathways that require a rate-limiting bond cleavage step. Even when an informed decision on DKIEs is made, the impact of deuteration might not be the one expected, and translating deuterated compounds from bench to bedside is quite challenging. The reasons behind drug failure of deuterated analogues are diverse and include: deuterium-promoted multidirectional metabolic switching (for example, d7-doxophylline145); reduced metabolite-induced efficacy in vivo (for example, d1-N-demethyldiazepam146, d6-δ-tocotrienol147); masking of DKIE by competing conjugating enzymes or nonmetabolic elimination mechanisms (for example, deuterated analogues of propofol148); and interspecies variability jeopardizing the predictivity of preclinical testing (for example, d3-imatinib149).
Thanks to the increased availability of deuterated reagents and synthetic methods (Box 3), which have enriched the repertoire of deuteration approaches, most active pharmaceutical ingredients (APIs) can be prepared with D in the desired soft spots. However, isotopic mixtures are inseparable using common purification techniques, and the challenge is to synthesize deuterated APIs with high isotopic purity at the soft spot (preferably >98%) and on a scale large enough to supply the market. Indeed, suboptimal isotopic purity of reagents and low-efficiency labelling reactions can result in under-deuteration of APIs, requiring extra steps, reiteration of the deuteration process and/or the use of deuterated solvents to prevent H to D exchange. Moreover, process chemists have to devise specific conditions to prevent over-deuteration at sites other than the soft spot or the generation of isotopologue impurities. In general, deuterated reagents cost more than non-deuterated reagents, and other deuterated building blocks may not be commercially available and may require specialized procedures, which can add costs and difficulty to the synthesis. It follows that the manufacturing costs for deuterated APIs are higher than for non-deuterated APIs, and in some instances the synthesis of a deuterated drug may not be financially sustainable. The challenges are not only synthetic but also analytical, as common spectroscopic techniques used to characterize organic compounds (liquid chromatography–mass spectrometry (LC/MS) and NMR) are insufficient to measure the precise location and quantity of deuterium in isotopic product mixtures. A less common method that can assist this determination is molecular rotational resonance (MRR)150, a technique that has not been fully adapted yet.
Currently, there are no established guidelines from the FDA or other governing bodies outlining how to address isotropic impurities in deuterated APIs in terms of analytical, toxicological and regulatory matters. This has led the International Consortium for Innovation & Quality in Pharmaceutical Development to recently establish a working group to frame the problem and develop viable practices151. As such, there is mounting concern that regulatory agencies may default to the use of ICHQ3a152 and treat isotopologues and isotopomers as regular impurities. If this were the case, it could make it very difficult for companies to meet specifications for isotopic impurities, as these depend on the isotopic purity of the D sources or deuterated pools from which the APIs are made.
It is also interesting to note that no official rule has been established so far by the WHO regarding the assignment of international non-proprietary names (INNs) to deuterated APIs153. To our knowledge, this issue has been recently discussed by the INN Programme and Classification of Medical Products Unit, which, according to the publicly available executive summary154, declared that “Ways in which deuterated substances should be named include the well-used deu(t)- prefix on a pre-existing or new INN, the incorporation of -deu- as an infix, or the use of a second word such as xxdeuter, where ‘xx’ could indicate the number of D atoms”. However, as no final decision has yet been made, at present any novel deuterated drug is conventionally named by adding the prefix deu-, regardless of the existence of its non-deuterated counterpart. There are, however, some exceptions to this convention that lack the prefix ‘deu-’, such as poyendarone155,156,157 and dosimertinib50, which are at early stages of development or are being developed in countries that have their own naming agencies.
Deuterium switches offer many opportunities, including the possibility to overcome the issues associated with pharmaceutical development of natural compounds158, as testified by CYB003 (Table 2), a deuterated analogue of the natural product psilocybin that was recently designated by the FDA as a breakthrough therapy for treatment-resistant depression and major depressive disorder. Of note, CYB003, the structure of which has not yet been disclosed, is currently being tested in a phase I/IIa trial in patients with major depressive disorder159. Similarly, several deuterated analogues of N,N′-dimethyltryptamine (for example, d8-DMT), a serotonergic hallucinogen found in several plants, are being developed with the goal of reducing monoamine oxidase A (MAO-A)-mediated metabolism and obtaining more stable inhalable formulations for the treatment of psychiatric or neurological disorders160.
However, the development of drugs resulting from a deuterium switch has become an increasingly challenging task for several reasons. As with all compounds, the patentability will have to be evaluated on a case-by-case basis based on the improvements made by the discoveries during development161,162,163. Another complication is that regulatory agencies require a well-established advantage of the deuterated drug in terms of effectiveness, safety profile or dosing frequency over the existing undeuterated compound before granting approval, and more rigorous head-to-head comparisons with the undeuterated drug are demanded to demonstrate a significant clinical added value. Finally, given that the cost of goods for deuterated drugs can be higher than those for their undeuterated counterparts, it is important that deuterated analogues offer value to patients and other stakeholders proportionate to their cost. For all these reasons, the use of D in drug discovery is now at a crossroads: deuterium switches are slowly going out of fashion, whereas de novo deuteration is becoming an integral part of drug discovery campaigns, where it is widely used to overcome PK liabilities, in addition to other ADMET property considerations, at an early development stage.
In conclusion, deuteration is an ever-evolving and exciting approach with manifold applications, including those beyond the pharmaceutical industry, which include neutron scattering for the investigation of polymers164, electronic materials and TV screens, the brightness of which is enhanced in the presence of the heavy isotope165, and fluorescence microscopy166,167, for which D leads to more stable and brighter fluorescent dyes. In this Review, we summarize recent advances in the use of deuteration in drug R&D, and we foresee that, in spite of the associated challenges, its systematic embedding into medicinal chemistry campaigns will improve the PK and safety profiles of innovative drugs and help to reduce attrition rates.
References
Meanwell, N. A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 54, 2529–2591 (2011).
Belleau, B., Burba, J., Pindell, M. & Reiffenstein, J. Effect of deuterium substitution in sympathomimetic amines on adrenergic responses. Science 133, 102–104 (1961).
Elison, C., Rapoport, H., Laursen, R. & Elliott, H. W. Effect of deuteration of N-CH3 group on potency and enzymatic N-demethylation of morphine. Science 134, 1078–1079 (1961).
Agranat, I., Caner, H. & Caldwell, J. Putting chirality to work: the strategy of chiral switches. Nat. Rev. Drug Discov. 1, 753–768 (2002).
Vitale, G. Deuterium drugs attract investment. CEN Glob. Enterp. 101, 10–10 (2023).
DeWitt, S. H. & Maryanoff, B. E. Deuterated drug molecules: focus on FDA-approved deutetrabenazine. Biochemistry 57, 472–473 (2018).
Keam, S. J. & Duggan, S. Donafenib: first approval. Drugs 81, 1915–1920 (2021).
Qian, H.-j et al. Safety, tolerability, and pharmacokinetics of VV116, an oral nucleoside analog against SARS-CoV-2, in Chinese healthy subjects. Acta Pharmacol. Sin. 43, 3130–3138 (2022).
Wrobleski, S. T. et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J. Med. Chem. 62, 8973–8995 (2019).
Liu, C. et al. Discovery of BMS-986202: a clinical Tyk2 inhibitor that binds to Tyk2 JH2. J. Med. Chem. 64, 677–694 (2021).
Lesage, A. et al. In vitro pharmacological profile of PHA-022121, a small molecule bradykinin B2 receptor antagonist in clinical development. Int. Immunopharmacol. 105, 108523 (2022).
Khan, A. J. et al. VX-984 is a selective inhibitor of non-homologous end joining, with possible preferential activity in transformed cells. Oncotarget 9, 25833–25841 (2018).
Harbeson, S. L. & Tung, R. D. Deuterium in drug discovery and development. Annu. Rep. Med. Chem. 46, 403–417 (2011).
Gant, T. G. Using deuterium in drug discovery: leaving the label in the drug. J. Med. Chem. 57, 3595–3611 (2014).
Liu, J. F. et al. in Platform Technologies in Drug Discovery and Validation (ed. Goodnow, R. A.) 519–542 (Academic, 2017). [Series Ed. Neidle, S. Annual Reports in Medicinal Chemistry Vol. 50].
Pirali, T., Serafini, M., Cargnin, S. & Genazzani, A. A. Applications of deuterium in medicinal chemistry. J. Med. Chem. 62, 5276–5297 (2019).
Cargnin, S., Serafini, M. & Pirali, T. A primer of deuterium in drug design. Future Med. Chem. 11, 2039–2042 (2019).
Brunning, A. Periodic graphics: the science and uses of deuterium (Poster). Chem. Eng. News https://cen.acs.org/physical-chemistry/Periodic-Graphics-science-uses-deuterium/99/i43 (2021).
Baillie, T. A. The use of stable isotopes in pharmacological research. Pharmacol. Rev. 33, 81–132 (1981).
Atzrodt, J., Derdau, V., Kerr, W. J. & Reid, M. Deuterium- and tritium-labelled compounds: applications in the life sciences. Angew. Chem. Int. Ed. Engl. 57, 1758–1784 (2018).
Urey, H. C., Brickwedde, F. G. & Murphy, G. M. A Hydrogen isotope of mass 2. Phys. Rev. 39, 164–165 (1932).
Gharibi, H. et al. Abnormal (hydroxy)proline deuterium content redefines hydrogen chemical mass. J. Am. Chem. Soc. 144, 2484–2487 (2022).
Jones, P. J. & Leatherdale, S. T. Stable isotopes in clinical research: safety reaffirmed. Clin. Sci. 80, 277–280 (1991).
Koletzko, B., Sauerwald, T. & Demmelmair, H. Safety of stable isotope use. Eur. J. Pediatr. 156, S12–S17 (1997).
Scheiner, S. & Čuma, M. Relative stability of hydrogen and deuterium bonds. J. Am. Chem. Soc. 118, 1511–1521 (1996).
Wiberg, K. B. The deuterium isotope effect. Chem. Rev. 55, 713–743 (1955).
Bell, R. P. Recent advances in the study of kinetic hydrogen isotope effects. Chem. Soc. Rev. 3, 513–544 (1974).
Krumbiegel, P. Large deuterium isotope effects and their use: a historical review. Isot. Environ. Health Stud. 47, 1–17 (2011).
Gjervig Jensen, K., Tornby Christoffersen, C., Graulund Hvenegaard, M., Didriksen, M. & Jørgensen, M. Distal kinetic deuterium isotope effect: phenyl ring deuteration attenuates N-demethylation of Lu AF35700. Bioorg. Med. Chem. Lett. 72, 128879 (2022).
Furge, L. L. & Guengerich, F. P. Cytochrome P450 enzymes in drug metabolism and chemical toxicology: an introduction. Biochem. Mol. Biol. Educ. 34, 66–74 (2006).
Nelson, S. D. & Trager, W. F. The use of deuterium isotope effects to probe the active site properties, mechanism of cytochrome P450-catalyzed reactions, and mechanisms of metabolically dependent toxicity. Drug Metab. Dispos. 31, 1481–1498 (2003).
Guengerich, F. P. Kinetic deuterium isotope effects in cytochrome P450 reactions. Methods Enzymol. 596, 217–238 (2017).
Johnson, K., Le, H. & Khojasteh, S. C. in Identification and Quantification of Drugs, Metabolites, Drug Metabolizing Enzymes, and Transporters (2nd edn) (eds Ma, S. & Chowdhury, S. K.) 439–460 (Elsevier, 2020).
Horning, M. G., Haegele, K. D., Sommer, K. R., Nowlin, J. & Stafford, M. Metabolic Switching of Drug Pathways as a Consequence of Deuterium Substitution (OSTI, 1975).
Regal, K. A., Kunze, K. L., Peter, R. M. & Nelson, S. D. Oxidation of caffeine by CYP1A2: isotope effects and metabolic switching. Drug. Metab. Dispos. 33, 1837–1844 (2005).
Benchekroun, Y., Dautraix, S., Desage, M. & Brazier, J. L. Deuterium isotope effects on caffeine metabolism. Eur. J. Drug Metab. Pharmacokinet. 22, 127–133 (1997).
Parente, R. M., Tarantino, P. M., Sippy, B. C. & Burdock, G. A. Pharmacokinetic, pharmacological, and genotoxic evaluation of deuterated caffeine. Food Chem. Toxicol. 160, 112774 (2022).
Sherman, M. M. et al. A double-blind, randomized, two-part, two-period crossover study to evaluate the pharmacokinetics of caffeine versus d9-caffeine in healthy subjects. Regul. Toxicol. Pharmacol. 133, 105194 (2022).
Bechalany, A. et al. Isotope effects on the lipophilicity of deuterated caffeines. Helv. Chim. Acta 72, 472–476 (1989).
Cherrah, Y. et al. Study of deuterium isotope effects on protein binding by gas chromatography/mass spectrometry. Caffeine and deuterated isotopomers. Biomed. Environ. Mass. Spectrom. 14, 653–657 (1987).
Pang, X., Peng, L. & Chen, Y. Effect of N-methyl deuteration on pharmacokinetics and pharmacodynamics of enzalutamide. J. Label. Compd. Radiopharm. 60, 401–409 (2017).
Li, X. et al. Phase I clinical trial of HC-1119: a deuterated form of enzalutamide. Int. J. Cancer 149, 1473–1482 (2021).
Bardin, E. et al. Modulators of CFTR. Updates on clinical development and future directions. Eur. J. Med. Chem. 213, 113195 (2021).
Garnock-Jones, K. P. Dextromethorphan/quinidine: in pseudobulbar affect. CNS Drugs 25, 435–445 (2011).
Garay, R. P. & Grossberg, G. T. AVP-786 for the treatment of agitation in dementia of the Alzheimer’s type. Expert Opin. Investig. Drugs 26, 121–132 (2017).
Wilkinson, S. T. & Sanacora, G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug. Discov. Today 24, 606–615 (2019).
Khoury, R. et al. AVP-786 as a promising treatment option for Alzheimer’s disease including agitation. Expert Opin. Pharmacother. 22, 783–795 (2021).
Khozin, S. et al. Osimertinib for the treatment of metastatic EGFR T790M mutation-positive non-small cell lung cancer. Clin. Cancer Res. 23, 2131–2135 (2017).
Dickinson, P. A. et al. Metabolic disposition of osimertinib in rats, dogs, and humans: insights into a drug designed to bind covalently to a cysteine residue of epidermal growth factor receptor. Drug Metab. Dispos. 44, 1201–1212 (2016).
Meng, Y. et al. Discovery of dosimertinib, a highly potent, selective, and orally efficacious deuterated EGFR targeting clinical candidate for the treatment of non-small-cell lung cancer. J. Med. Chem. 64, 925–937 (2021).
Ghanayem, B. I., Burka, L. T. & Matthews, H. B. Metabolic basis of ethylene glycol monobutyl ether (2-butoxyethanol) toxicity: role of alcohol and aldehyde dehydrogenases. J. Pharmacol. Exp. Ther. 242, 222–231 (1987).
Schneider, F. et al. Pharmacokinetics, metabolism and safety of deuterated L-DOPA (SD-1077)/carbidopa compared to L-DOPA/carbidopa following single oral dose administration in healthy subjects. Br. J. Clin. Pharmacol. 84, 2422–2432 (2018).
Pestov, N. B. et al. Control of lysyl oxidase activity through site-specific deuteration of lysine. Bioorg. Med. Chem. Lett. 21, 255–258 (2011).
Blum, E. et al. Rational alteration of pharmacokinetics of chiral fluorinated and deuterated derivatives of emixustat for retinal therapy. J. Med. Chem. 64, 8287–8302 (2021).
Manevski, N., King, L., Pitt, W. R., Lecomte, F. & Toselli, F. Metabolism by aldehyde oxidase: drug design and complementary approaches to challenges in drug discovery. J. Med. Chem. 62, 10955–10994 (2019).
Lolkema, M. P. et al. The c-Met tyrosine kinase inhibitor JNJ-38877605 causes renal toxicity through species-specific insoluble metabolite formation. Clin. Cancer Res. 21, 2297–2304 (2015).
Zhan, Z., Peng, X., Sun, Y., Ai, J. & Duan, W. Evaluation of deuterium-labeled JNJ38877605: pharmacokinetic, metabolic, and in vivo antitumor profiles. Chem. Res. Toxicol. 31, 1213–1218 (2018).
DeWitt, S., Czarnik, A. W. & Jacques, V. Deuterium-enabled chiral switching (DECS) yields chirally pure drugs from chemically interconverting racemates. ACS Med. Chem. Lett. 11, 1789–1792 (2020).
Maltais, F. et al. In vitro and in vivo isotope effects with hepatitis C protease inhibitors: enhanced plasma exposure of deuterated telaprevir versus telaprevir in rats. J. Med. Chem. 52, 7993–8001 (2009).
Jacques, V., Czarnik, A. W., Judge, T. M., Van der Ploeg, L. H. T. & DeWitt, S. H. Differentiation of antiinflammatory and antitumorigenic properties of stabilized enantiomers of thalidomide analogs. Proc. Natl Acad. Sci. USA 112, E1471–E1479 (2015).
Jacques, V. et al. Deuterium-stabilized (R)-pioglitazone (PXL065) is responsible for pioglitazone efficacy in NASH yet exhibits little to no PPARγ activity. Hepatol. Commun. 5, 1412–1425 (2021).
Monternier, P.-A. et al. Therapeutic potential of deuterium-stabilized (R)-pioglitazone — PXL065 — for X-linked adrenoleukodystrophy. J. Inherit. Metab. Dis. 45, 832–847 (2022).
Jansen-van Vuuren, R. D., Jedlovčnik, L., Košmrlj, J., Massey, T. E. & Derdau, V. Deuterated drugs and biomarkers in the COVID-19 pandemic. ACS Omega 7, 41840–41858 (2022).
Quan, B.-X. et al. An orally available Mpro inhibitor is effective against wild-type SARS-CoV-2 and variants including Omicron. Nat. Microbiol. 7, 716–725 (2022).
Charbel Issa, P., Barnard, A. R., Herrmann, P., Washington, I. & MacLaren, R. E. Rescue of the Stargardt phenotype in Abca4 knockout mice through inhibition of vitamin A dimerization. Proc. Natl Acad. Sci. USA 112, 8415–8420 (2015).
Kaufman, Y., Ma, L. & Washington, I. Deuterium enrichment of vitamin A at the C20 position slows the formation of detrimental vitamin A dimers in wild-type rodents. J. Biol. Chem. 286, 7958–7965 (2011).
Saad, L., & Washington, I. Can vitamin A be improved to prevent blindness due to age-related macular degeneration, Stargardt disease and other retinal dystrophies? Adv. Exp. Med. Biol. 854, 355–361 (2016).
Brenna, J. T. et al. Plasma and red blood cell membrane accretion and pharmacokinetics of RT001 (bis-allylic 11,11-D2-linoleic acid ethyl ester) during long term dosing in patients. J. Pharm. Sci. 109, 3496–3503 (2020).
Zesiewicz, T. et al. Randomized, clinical trial of RT001: early signals of efficacy in Friedreich’s ataxia. Mov. Disord. 33, 1000–1005 (2018).
Angelova, P. R. et al. RT001 in progressive supranuclear palsy — clinical and in-vitro observations. Antioxidants 10, 1021 (2021).
Ben Abu, N. et al. Sweet taste of heavy water. Commun. Biol. 4, 440 (2021).
Urey, H. C. & Failla, G. Concerning the taste of heavy water. Science 81, 273–273 (1935).
Belz, T. F. et al. Enhancement of a heroin vaccine through hapten deuteration. J. Am. Chem. Soc. 142, 13294–13298 (2020).
Timmins, G. S. Deuterated drugs; updates and obviousness analysis. Expert Opin. Ther. Pat. 27, 1353–1361 (2017).
Schmidt, C. First deuterated drug approved. Nat. Biotechnol. 35, 493–494 (2017).
Hayden, M. R., Leavitt, B. R., Yasothan, U. & Kirkpatrick, P. Tetrabenazine. Nat. Rev. Drug Discov. 8, 17–18 (2009).
Schneider, F. et al. Pharmacokinetic and metabolic profile of deutetrabenazine (TEV-50717) compared with tetrabenazine in healthy volunteers. Clin. Transl. Sci. 13, 707–717 (2020).
Schneider, F. et al. Pharmacokinetics of deutetrabenazine and tetrabenazine: dose proportionality and food effect. Clin. Pharmacol. Drug Dev. 10, 647–659 (2021).
Gupta, H. et al. Deutetrabenazine for the treatment of chorea associated with Huntington’s disease. Health Psychol. Res. 10, 36040 (2022).
Coffey, B. et al. Efficacy and safety of fixed-dose deutetrabenazine in children and adolescents for tics associated with Tourette syndrome: a randomized clinical trial. JAMA Netw. Open 4, e2129397 (2021).
Jankovic, J. et al. Safety and efficacy of flexible-dose deutetrabenazine in children and adolescents with Tourette syndrome: a randomized clinical trial. JAMA Netw. 4, e2128204 (2021).
Group, H. S. Effect of deutetrabenazine on chorea among patients with Huntington disease: a randomized clinical trial. J. Am. Med. Assoc. 316, 40–50 (2016).
Frank, S. et al. Safety of converting from tetrabenazine to deutetrabenazine for the treatment of chorea. JAMA Neurol. 74, 977–982 (2017).
Rodrigues, F. B. & Wild, E. J. Huntington’s disease clinical trials corner: February 2018. J. Huntingt. Dis. 7, 89–98 (2018).
Hauser, R. A. et al. Long-term deutetrabenazine treatment for tardive dyskinesia is associated with sustained benefits and safety: a 3-year, open-label extension study. Front. Neurol. 13, 773999 (2022).
Anderson, K. E. et al. Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Psychiatry 4, 595–604 (2017).
Fernandez, H. H. et al. Long-term safety and efficacy of deutetrabenazine for the treatment of tardive dyskinesia. J. Neurol. Neurosurg. Psychiatry 90, 1317–1323 (2019).
Claassen, D. O. et al. Indirect tolerability comparison of deutetrabenazine and etrabenazine for Huntington disease. J. Clin. Mov. Disord. 4, 3 (2017).
Claassen, D. O. et al. Real-world adherence to tetrabenazine or deutetrabenazine among patients with Huntington’s disease: a retrospective database analysis. Neurol. Ther. 11, 435–448 (2022).
Rodrigues, F. B., Duarte, G. S., Costa, J., Ferreira, J. J. & Wild, E. J. Tetrabenazine versus deutetrabenazine for Huntington’s disease: twins or distant cousins? Mov. Disord. Clin. Pract. 4, 582–585 (2017).
Rodrigues, F. B., Duarte, G. S., Costa, J., Ferreira, J. J. & Wild, E. J. Meta-research metrics matter: letter regarding article “indirect tolerability comparison of deutetrabenazine and tetrabenazine for Huntington disease”. J. Clin. Mov. Disord. 4, 19 (2017).
US DHHS, FDA, CDER. Guidance for Industry: Applications covered by Section 505(b)2 https://www.fda.gov/media/72419/download (1999).
Freije, I., Lamouche, S. & Tanguay, M. Review of drugs approved via the 505(b)(2) pathway: uncovering drug development trends and regulatory requirements. Ther. Innov. Regul. Sci. 54, 128–138 (2020).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 7, 6 (2021).
Liu, J. et al. Safety, pharmacokinetics and efficacy of donafenib in treating advanced hepatocellular carcinoma: report from a phase 1b trial. Pharmazie 74, 688–693 (2019).
Li, X. et al. A phase I dose-escalation, pharmacokinetics and food-effect study of oral donafenib in patients with advanced solid tumours. Cancer Chemother. Pharmacol. 85, 593–604 (2020).
Qin, S. et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II–III trial. J. Clin. Oncol. 39, 3002–3011 (2021).
Li, Q. & Zhu, H. Donafenib treatment for hepatocellular carcinoma: a case report. Medicine 100, e26373 (2021).
Meng, R., Cao, Y., Zhou, T., Hu, H. & Qiu, Y. The cost effectiveness of donafenib compared with sorafenib for the first-line treatment of unresectable or metastatic hepatocellular carcinoma in China. Front. Public Health 10, 794131 (2022).
Kokic, G. et al. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 12, 279 (2021).
Mehellou, Y., Rattan, H. S. & Balzarini, J. The ProTide prodrug technology: from the concept to the clinic. J. Med. Chem. 61, 2211–2226 (2018).
Xie, J. & Wang, Z. Can remdesivir and its parent nucleoside GS-441524 be potential oral drugs? An in vitro and in vivo DMPK assessment. Acta Pharm. Sin. B 11, 1607–1616 (2021).
Xie, Y. et al. Design and development of an oral remdesivir derivative VV116 against SARS-CoV-2. Cell Res. 31, 1212–1214 (2021).
Shen, Y. et al. An open, prospective cohort study of VV116 in Chinese participants infected with SARS-CoV-2 Omicron variants. Emerg. Microbes Infect. 11, 1518–1523 (2022).
Zhang, R. et al. Oral remdesivir derivative VV116 is a potent inhibitor of respiratory syncytial virus with efficacy in mouse model. Signal Transduct. Target. Ther. 7, 123 (2022).
Chessari, G. et al. Structure-based design of potent and orally active isoindolinone inhibitors of MDM2-p53 protein–protein interaction. J. Med. Chem. 64, 4071–4088 (2021).
Purushottamachar, P., Thomas, E., Thankan, R. S. & Njar, V. C. O. Novel deuterated Mnk1/2 protein degrader VNLG-152R analogs: synthesis, in vitro anti-TNBC activities and pharmacokinetics in mice. Eur. J. Med. Chem. 238, 114441 (2022).
Dampalla, C. S. et al. Structure-guided design of potent inhibitors of SARS-CoV-2 3CL protease: structural, biochemical, and cell-based studies. J. Med. Chem. 64, 17846–17865 (2021).
Dampalla, C. S. et al. Structure-guided design of potent spirocyclic inhibitors of severe acute respiratory syndrome coronavirus-2 3C-like protease. J. Med. Chem. 65, 7818–7832 (2022).
Turcu, A. L. et al. Design, synthesis, and in vitro and in vivo characterization of new memantine analogs for Alzheimer’s disease. Eur. J. Med. Chem. 236, 114354 (2022).
Yang, T. et al. Identification of a novel 2,8-diazaspiro[4.5]decan-1-one derivative as a potent and selective dual TYK2/JAK1 inhibitor for the treatment of inflammatory bowel disease. J. Med. Chem. 65, 3151–3172 (2022).
King, B. et al. A phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of the oral Janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24-week results. J. Am. Acad. Dermatol. 85, 379–387 (2021).
Liu, L. et al. Design and evaluation of [18F]CHDI-650 as a positron emission tomography ligand to image mutant Huntingtin aggregates. J. Med. Chem. 66, 641–656 (2023).
Schwartz, D. M. et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 16, 843–862 (2017).
Attwood, M. M., Fabbro, D., Sokolov, A. V., Knapp, S. & Schiöth, H. B. Trends in kinase drug discovery: targets, indications and inhibitor design. Nat. Rev. Drug Discov. 20, 839–861 (2021).
Kragstrup, T. W. et al. Waiting for JAK inhibitor safety data. RDM Open 8, e002236 (2022).
Nogueira, M., Puig, L. & Torres, T. JAK inhibitors for treatment of psoriasis: focus on selective TYK2 inhibitors. Drugs 80, 341–352 (2020).
Jo, C. E., Gooderham, M. & Beecker, J. TYK 2 inhibitors for the treatment of dermatologic conditions: the evolution of JAK inhibitors. Int. J. Dermatol. 61, 139–147 (2022).
Dendrou, C. A. et al. Resolving TYK2 locus genotype-to-phenotype differences in autoimmunity. Sci. Transl. Med. 8, 363ra149 (2016).
Schlapbach, C. & Conrad, C. TYK-ing all the boxes in psoriasis. J. Allergy Clin. Immunol. 149, 1936–1939 (2022).
Catlett, I. M. et al. Molecular and clinical effects of selective tyrosine kinase 2 inhibition with deucravacitinib in psoriasis. J. Allergy Clin. Immunol. 149, 2010–2020.e8 (2022).
Burke, J. R. et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci. Transl. Med. 11, eaaw1736 (2019).
Catlett, I. et al. SAT0226. A first-in-human, study of BMS-986165, a selective, potent, allosteric small molecule inhibitor of tyrosine kinase 2. Ann. Rheum. Dis. 76, 859 (2017).
Papp, K. et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N. Engl. J. Med. 379, 1313–1321 (2018).
Thaçi, D. et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol. Ther. 12, 495–510 (2022).
Chimalakonda, A. et al. Lack of electrocardiographic effects of deucravacitinib in healthy subjects. Clin. Pharmacol. Drug Dev. 11, 442–453 (2022).
Strober, B. et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 POETYK PSO-2 trial. J. Am. Acad. Dermatol. 88, 29–39 (2023).
Winthrop, K. L. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 13, 234–243 (2017).
Mullard, A. First de novo deuterated drug poised for approval. Nat. Rev. Drug Discov. 21, 623–625 (2022).
Mease, P. J. et al. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann. Rheum. Dis. 81, 815–822 (2022).
Hannon, C. W., McCourt, C., Lima, H. C., Chen, S. & Bennett, C. Interventions for cutaneous disease in systemic lupus erythematosus. Cochrane Database Syst. Rev. 3, CD007478 (2021).
King, Brett et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata. J. Am. Acad. Dermatol. 87, 306–313 (2022).
Zuraw, B. L. HAE therapies: past present and future. Allergy Asthma Clin. Immunol. 6, 23 (2010).
Lesage, A., Loenders, B. & Knolle, J. PHA-022121, a first in class oral bradykinin B2 receptor antagonist in clinical development: proof of concept study in a translational monkey bradykinin challenge model. J. Allergy Clin. Immunol. 145, AB346 (2020).
Timme, C. R., Rath, B. H., O’Neill, J. W., Camphausen, K. & Tofilon, P. J. The DNA-PK inhibitor VX-984 enhances the radiosensitivity of glioblastoma cells grown in vitro and as orthotopic xenografts. Mol. Cancer Ther. 17, 1207–1216 (2018).
Meanwell, N. A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 61, 5822–5880 (2018).
Pan, Y. The darck of fluorine. ACS Med. Chem. Lett. 10, 1016–1019 (2019).
Han, J. et al. Chemical aspects of human and environmental overload with fluorine. Chem. Rev. 121, 4678–4742 (2021).
Wang, X.-M. et al. Effect of deuteration on the single dose pharmacokinetic properties and postoperative analgesic activity of methadone. Drug. Metab. Pharmacokinet. 47, 100477 (2022).
Sun, L.-Q. et al. Discovery of BMS-986144, a third-generation, pan-genotype NS3/4A protease inhibitor for the treatment of hepatitis C virus infection. J. Med. Chem. 63, 14740–14760 (2020).
Spock, M. et al. Discovery of VU6028418: a highly selective and orally bioavailable M4 muscarinic acetylcholine receptor antagonist. ACS Med. Chem. Lett. 12, 1342–1349 (2021).
Xu, Z. et al. Evaluation of efficacy and safety after replacement of methyl hydrogen with deuterium at methyl formate of clopidogrel. Eur. J. Pharm. Sci. 172, 106157 (2022).
Shvartsbart, A. et al. Discovery of potent and selective inhibitors of wild-type and gatekeeper mutant fibroblast growth factor receptor (FGFR) 2/3. J. Med. Chem. 65, 15433–15442 (2022).
Zhu, Y., Zhou, J. & Jiao, B. Deuterated clopidogrel analogues as a new generation of antiplatelet agents. ACS Med. Chem. Lett. 4, 349–352 (2013).
Aprile, S. et al. An unexpected deuterium-induced metabolic switch in doxophylline. ACS Med. Chem. Lett. 13, 1278–1285 (2022).
Marcucci, F., Mussoni, E., Martelli, P., Guaitani, A. & Garattini, S. Metabolism and anticonvulsant activity of deuterated N-demethyldiazepam. J. Pharm. Sci. 62, 1900–1902 (1973).
Liu, X. et al. Deuteration of the farnesyl terminal methyl groups of δ-tocotrienol and its effects on the metabolic stability and ability of inducing G-CSF production. Bioorg. Med. Chem. 28, 115498 (2020).
Helfenbein, J. et al. Isotopic effect study of propofol deuteration on the metabolism, activity, and toxicity of the anesthetic. J. Med. Chem. 45, 5806–5808 (2002).
Manley, P. W., Blasco, F., Mestan, J. & Aichholz, R. The kinetic deuterium isotope effect as applied to metabolic deactivation of imatinib to the des-methyl metabolite, CGP74588. Bioorg. Med. Chem. 21, 3231–3239 (2013).
Vang, Z. P. et al. Copper-catalyzed transfer hydrodeuteration of aryl alkenes with quantitative isotopomer purity analysis by molecular rotational resonance spectroscopy. J. Am. Chem. Soc. 143, 7707–7718 (2021).
Czeskis, B. et al. Deuterated active pharmaceutical ingredients: a science-based proposal for synthesis, analysis, and control. Part 1: framing the problem. J. Label. Compd. Radiopharm. 62, 690–694 (2019).
ICH. Impurities in new drug substrates Q3A(R2). ICH Harmonised Tripartite Guideline https://database.ich.org/sites/default/files/Q3A%28R2%29%20Guideline.pdf (2006).
Serafini, M. et al. What’s in a name? Drug nomenclature and medicinal chemistry trends using INN publications. J. Med. Chem. 64, 4410–4429 (2021).
World Health Organization. Executive summary. INN Working Doc. 21.533 WHO https://cdn.who.int/media/docs/default-source/international-nonproprietary-names-(inn)/73rd_executive_summary.pdf (2021).
Karkhanis, A. V. et al. Site-directed deuteration of dronedarone preserves cytochrome P4502J2 activity and mitigates its cardiac adverse effects in canine arrhythmic hearts. Acta Pharm. Sin. B 12, 3905–3923 (2022).
Tang, L. W. T., Lim, R. Y. R., Venkatesan, G. & Chan, E. C. Y. Rational deuteration of dronedarone attenuates its toxicity in human hepatic HepG2 cells. Toxicol. Res. 11, 311–324 (2022).
Kambayashi, R. et al. An exploratory analysis of effects of poyendarone, a deuterated analogue of dronedarone, on the canine model of paroxysmal atrial fibrillation. Naunyn Schmiedebergs Arch. Pharmacol. 394, 1103–1112 (2021).
Marks, M., & Cohen, I. G. Patents on psychedelics: the next legal battlefront of drug development. Harv. Law Rev. Forum 135, 212–35 (2022).
Kargbo, R. B. Psilocybin therapeutic research: the present and future paradigm. ACS Med. Chem. Lett. 11, 399–402 (2020).
Kargbo, R. B. Application of deuterated N,N-dimethyltryptamine in the potential treatment of psychiatric and neurological disorders. ACS Med. Chem. Lett. 13, 1402–1404 (2022).
Timmins, G. S. Deuterated drugs: where are we now? Expert Opin. Ther. Pat. 24, 1067–1075 (2014).
Buteau, K. C. Deuterated drugs: unexpectedly nonobvious? J. High Tech. L. 10, 22 (2010).
Furrow, M., & Austin, E. Protecting deuterated drugs. Intellectual Property Magazine 35–36 https://www.venable.com/-/media/035_036ipm_february_2018fo1.pdf (Feb 2018).
Li, L., Jakowski, J., Do, C. & Hong, K. Deuteration and polymers: rich history with great potential. Macromolecules 54, 3555–3584 (2021).
Tullo, A. H. Your next TV could contain uncommon isotopes. CEN Glob. Enterp. 100, 21–22 (2022).
Grimm, J. B. et al. A general method to improve fluorophores using deuterated auxochromes. JACS Au 1, 690–696 (2021).
Roßmann, K. et al. N-methyl deuterated rhodamines for protein labelling in sensitive fluorescence microscopy. Chem. Sci. 13, 8605–8617 (2022).
Harbeson, S. L. et al. Altering metabolic profiles of drugs by precision deuteration 2: discovery of a deuterated analog of ivacaftor with differentiated pharmacokinetics for clinical development. J. Pharmacol. Exp. Ther. 362, 359–367 (2017).
Looker, A. R. et al. Utilizing O-quinone methide chemistry: synthesis of d9-ivacaftor. J. Org. Chem. 85, 501–507 (2020).
Danese, S. & Peyrin-Biroulet, L. Selective tyrosine kinase 2 inhibition for treatment of inflammatory bowel disease: new hope on the rise. Inflamm. Bowel Dis. 27, 2023–2030 (2021).
Morand, E. et al. Deucravacitinib, a tyrosine kinase 2 inhibitor, in systemic lupus erythematosus: a phase II, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 75, 242–252 (2023).
No authors listed. Vertex ramps up CRISPR repair. Nat. Biotechnol. 37, 205 (2019).
Dittrich, C. et al. Phase I and pharmacokinetic study of BIBX 1382 BS, an epidermal growth factor receptor (EGFR) inhibitor, given in a continuous daily oral administration. Eur. J. Cancer 38, 1072–1080 (2002).
Diamond, S. et al. Species-specific metabolism of SGX523 by aldehyde oxidase and the toxicological implications. Drug Metab. Dispos. 38, 1277–1285 (2010).
Zheng, J. et al. Pharmacokinetics and disposition of momelotinib revealed a disproportionate human metabolite—resolution for clinical development. Drug Metab. Dispos. 46, 237 (2018).
Pryde, D. C. et al. Aldehyde oxidase: an enzyme of emerging importance in drug discovery. J. Med. Chem. 53, 8441–8460 (2010).
Sharma, R. et al. Deuterium isotope effects on drug pharmacokinetics. I. System-dependent effects of specific deuteration with aldehyde oxidase cleared drugs. Drug Metab. Dispos. 40, 625–634 (2012).
Kopf, S. et al. Recent developments for the deuterium and tritium labeling of organic molecules. Chem. Rev. 122, 6634–6718 (2022).
Treitler, D. S. et al. Development of a commercial process for deucravacitinib, a deuterated API for TYK2 inhibition. Org. Process. Res. Dev. 26, 1202–1222 (2022).
Atzrodt, J., Derdau, V., Fey, T. & Zimmermann, J. The renaissance of H/D exchange. Angew. Chem. Int. Ed. 46, 7744–7765 (2007).
Atzrodt, J., Derdau, V., Kerr, W. J. & Reid, M. C−H functionalisation for hydrogen isotope exchange. Angew. Chem. Int. Ed. 57, 3022–3047 (2018).
Prakash, G., Paul, N., Oliver, G. A., Werz, D. B. & Maiti, D. C–H deuteration of organic compounds and potential drug candidates. Chem. Soc. Rev. 51, 3123–3163 (2022).
Rowbotham, J. S., Ramirez, M. A., Lenz, O., Reeve, H. A. & Vincent, K. A. Bringing biocatalytic deuteration into the toolbox of asymmetric isotopic labelling techniques. Nat. Commun. 11, 1454 (2020).
Loh, Y. Y. et al. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science 358, 1182–1187 (2017).
Zhou, R., Ma, L., Yang, X. & Cao, J. Recent advances in visible-light photocatalytic deuteration reactions. Org. Chem. Front. 8, 426–444 (2021).
Shi, Q. et al. Visible-light mediated catalytic asymmetric radical deuteration at non-benzylic positions. Nat. Commun. 13, 4453 (2022).
Norcott, P. L. Current electrochemical approaches to selective deuteration. ChemComm 58, 2944–2953 (2022).
Li, N., Li, Y., Wu, X., Zhu, C. & Xie, J. Radical deuteration. Chem. Soc. Rev. 51, 6291–6306 (2022).
Steverlynck, J., Sitdikov, R. & Rueping, M. The deuterated “Magic Methyl” group: a guide to site-selective trideuteromethyl incorporation and labeling by using CD3 reagents. Chem. Eur. J. 27, 11751–11772 (2021).
Sun, Q. & Soulé, J.-F. Broadening of horizons in the synthesis of CD3-labeled molecules. Chem. Soc. Rev. 50, 10806–10835 (2021).
Wang, L., Xia, Y., Derdau, V. & Studer, A. Remote site-selective radical C(sp3)−H monodeuteration of amides using D2O. Angew. Chem. Int. Ed. 60, 18645–18650 (2021).
Acknowledgements
This work is part of the project NODES, which has received funding from the MUR-M4C2 1.5 of Piano Nazionale di Ripresa e Resilienza (PNRR) with grant agreement no. ECS00000036.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
T.P. is co-founder and shareholder of the start-up company ChemICare, Italy. B.D.M. is senior scientific fellow in process chemistry at Vertex Pharmaceuticals (Boston, MA, USA) and currently holds shares of stock and options in the company. R.M.C.D.M. declares no competing interests.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Volker Derdau and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov
Glossary
- Bioisosterism
-
Bioisosteres are structurally distinct compounds bearing atoms or groups that owing to chemical and/or physical similarities elicit broadly similar biological effects.
- Chiral switch
-
A term used to describe the development of a single enantiomer of a previously approved racemic drug with the goal of improving on the characteristics of the racemic version.
- Enantiomer
-
One of two stereoisomers that cannot be superimposed on each other and are mirror images of each other.
- Enantiomerization
-
The process of interconversion of one enantiomer into the other.
- Epimerization
-
The process in stereochemistry in which there is a change in the configuration of only one stereocentre in a compound that has more than one stereocentre.
- Epimers
-
Diastereomers that contain more than one stereocentre and differ from each other in the absolute configuration at only one stereocentre.
- Isotopes
-
Species of the same element that differ in the number of neutrons in the nucleus.
- Isotopologues
-
Molecular entities that differ only in isotopic composition (number of isotopic substitutions).
- Isotopomers
-
(Also known as isotopic isomers). Isomers having the same number of each isotope, but differing in their positions.
- Metabolic shunting
-
A shift towards a desirable and non-toxic metabolic pathway.
- Metabolic switching
-
A shift towards a collateral and undesired metabolic pathway.
- Soft spots
-
Sites on molecules that are vulnerable to metabolism by enzymes such as those in the cytochrome P450 family.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Di Martino, R.M.C., Maxwell, B.D. & Pirali, T. Deuterium in drug discovery: progress, opportunities and challenges. Nat Rev Drug Discov 22, 562–584 (2023). https://doi.org/10.1038/s41573-023-00703-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-023-00703-8
- Springer Nature Limited
This article is cited by
-
Stable isotope applications in drug development and the elucidation of mechanisms of drug metabolism
Medicinal Chemistry Research (2023)