Abstract
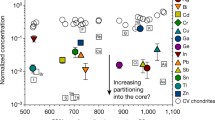
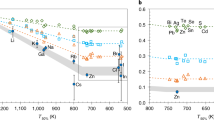
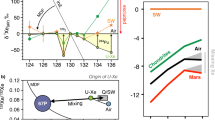
How and when Earth’s volatile content was established is controversial with several mechanisms postulated, including planetesimal evaporation, core formation and the late delivery of undifferentiated chondrite-like materials. The isotopes of volatile elements such as sulfur can be fractionated during planetary accretion and differentiation and thus are potential tracers of these processes. Using first-principles calculations, we examine sulfur isotope fractionation during core formation and planetesimal evaporation. We find no measurable sulfur isotope fractionation between silicate and metallic melts at core-forming conditions, indicating that the observed light sulfur isotope composition of the bulk silicate Earth relative to chondrites cannot be explained by metal–silicate fractionation. Our thermodynamic calculations show that sulfur evaporates mostly as H2S during planetesimal evaporation when nebular H2 is present. The observed bulk Earth sulfur isotope signature and abundance can be reproduced by evaporative loss of about 90% sulfur mainly as H2S from molten planetesimals before nebular H2 is dissipated. The heavy sulfur isotope composition of the Moon relative to the Earth is consistent with evaporative sulfur loss under 94–98% saturation condition during the Moon-forming giant impact. In summary, volatile evaporation from molten planetesimals before Earth’s formation probably played a key role in establishing Earth’s volatile element content.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available in the article and Supplementary Information files. All new data associated with this paper will be made publicly available at https://doi.org/10.6084/m9.figshare.16566336.v1.
Code availability
The Vienna Ab Initio Simulation Package is a proprietary software available for purchase at https://www.vasp.at/.
References
Dauphas, N. The isotopic nature of the Earth’s accreting material through time. Nature 541, 521–524 (2017).
Wood, B. J., Walter, M. J. & Wade, J. Accretion of the Earth and segregation of its core. Nature 441, 825–833 (2006).
Schonbachler, M., Carlson, R. W., Horan, M. F., Mock, T. D. & Hauri, E. H. Heterogeneous accretion and the moderately volatile element budget of Earth. Science 328, 884–887 (2010).
Kleine, T. et al. Hf–W chronology of the accretion and early evolution of asteroids and terrestrial planets. Geochim. Cosmochim. Acta 73, 5150–5188 (2009).
Norris, C. A. & Wood, B. J. Earth’s volatile contents established by melting and vaporization. Nature 549, 507–510 (2017).
Hin, R. C. et al. Magnesium isotope evidence that accretional vapour loss shapes planetary compositions. Nature 549, 511–527 (2017).
Rose-Weston, L., Brenan, J. M., Fei, Y., Secco, R. A. & Frost, D. J. Effect of pressure, temperature, and oxygen fugacity on the metal–silicate partitioning of Te, Se, and S: implications for Earth differentiation. Geochim. Cosmochim. Acta 73, 4598–4615 (2009).
Boujibar, A. et al. Metal–silicate partitioning of sulphur, new experimental and thermodynamic constraints on planetary accretion. Earth Planet. Sci. Lett. 391, 42–54 (2014).
Suer, T.-A., Siebert, J., Remusat, L., Menguy, N. & Fiquet, G. A sulfur-poor terrestrial core inferred from metal–silicate partitioning experiments. Earth Planet. Sci. Lett. 469, 84–97 (2017).
O’Neill, H. S. The origin of the Moon and the early history of the Earth—a chemical model. Part 1: the Moon. Geochim. Cosmochim. Acta 55, 1135–1157 (1991).
Braukmüller, N., Wombacher, F., Funk, C. & Münker, C. Earth’s volatile element depletion pattern inherited from a carbonaceous chondrite-like source. Nat. Geosci. 12, 564–568 (2019).
Lodders, K. Solar System abundances and condensation temperatures of the elements. Astrophys. J. 591, 1220–1247 (2003).
Wang, Z. & Becker, H. Ratios of S, Se and Te in the silicate Earth require a volatile-rich late veneer. Nature 499, 328–331 (2013).
Yierpan, A., König, S., Labidi, J. & Schoenberg, R. Selenium isotope and S–Se–Te elemental systematics along the Pacific–Antarctic ridge: role of mantle processes. Geochim. Cosmochim. Acta 249, 199–224 (2019).
Labidi, J., Cartigny, P. & Moreira, M. Non-chondritic sulphur isotope composition of the terrestrial mantle. Nature 501, 208–211 (2013).
Labidi, J., Cartigny, P., Hamelin, C., Moreira, M. & Dosso, L. Sulfur isotope budget (32S, 33S, 34S and 36S) in Pacific–Antarctic ridge basalts: a record of mantle source heterogeneity and hydrothermal sulfide assimilation. Geochim. Cosmochim. Acta 133, 47–67 (2014).
Gao, X. & Thiemens, M. H. Isotopic composition and concentration of sulfur in carbonaceous chondrites. Geochim. Cosmochim. Acta 57, 3159–3169 (1993).
Gao, X. & Thiemens, M. H. Variations of the isotopic composition of sulfur in enstatite and ordinary chondrites. Geochim. Cosmochim. Acta 57, 3171–3176 (1993).
Labidi, J., Farquhar, J., Alexander, C. M. O. D., Eldridge, D. L. & Oduro, H. Mass independent sulfur isotope signatures in CMs: implications for sulfur chemistry in the early Solar System. Geochim. Cosmochim. Acta 196, 326–350 (2017).
Labidi, J. et al. Experimentally determined sulfur isotope fractionation between metal and silicate and implications for planetary differentiation. Geochim. Cosmochim. Acta 175, 181–194 (2016).
Fischer, R. A. et al. High pressure metal–silicate partitioning of Ni, Co, V, Cr, Si, and O. Geochim. Cosmochim. Acta 167, 177–194 (2015).
Sanloup, C. et al. Structural change in molten basalt at deep mantle conditions. Nature 503, 104–107 (2013).
Sun, N., Stixrude, L., Koker, Nde & Karki, B. B. First principles molecular dynamics simulations of diopside (CaMgSi2O6) liquid to high pressure. Geochim. Cosmochim. Acta 75, 3792–3802 (2011).
De Koker, N. Structure, thermodynamics, and diffusion in CaAl2Si2O8 liquid from first-principles molecular dynamics. Geochim. Cosmochim. Acta 74, 5657–5671 (2010).
McEwing, C., Thode, H. & Rees, C. Sulphur isotope effects in the dissociation and evaporation of troilite: a possible mechanism for 34S enrichment in lunar soils. Geochim. Cosmochim. Acta 44, 565–571 (1980).
Young, E. D. et al. Near-equilibrium isotope fractionation during planetesimal evaporation. Icarus 323, 1–15 (2019).
Bigeleisen, J. & Mayer, M. G. Calculation of equilibrium constants for isotopic exchange reactions. J. Chem. Phys. 15, 261 (1947).
Nash, W. M., Smythe, D. J. & Wood, B. J. Compositional and temperature effects on sulfur speciation and solubility in silicate melts. Earth Planet. Sci. Lett. 507, 187–198 (2019).
Jugo, P. J., Wilke, M. & Botcharnikov, R. E. Sulfur K-edge XANES analysis of natural and synthetic basaltic glasses: implications for S speciation and S content as function of oxygen fugacity. Geochim. Cosmochim. Acta 74, 5926–5938 (2010).
Wadhwa, M. Redox conditions on small bodies, the Moon and Mars. Rev. Mineral. Geochem. 68, 493–510 (2008).
McCammon, C. Geochemistry: the paradox of mantle redox. Science 308, 807–808 (2005).
Rubie, D. C., Nimmo, F. & Melosh, H. J. in Treatise on Geophysics 2nd edn, Vol. 9 (ed. Schubert, G.) 43–79 (Elsevier, 2015); https://doi.org/10.1016/B978-0-444-53802-4.00154-8
Righter, K. Prediction of metal–silicate partition coefficients for siderophile elements: an update and assessment of PT conditions for metal–silicate equilibrium during accretion of the Earth. Earth Planet. Sci. Lett. 304, 158–167 (2011).
Rubie, D. C. et al. Accretion and differentiation of the terrestrial planets with implications for the compositions of early-formed Solar System bodies and accretion of water. Icarus 248, 89–108 (2015).
Grewal, D. S., Dasgupta, R., Sun, C., Tsuno, K. & Costin, G. Delivery of carbon, nitrogen, and sulfur to the silicate Earth by a giant impact. Sci. Adv. 5, eaau3669 (2019).
Varas-Reus, M. I., König, S., Yierpan, A., Lorand, J.-P. & Schoenberg, R. Selenium isotopes as tracers of a late volatile contribution to Earth from the outer Solar System. Nat. Geosci. 12, 779–782 (2019).
Sharp, Z. D. Nebular ingassing as a source of volatiles to the terrestrial planets. Chem. Geol. 448, 137–150 (2017).
McDonough, W. F. & Sun, S.-s The composition of the Earth. Chem. Geol. 120, 223–253 (1995).
Mann, U., Frost, D. J., Rubie, D. C., Becker, H. & Audétat, A. Partitioning of Ru, Rh, Pd, Re, Ir and Pt between liquid metal and silicate at high pressures and high temperatures—implications for the origin of highly siderophile element concentrations in the Earth’s mantle. Geochim. Cosmochim. Acta 84, 593–613 (2012).
Nie, N. X. & Dauphas, N. Vapor drainage in the protolunar disk as the cause for the depletion in volatile elements of the Moon. Astrophys. J. Lett. 884, L48 (2019).
Wang, K. & Jacobsen, S. B. Potassium isotopic evidence for a high-energy giant impact origin of the Moon. Nature 538, 487–490 (2016).
Paniello, R. C., Day, J. M. D. & Moynier, F. Zinc isotopic evidence for the origin of the Moon. Nature 490, 376–379 (2012).
Lock, S. J. et al. The origin of the Moon within a terrestrial synestia. J. Geophys. Res. Planets 123, 910–951 (2018).
Schaefer, L., Lodders, K. & Fegley, B. Vaporization of the Earth: application to exoplanet atmospheres. Astrophys. J. 755, 41 (2012).
Day, J. M. D. Geochemical constraints on residual metal and sulfide in the sources of lunar mare basalts. Am. Mineral. 103, 1734–1740 (2018).
Saal, A. E. & Hauri, E. H. Large sulfur isotope fractionation in lunar volcanic glasses reveals the magmatic differentiation and degassing of the Moon. Sci. Adv. 7, 1–12 (2021).
Wing, B. A. & Farquhar, J. Sulfur isotope homogeneity of lunar mare basalts. Geochim. Cosmochim. Acta 170, 266–280 (2015).
Franz, H. B. et al. Isotopic links between atmospheric chemistry and the deep sulphur cycle on Mars. Nature 508, 364–368 (2014).
Rai, V. K., Jackson, T. L. & Thiemens, M. H. Photochemical mass-independent sulfur isotopes in achondritic meteorites. Science 309, 1062–1065 (2005).
Wu, N., Farquhar, J., Dottin, J. W. & Magalhães, N. Sulfur isotope signatures of eucrites and diogenites. Geochim. Cosmochim. Acta 233, 1–13 (2018).
Defouilloy, C., Cartigny, P., Assayag, N., Moynier, F. & Barrat, J.-A. High-precision sulfur isotope composition of enstatite meteorites and implications of the formation and evolution of their parent bodies. Geochim. Cosmochim. Acta 172, 393–409 (2016).
Antonelli, M. A. et al. Early inner Solar System origin for anomalous sulfur isotopes in differentiated protoplanets. Proc. Natl Acad. Sci. USA 111, 17749–17754 (2014).
Dottin, J. W., Farquhar, J. & Labidi, J. Multiple sulfur isotopic composition of main group pallasites support genetic links to IIIAB iron meteorites. Geochim. Cosmochim. Acta 224, 276–281 (2018).
Wang, W., Wu, Z. & Huang, F. Equilibrium barium isotope fractionation between minerals and aqueous solution from first-principles calculations. Geochim. Cosmochim. Acta 292, 64–77 (2021).
Wang, S.-J. et al. Nickel isotopic evidence for late-stage accretion of Mercury-like differentiated planetary embryos. Nat. Commun. 12, 294 (2021).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Caracas, R., Hirose, K., Nomura, R. & Ballmer, M. D. Melt–crystal density crossover in a deep magma ocean. Earth Planet. Sci. Lett. 516, 202–211 (2019).
Petaev, M. I. The GRAINS thermodynamic and kinetic code for modeling nebular condensation. Calphad 33, 317–327 (2009).
Acknowledgements
This work is supported by the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB41000000), Natural Science Foundation of China (41925017 and 41721002), and the Fundamental Research Funds for the Central Universities (WK2080000144). W.W. acknowledges support from the UCL–Carnegie Postdoctoral Scholarship. S.H. and M.L. acknowledge support from NSF AST-1910955 and EAR-1942042. Part of the calculations were conducted at the Supercomputing Center of the University of Science and Technology of China.
Author information
Authors and Affiliations
Contributions
W.W. and C.-H.L. conceived and designed this project. W.W. performed the theoretical calculations. S.H. and M.L. did the GRAINS calculations. W.W. wrote the manuscript with the help of C.-H.L., and all authors contributed to the discussion of the results and revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Geoscience thanks Yuan Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Rebecca Neely, in collaboration with the Nature Geoscience team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Radial distribution functions g(r) and coordination numbers (CN) for S-O, S-Mg, and S-Si pairs in Mg32Si32O96SO2 silicate melt at different pressures.
Extended Data Fig. 2
Radial distribution functions g(r) and coordination numbers (CN) for S-O, S-Mg, and S-Si pairs in Mg32Si32O95S silicate melt at different pressures.
Extended Data Fig. 3
Radial distribution function g(r) and coordination number (CN) for S-Fe pair in Fe97S3 metallic melt at different pressures.
Extended Data Fig. 4
Radial distribution function g(r) and coordination number (CN) for S-Mg/Fe/Si/Ca/Al/O in Mg41Ca2Fe5Si32Al4O117 melt at 46.59 GPa and S-Fe/Ni/Si/C/O pairs in Fe87Ni4Si10O2C2S3 melt at 41.81 GPa.
Extended Data Fig. 5 Average force constants <F > of S in silicate and metallic melts at different pressures.
Mg32Si32O95S and Mg32Si32O96SO2 represent the S-bearing silicate melts under extremely reducing and oxidizing conditions, respectively. The green star and pentagon are the <F > of S in Mg41Ca2Fe5Si32Al4O117S and Fe87Ni4Si10O2C2S3 melts, respectively.
Extended Data Fig. 6
The reduced partition function ratios (103lnβ) of 34S/32S of Fe97S3, Mg32Si32O95S, and Mg32Si32O96SO2 melts at 0, 30, 60, 90 GPa.
Extended Data Fig. 7 The fractions of major S species in the vapour phase at 1e-4 bar and different temperatures (1000-1600 K).
(a) solar abundance, (b) solar abundance with H concentration decreased by one order of magnitude, (c) solar abundance with H concentration decreased by four orders of magnitude.
Extended Data Fig. 8 Equilibrium sulfur isotope fractionation factors (103lnαvapor-melt of 34S/32S) between vapour phase and silicate melt (Mg32Si32O95S).
The 103lnβ of silicate melt is derived from the force constant of S in Mg32Si32O95S silicate melt. The 103lnβ of vapour phase is estimated based on the <F > of each important S species (Extended Data Table 1) and their fractions in the vapour phase (Extended Data Table 2).
Supplementary information
Supplementary Information
Supplementary text, Tables 1 and 2 and Figs. 1–6.
Rights and permissions
About this article
Cite this article
Wang, W., Li, CH., Brodholt, J.P. et al. Sulfur isotopic signature of Earth established by planetesimal volatile evaporation. Nat. Geosci. 14, 806–811 (2021). https://doi.org/10.1038/s41561-021-00838-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-021-00838-6
- Springer Nature Limited
This article is cited by
-
Earth’s volatile depletion trend is consistent with a high-energy Moon-forming impact
Communications Earth & Environment (2023)
-
Late veneer and the origins of volatiles of Earth
Acta Geochimica (2022)
-
Sulfur evaporation in planetesimals
Nature Geoscience (2021)