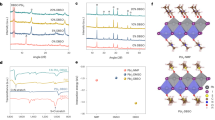
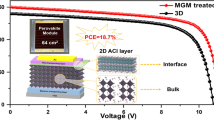
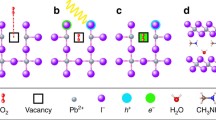
Abstract
The manufacturing of perovskite solar cells under ambient conditions is desirable, yet the efficiency of p–i–n perovskite solar cells fabricated in air still lags behind those made in an inert atmosphere. Here we introduce an ionic pair stabilizer, dimethylammonium formate (DMAFo), into the perovskite precursor solution to prevent the degradation of perovskite precursors. DMAFo inhibits the oxidization of iodide ions and deprotonation of organic cations, improving the crystallinity and reducing defects in the resulting perovskite films. We show the generation of additional p-type defects during ambient air fabrication that suggests the need for improving bulk properties of the perovskite film beyond surface passivation. Upon addition of DMAFo, we demonstrate that the efficiency of inverted p–i–n solar cells based on perovskite layers with 1.53-eV and 1.65-eV bandgaps fabricated under ambient conditions (25–30 °C, 35–50% relative humidity) increases by 15–20%. We achieve a certified stabilized efficiency of 24.72% for the 1.53-eV cell, on a par with state-of-the-art counterparts fabricated in an inert atmosphere.




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in the published article and its Supplementary Information. Source data are provided with this paper.
References
Polman, A., Knight, M., Garnett, E. C., Ehrler, B. & Sinke, W. C. Photovoltaic materials: present efficiencies and future challenges. Science 352, aad4424 (2016).
National Renewable Energy Laboratory Best Research-cell Efficiency Chart (NREL, 2023); https://www.nrel.gov/pv/cell-efficiency.html
Park, J. et al. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 616, 724–730 (2023).
Chen, H. et al. Quantum-size-tuned heterostructures enable efficient and stable inverted perovskite solar cells. Nat. Photonics 16, 352–358 (2022).
Jiang, Q. et al. Surface reaction for efficient and stable inverted perovskite solar cells. Nature 611, 278–283 (2022).
Li, Z. et al. Organometallic-functionalized interfaces for highly efficient inverted perovskite solar cells. Science 376, 416–420 (2022).
Peng, W. et al. Reducing nonradiative recombination in perovskite solar cells with a porous insulator contact. Science 379, 683–690 (2023).
Bush, K. A. et al. 23.6%-efficient monolithic perovskite/silicon tandem solar cells with improved stability. Nat. Energy 2, 17009 (2017).
Xu, J. et al. Triple-halide wide-band gap perovskites with suppressed phase segregation for efficient tandems. Science 367, 1097–1104 (2020).
Tan, Q. et al. Inverted perovskite solar cells using dimethylacridine-based dopants. Nature https://doi.org/10.1038/s41586-023-06207-0 (2023).
Zhang, S. et al. Minimizing buried interfacial defects for efficient inverted perovskite solar cells. Science 380, 404–409 (2023).
Chen, S. et al. Stabilizing perovskite-substrate interfaces for high-performance perovskite modules. Science 373, 902–907 (2021).
Li, M. et al. Stabilizing perovskite precursor by synergy of functional groups for NiOx-based inverted solar cells with 23.5% efficiency. Angew. Chem. Int. Ed. Engl. 61, e202206914 (2022).
Sveinbjörnsson, K. et al. Ambient air-processed mixed-ion perovskites for high-efficiency solar cells. J. Mater. Chem. A 4, 16536–16545 (2016).
Krishna, B. G., Sundar Ghosh, D. & Tiwari, S. Progress in ambient air-processed perovskite solar cells: insights into processing techniques and stability assessment. Sol. Energy 224, 1369–1395 (2021).
Jiang, Q. et al. Planar-structure perovskite solar cells with efficiency beyond 21. Adv. Mater. 29, 1703852 (2017).
Kim, Y. C. et al. Beneficial effects of PbI2 incorporated in organo-lead halide perovskite solar cells. Adv. Energy Mater. 6, 1502104 (2016).
Du, Y. et al. Ionic liquid treatment for highest-efficiency ambient printed stable all-inorganic CsPbI3 perovskite solar cells. Adv. Mater. 34, e2106750 (2022).
Akin, S., Akman, E. & Sonmezoglu, S. FAPbI3-based perovskite solar cells employing hexyl-based ionic liquid with an efficiency over 20% and excellent long-term stability. Adv. Funct. Mater. 30, 2002964 (2020).
Li, T. et al. Multiple functional groups synergistically improve the performance of inverted planar perovskite solar cells. Nano Energy 82, 105742 (2021).
Wang, Y. et al. Efficient and stable perovskite solar cells by fluorinated ionic liquid-induced component interaction. Sol. RRL 5, 2000582 (2020).
Bai, S. et al. Planar perovskite solar cells with long-term stability using ionic liquid additives. Nature 571, 245–250 (2019).
Lin, Y. H. et al. A piperidinium salt stabilizes efficient metal-halide perovskite solar cells. Science 369, 96–102 (2020).
Du, M. et al. High-pressure nitrogen-extraction and effective passivation to attain highest large-area perovskite solar module efficiency. Adv. Mater. 32, e2004979 (2020).
Zhu, X. et al. Ionic-liquid-perovskite capping layer for stable 24.33%-efficient solar cell. Adv. Energy Mater. 12, 2103491 (2021).
Caprioglio, P. et al. Bi-functional interfaces by poly(ionic liquid) treatment in efficient pin and nip perovskite solar cells. Energy Environ. Sci. 14, 4508–4522 (2021).
Zhu, X. et al. High-efficiency perovskite solar cells with imidazolium-based ionic liquid for surface passivation and charge transport. Angew. Chem. Int. Ed. Engl. 60, 4238–4244 (2021).
Hui, W. et al. Stabilizing black-phase formamidinium perovskite formation at room temperature and high humidity. Science 371, 1359–1364 (2021).
Chao, L. et al. Direct and stable α-phase formation via ionic liquid solvation for formamidinium-based perovskite solar cells. Joule 6, 2203–2217 (2022).
Chao, L. F. et al. Room-temperature molten salt for facile fabrication of efficient and stable perovskite solar cells in ambient air. Chem 5, 995–1006 (2019).
McMeekin, D. P. et al. Intermediate-phase engineering via dimethylammonium cation additive for stable perovskite solar cells. Nat. Mater. 22, 73–83 (2023).
Dou, B. et al. Degradation of highly alloyed metal halide perovskite precursor inks: mechanism and storage solutions. ACS Energy Lett. 3, 979–985 (2018).
Noel, N. K. et al. Unveiling the influence of pH on the crystallization of hybrid perovskites, delivering low voltage loss photovoltaics. Joule 1, 328–343 (2017).
Meng, X. et al. Highly reproducible and efficient FASnI3 perovskite solar cells fabricated with volatilizable reducing solvent. J. Phys. Chem. Lett. 11, 2965–2971 (2020).
Jeong, J. et al. Pseudo-halide anion engineering for alpha-FAPbI3 perovskite solar cells. Nature 592, 381–385 (2021).
Li, Z. H. et al. 4-hydrazinobenzoic-acid antioxidant for high-efficiency Sn-Pb alloyed perovskite solar cells. Energy Technol. 10, 2200217 (2022).
Li, G. X. et al. Ionic liquid stabilizing high-efficiency tin halide perovskite solar cells. Adv. Energy Mater. 11, 2101539 (2021).
Niu, T. T. et al. Ionic liquids-enabled efficient and stable perovskite photovoltaics: progress and challenges. ACS Energy Lett. 6, 1453–1479 (2021).
Yang, W. S. et al. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 348, 1234–1237 (2015).
Wu, W. Q. et al. Bilateral alkylamine for suppressing charge recombination and improving stability in blade-coated perovskite solar cells. Sci. Adv. 5, eaav8925 (2019).
Kerner, R. A., Xu, Z. J., Larson, B. W. & Rand, B. P. The role of halide oxidation in perovskite halide phase separation. Joule 5, 2273–2295 (2021).
Zhang, W. et al. Enhanced optoelectronic quality of perovskite thin films with hypophosphorous acid for planar heterojunction solar cells. Nat. Commun. 6, 10030 (2015).
Chen, S., Xiao, X., Gu, H. & Huang, J. Iodine reduction for reproducible and high-performance perovskite solar cells and modules. Sci. Adv. 7, eabe8130 (2021).
Gratia, P. et al. The many faces of mixed ion perovskites: unraveling and understanding the crystallization process. ACS Energy Lett. 2, 2686–2693 (2017).
Meng, H. et al. Chemical composition and phase evolution in DMAI-derived inorganic perovskite solar cells. ACS Energy Lett. 5, 263–270 (2019).
Shao, Z. et al. Cs4PbI6-mediated synthesis of thermodynamically stable FA0.15Cs0.85PbI3 perovskite solar cells. Adv. Mater. 32, e2001054 (2020).
Stolterfoht, M. et al. Visualization and suppression of interfacial recombination for high-efficiency large-area pin perovskite solar cells. Nat. Energy 3, 847–854 (2018).
Hoke, E. T. et al. Reversible photo-induced trap formation in mixed-halide hybrid perovskites for photovoltaics. Chem. Sci. 6, 613–617 (2015).
An, K. et al. Direct observation of crystal engineering in perovskite solar cells in a moisture-free environment using conductive atomic force microscopy and friction force microscopy. J. Phys. Chem. C 124, 4946–4952 (2020).
Fairfield, D. J. et al. Structure and chemical stability in perovskite–polymer hybrid photovoltaic materials. J. Mater. Chem. A 7, 1687–1699 (2019).
Tan, S. et al. Stability-limiting heterointerfaces of perovskite photovoltaics. Nature 605, 268–273 (2022).
Tong, J. H. et al. Carrier control in Sn-Pb perovskites via 2D cation engineering for all-perovskite tandem solar cells with improved efficiency and stability. Nat. Energy 7, 642–651 (2022).
Sproul, A. B. Dimensionless solution of the equation describing the effect of surface recombination on carrier decay in semiconductors. J. Appl. Phys. 76, 2851–2854 (1994).
Chen, H. et al. Regulating surface potential maximizes voltage in all-perovskite tandems. Nature 613, 676–681 (2023).
Zhu, Z. J. et al. Correlating the perovskite/polymer multi-mode reactions with deep-level traps in perovskite solar cells. Joule 6, 2849–2868 (2022).
Tang, R. et al. Hydrothermal deposition of antimony selenosulfide thin films enables solar cells with 10% efficiency. Nat. Energy 5, 587–595 (2020).
Chen, J. & Park, N. G. Causes and solutions of recombination in perovskite solar cells. Adv. Mater. 31, e1803019 (2019).
Cao, Q. et al. Efficient and stable inverted perovskite solar cells with very high fill factors via incorporation of star-shaped polymer. Sci. Adv. 7, eabg0633 (2021).
Yan, L. et al. Fabrication of perovskite solar cells in ambient air by blocking perovskite hydration with guanabenz acetate salt. Nat. Energy 8, 1158–1167 (2023).
Shi, L. et al. Gas chromatography-mass spectrometry analyses of encapsulated stable perovskite solar cells. Science 368, eaba2412 (2020).
Saliba, M. et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 354, 206–209 (2016).
Mosconi, E., Meggiolaro, D., Snaith, H. J., Stranks, S. D. & De Angelis, F. Light-induced annihilation of Frenkel defects in organo-lead halide perovskites. Energy Environ. Sci. 9, 3180–3187 (2016).
Khenkin, M. V. et al. Consensus statement for stability assessment and reporting for perovskite photovoltaics based on ISOS procedures. Nat. Energy 5, 35–49 (2020).
Acknowledgements
We acknowledge the staff of the Shanghai Synchrotron Radiation Facility (SSRF, BL14B1, BL14W1 and BL03HB beamline, project number 2020-SSRF-PT-014606), J. Xue (National Synchrotron Radiation Laboratory, University of Science and Technology of China) for helpful discussion and the Anhui Absorption Spectroscopy Analysis Instrument Co. for XAFS measurements and analysis. This work was partially carried out at the Instruments Center for Physical Science, University of Science and Technology of China. Funding: the work was supported by the National Natural Science Foundation of China (number 52172246); Anhui Province Industrial Innovation Project (AHZDCYCX-LSDT2023-09); National Key R&D Program of China (number 2021YFF0501900); the Institute of Energy, Hefei Comprehensive National Science Center (number 21KZS212); the Cornerstone Science Foundation through an XPLORER PRIZE to Jixian Xu and Fundamental research funds for the central universities (WK3430000009).
Author information
Authors and Affiliations
Contributions
Jixian Xu conceived and directed the project. M.D.M., H.M. and Jixian Xu reviewed the experiment, analysed the data and wrote the manuscript, and all authors discussed the results and commented on the manuscript. H.M. synthesized the ionic liquids, fabricated films/devices and conducted XRD/UV–vis/GIWAXs/PLQY/stability measurements. K.M. and W.P. helped fabricate and characterize the devices. F. Cai performed the device simulations. C.X. and F. Cao conducted KPFM characterizations. Z.S. helped carry out GIWAXS, EXAFS and analyse data. S.Y. and Z.Z. carried out the NMR measurement. X.W., K.Z. and Y.G. performed density functional theory calculations. T.L. and X.F. helped collect and analyse XPS data. Jiahang Xu and W.C. helped synthesize and characterize the ionic liquids.
Corresponding author
Ethics declarations
Competing interests
M.D.M. is an adviser to Swift Solar. University of Science and Technology of China (USTC) has filed patents related to the subject matter of this manuscript. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Dong Suk Kim, Min Kim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24, Notes 1–10, Tables 1–3 and references.
Supplementary Data 1
Statistical source data for Supplementary Fig. 13.
Source data
Source Data Fig. 4
Source data for Fig. 4k,m.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meng, H., Mao, K., Cai, F. et al. Inhibition of halide oxidation and deprotonation of organic cations with dimethylammonium formate for air-processed p–i–n perovskite solar cells. Nat Energy 9, 536–547 (2024). https://doi.org/10.1038/s41560-024-01471-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-024-01471-4
- Springer Nature Limited