Abstract
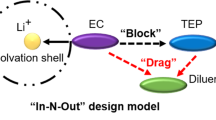
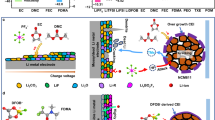
The current high-energy lithium metal batteries are limited by their safety and lifespan owing to the lack of suitable electrolyte solutions. Here we report a synergy of fluorinated co-solvent and gelation treatment by a butenoxycyclotriphosphazene (BCPN) monomer, which facilitates the use of ether-based electrolyte solutions for high-energy lithium metal batteries. We show that the safety risks of fire and electrolyte leakage are eliminated by the fluorinated co-solvent and fireproof polymeric matrices. The compatibility with high-energy cathodes is realized by a well-tailored Li+ solvation sheath, along with BCPN-derived protective surface films developed on the cathodes. Our Li | |LiNi0.8Co0.1Mn0.1O2 cells reach high-capacity retention, superior low-temperature performance, good cyclability under high pressure and steady power supply under abusive conditions. Our electrolyte design concept provides a promising path for high energetic, durable and safe rechargeable Li batteries.






Similar content being viewed by others
Data availability
All data are available in the main text or the Supplementary Information.
References
Dunn, B. et al. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Cui, C. et al. A highly reversible, dendrite-free lithium metal anode enabled by a lithium–fluoride-enriched interphase. Adv. Mater. 32, 1906427 (2020).
Liu, B. et al. Advancing lithium metal batteries. Joule 2, 833–845 (2018).
Makhlooghiazad, F. et al. Zwitterionic materials with disorder and plasticity and their application as non-volatile solid or liquid electrolytes. Nat. Mater. 21, 228–236 (2022).
Xiao, J. How lithium dendrites form in liquid batteries. Science 366, 426–427 (2019).
Koch, V. R. et al. The stability of the secondary lithium electrode in tetrahydrofuran-based electrolytes. J. Electrochem. Soc. 125, 1371–1377 (1978).
Holoubek, J. et al. Tailoring electrolyte solvation for Li metal batteries cycled at ultra-low temperature. Nat. Energy 6, 303–313 (2021).
Park, M. S. et al. A highly reversible lithium metal anode. Sci. Rep. 4, 3815 (2014).
Zhou, D. et al. Polymer electrolytes for lithium-based batteries: advances and prospects. Chem 5, 2326–2352 (2019).
Zhang, Z. et al. Fluorinated electrolytes for 5 V lithium-ion battery chemistry. Energy Environ. Sci. 6, 1806–1810 (2013).
Fan, X. et al. Highly fluorinated interphases enable high-voltage Li-metal batteries. Chem 4, 174–185 (2018).
Jaumaux, P. et al. Non-flammable liquid and quasi-solid electrolytes toward highly-safe alkali metal-based batteries. Adv. Funct. Mater. 31, 2008644 (2021).
Chen, S. et al. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes. Adv. Mater. 30, 1706102 (2018).
Tan, L. et al. Intrinsic nonflammable ether electrolytes for ultrahigh-voltage lithium metal batteries enabled by chlorine functionality. Angew. Chem. Int. Ed. 61, e202203693 (2022).
Wang, X. et al. Nonflammable trimethyl phosphate solvent-containing electrolytes for lithium-ion batteries: I. fundamental properties. J. Electrochem. Soc. 148, A1058 (2001).
Zheng, Q. et al. A cyclic phosphate-based battery electrolyte for high voltage and safe operation. Nat. Energy 5, 291–298 (2020).
Guan, X. et al. In-situ crosslinked single ion gel polymer electrolyte with superior performances for lithium metal batteries. Chem. Eng. J. 382, 122935 (2020).
Miao, R. et al. PVDF-HFP-based porous polymer electrolyte membranes for lithium-ion batteries. J. Power Sources 184, 420–426 (2008).
Vondrák, J. et al. Gel polymer electrolytes based on PMMA. Electrochim. Acta 46, 2047–2048 (2001).
Xu, G. et al. Prescribing functional additives for treating the poor performances of high-voltage (5 V-class) LiNi0.5Mn1.5O4/MCMB Li-ion batteries. Adv. Energy Mater. 8, 1701398 (2018).
He, M. et al. High voltage LiNi0.5Mn0.3Co0.2O2/graphite cell cycled at 4.6 V with a FEC/HFDEC-based electrolyte. Adv. Energy Mater. 7, 1700109 (2017).
von Aspern, N. et al. Fluorine and lithium: ideal partners for high-performance rechargeable battery electrolytes. Angew. Chem. Int. Ed. 58, 15978–16000 (2019).
Yamada, Y. et al. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 4, 269–280 (2019).
Chen, X. et al. Atomic insights into the fundamental interactions in lithium battery electrolytes. Acc. Chem. Res. 53, 1992–2002 (2020).
Kim, S. C. et al. Potentiometric measurement to probe solvation energy and its correlation to lithium battery cyclability. J. Am. Chem. Soc. 143, 10301–10308 (2021).
Abraham, K. M. et al. Highly conductive PEO-like polymer electrolytes. Chem. Mater. 9, 1978–1988 (1997).
Zhou, D. et al. Stable conversion chemistry-cased lithium metal batteries enabled by hierarchical multifunctional polymer electrolytes with near-single ion conduction. Angew. Chem. Int. Ed. 58, 6001–6006 (2019).
Chao, P. et al. Novel phosphorus–nitrogen–silicon flame retardants and their application in cycloaliphatic epoxy systems. Polym. Chem. 6, 2977–2985 (2015).
Chen, S. et al. Progress and future prospects of high-voltage and high-safety electrolytes in advanced lithium batteries: from liquid to solid electrolytes. J. Mater. Chem. A 6, 11631–11663 (2018).
Adams, B. D. et al. Accurate determination of coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv. Energy Mater. 8, 1702097 (2018).
Jiang, L. et al. Inhibiting solvent co-intercalation in a graphite anode by a localized high-concentration electrolyte in fast-charging batteries. Angew. Chem. Int. Ed. 60, 3402–3406 (2021).
Li, Y. et al. High-safety and high-voltage lithium metal batteries enabled by a nonflammable ether-based electrolyte with phosphazene as a cosolvent. ACS Appl. Mater. Interfaces 13, 10141–10148 (2021).
Boukamp, B. A. et al. Fast ionic conductivity in lithium nitride. Mater. Res. Bull. 13, 23–32 (1978).
Feng, Z. et al. In-situ formation of hybrid Li3PO4–AlPO4–Al(PO3)3 coating layer on LiNi0.8Co0.1Mn0.1O2 cathode with enhanced electrochemical properties for lithium-ion battery. Chem. Eng. J. 382, 122959 (2020).
McBrayer, J. D. et al. Mechanical studies of the solid electrolyte interphase on anodes in lithium and lithium ion batteries. Nanotechnology 32, 502005 (2021).
Chen, C. et al. Impact of dual-layer solid-electrolyte interphase inhomogeneities on early-stage defect formation in Si electrodes. Nat. Commun. 11, 3283 (2020).
Yoon, I. et al. Measurement of mechanical and fracture properties of solid electrolyte interphase on lithium metal anodes in lithium ion batteries. Energy Stor. Mater. 25, 296–304 (2020).
Verma, A. et al. Mechanistic analysis of mechano-electrochemical interaction in silicon electrodes with surface film. J. Electrochem. Soc. 164, A3570 (2017).
Zheng, J. et al. 3D visualization of inhomogeneous multi-layered structure and Young’s modulus of the solid electrolyte interphase (SEI) on silicon anodes for lithium ion batteries. Phys. Chem. Chem. Phys. 16, 13229–13238 (2014).
Zou, L. et al. Solid–liquid interfacial reaction trigged propagation of phase transition from surface into bulk lattice of Ni-rich layered cathode. Chem. Mater. 30, 7016–7026 (2018).
Li, Y. et al. High-safety and high-voltage lithium metal batteries enabled by a nonflammable ether-based electrolyte with phosphazene as a cosolvent. ACS Appl. Mater. Inter. 13, 10141–10148 (2021).
Morita, M. et al. Anodic behavior of aluminum current collector in LiTFSI solutions with different solvent compositions. J. Power Sources 119–121, 784–788 (2003).
von Wald Cresce, A. et al. Anion solvation in carbonate-based electrolytes. J. Phys. Chem. C. 119, 27255–27264 (2015).
Liu, X. et al. Conformal prelithiation nanoshell on LiCoO2 enabling high-energy lithium-ion batteries. Nano Lett. 20, 4558–4565 (2020).
Ren, X. et al. Enabling high-voltage lithium-metal batteries under practical conditions. Joule 3, 1662–1676 (2019).
Chen, S. et al. Strongly solvating ether electrolytes for high-voltage lithium metal batteries. ACS Appl. Mater. Interfaces 15, 13155–13164 (2023).
Gwon, H. et al. Recent progress on flexible lithium rechargeable batteries. Energy Environ. Sci. 7, 538–551 (2014).
Li, W. et al. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 46, 3006–3059 (2017).
Liu, X. et al. Thermal runaway of lithium-ion batteries without internal short circuit. Joule 2, 2047–2064 (2018).
Feng, X. et al. Mitigating thermal runaway of lithium-ion batteries. Joule 4, 743–770 (2020).
Wu, J. et al. A synergistic exploitation to produce high-voltage quasi-solid-state lithium metal batteries. Nat. Commun. 12, 5746 (2021).
Xu, K. “Charge-transfer” process at graphite/electrolyte interface and the solvation sheath structure of Li+ in nonaqueous electrolytes. J. Electrochem. Soc. 154, A162 (2007).
Xu, K. et al. Solvation sheath of Li+ in nonaqueous electrolytes and its implication of graphite/electrolyte interface chemistry. J. Phys. Chem. C. 111, 7411–7421 (2007).
Zhang, L. et al. Synthesis design of interfacial nanostructure for nickel–rich layered cathodes. Nano Energy 97, 107119 (2022).
Wu, Y. et al. High-voltage and high-safety practical lithium batteries with ethylene carbonate-free electrolyte. Adv. Energy Mater. 11, 2102299 (2021).
Ji, Y. et al. Toward a stable electrochemical interphase with enhanced safety on high-voltage LiCoO2 cathode: a case of phosphazene additives. J. Power Sources 359, 391–399 (2017).
Wan, T. H. et al. Influence of the discretization methods on the distribution of relaxation times deconvolution: implementing radial basis functions with DRTtools. Electrochim. Acta 184, 483–499 (2015).
Lu, Y. et al. The timescale identification decoupling complicated kinetic processes in lithium batteries. Joule 6, 1172–1198 (2022).
Thompson, A. P. et al. LAMMPS—a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 271, 108171 (2022).
Jorgensen, W. L. et al. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Bayly, C. I. et al. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges—the RESP model. J. Phys. Chem. 97, 10269–10280 (1993).
Park, C. et al. Molecular simulations of electrolyte structure and dynamics in lithium–sulfur battery solvents. J. Power Sources 373, 70–78 (2018).
Martínez, L. et al. PACKMOL: a package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 30, 2157–2164 (2009).
Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mat. Sci. Eng. 18, 015012 (2009).
Holoubek, J. et al. Tailoring electrolyte solvation for Li metal batteries cycled at ultra-low temperature. Nat. Energy 6, 303–313 (2021).
Hockney, R. W. et al. Computer Simulation Using Particles (CRC Press, 1988).
Gaussian 16 rev. C.01 (Gaussian Inc., 2016).
Lu, T. et al. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Acknowledgements
B.L. acknowledges support by the National Natural Science Foundation of China (number 52072208 and number 52261160384), Shenzhen Science and Technology Program (KCXFZ20211020163810015), the Fundamental Research Project of Shenzhen (number JCYJ20220818101004009), Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01N111) and Shenzhen Outstanding Talents Training Fund. G.W. acknowledges the support from Australian Research Council (ARC) Discovery Projects (DP200101249 and DP210101389) and the ARC Research Hub for Integrated Energy Storage Solutions (IH180100020). B.S. acknowledges the financial support from the Australian Research Council (ARC) through the ARC Future Fellowship (FT220100561).
Author information
Authors and Affiliations
Contributions
D.Z., M.A., B.L. and G.W. conceived and designed this work. Y.M. performed the experiments and wrote the manuscript. Y.G. and Y.T. carried out the computations. R.L., Y.W., B.S., F.K. and D.A. discussed the results and participated in the preparation of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Feifei Shi, Hsisheng Teng, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–48, Tables 1–9 and Notes 1–4.
Supplementary Video 1
Combustion test of pure ether electrolyte.
Supplementary Video 2
Combustion test of ether–SFE electrolyte.
Supplementary Video 3
Combustion test of NGPE.
Supplementary Video 4
Leakage test of liquid electrolyte and NGPE.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meng, Y., Zhou, D., Liu, R. et al. Designing phosphazene-derivative electrolyte matrices to enable high-voltage lithium metal batteries for extreme working conditions. Nat Energy 8, 1023–1033 (2023). https://doi.org/10.1038/s41560-023-01339-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01339-z
- Springer Nature Limited