Abstract
Fossilized lipids offer a rare glimpse into ancient ecosystems. 2-Methylhopanes in sedimentary rocks were once used to infer the importance of cyanobacteria as primary producers throughout geological history. However, the discovery of hopanoid C-2 methyltransferase (HpnP) in Alphaproteobacteria led to the downfall of this molecular proxy. In the present study, we re-examined the distribution of HpnP in a new phylogenetic framework including recently proposed candidate phyla and re-interpreted a revised geological record of 2-methylhopanes based on contamination-free samples. We show that HpnP was probably present in the last common ancestor of cyanobacteria, while the gene appeared in Alphaproteobacteria only around 750 million years ago (Ma). A subsequent rise of sedimentary 2-methylhopanes around 600 Ma probably reflects the expansion of Alphaproteobacteria that coincided with the rise of eukaryotic algae—possibly connected by algal dependency on microbially produced vitamin B12. Our findings re-establish 2-methylhopanes as cyanobacterial biomarkers before 750 Ma and thus as a potential tool to measure the importance of oxygenic cyanobacteria as primary producers on early Earth. Our study illustrates how genetics can improve the diagnostic value of biomarkers and refine the reconstruction of early ecosystems.
Similar content being viewed by others
Main
Cyanobacteria played a crucial role in transforming Earth from its initial anoxic state to a modern, oxygenated system, capable of sustaining increasingly complex life1,2. Because cyanobacteria were presumably the only relevant group of oxygenic primary producers for much of the Precambrian3, their fossilized remnants can serve as an indicator for the presence of oxygenic photosynthesis and relative variations in primary production and carbon burial in the geological past. Yet taphonomic alteration and non-quantitative preservation of cyanobacterial microfossils render their use in Precambrian reconstructions complicated4. By contrast, lipid molecules from cyanobacterial cellular membranes are less susceptible to taphonomic bias in environments that favour the preservation of organic matter. Thus, fossilized lipids in the form of biomarker hydrocarbons—in this case hopanoids—can provide important semi-quantitative information for reconstructing the ecological importance of cyanobacteria in the deep past5, thereby framing the environmental context that is crucially needed for better understanding the evolution of increasingly complex life.
Hopanoids are triterpenoid lipids predominantly produced by aerobic bacteria and considered functional analogues of eukaryotic sterols6,7. In contrast to the overall wide distribution of hopanoids in bacteria, hopanoids methylated at the C-2 position (2-methylhopanoids) have a much narrower taxonomical representation and were once extensively explored as taxon-specific biomarkers for cyanobacteria8. A cyanobacterial association was further supported by frequently observed concurrent records of elevated 2-methylhopane abundance and stable nitrogen isotope ratios suggestive of nitrogen fixation9,10—cyanobacteria being the major diazotrophs in modern oceans11. Although the physiological roles of methylhopanoids are not yet fully understood, protective roles against environmental stress have been suggested12,13. Precambrian rocks were found to generally contain higher proportions of 2-methylated hopanoids relative to Phanerozoic sediments, and this observation was interpreted to reflect an elevated proportion of cyanobacteria as Precambrian primary producers8,14. Also throughout the Phanerozoic, systematic trends and fluctuations in 2-methylhopane abundances relative to non-methylated regular hopanes (2-methylhopane index; 2-MHI) were widely used as an indicator of environmental stress and cyanobacterial proliferation9,15. Discovery of hopanoid C-2 methyltransferase (HpnP), however, called such interpretations into question by revealing the capacity for 2-methylhopanoid biosynthesis in both Cyanobacteria and Alphaproteobacteria16,17. It was even suggested that HpnP first evolved within aerobic Alphaproteobacteria18 and was only later horizontally transferred to Cyanobacteria. These inferences ultimately led to much uncertainty, debate and a questionable utility of ecological proxies involving 2-methylhopanoids.
Here we report on the existence of diagnostic time windows for fossil 2-methylhopanes in the geological past. Numerous new bacterial phyla have recently been found to possess hopanoid-producing genes19, and this urges a re-evaluation of 2-methylhopanoid distribution in bacteria and their geobiological implications. We re-examined the distribution of HpnP in the bacterial domain and its evolutionary history using up-to-date genomic datasets and an integrated approach towards phylogenetics20,21. In addition, by now, we know that pervasive contamination can overprint Precambrian lipid signatures, and doubts have been raised about previously published Precambrian biomarker records22. Hence, our molecular data were combined with new analyses of sedimentary 2-methylhopanes to provide an integrated account of 2-methylhopane production through Earth history.
Results and discussion
Hopanoid C-2 methyltransferase in bacteria
HpnP is mostly distributed across a subset of bacteria that possess squalene cyclase (SC), which creates the non-methylated hopanoid structure. Nearly all species that possess both the SC and HpnP genes retain only a single copy of each gene. Synteny (gene co-localization) between the SC and HpnP genes was not observed in any phylum, and thus these two genes may be independently inherited. In the current study, SC was found in 31 bacterial phyla, whereas HpnP was found in 12 phyla (Fig. 1a). This contrasts with only four phyla that were previously known to harbour HpnP-containing species23,24. Yet, the distribution of SC and HpnP is sporadic in most phyla that contain these two enzymes, and HpnP in particular is concentrated in three phyla: Rokubacteria, Alphaproteobacteria and Cyanobacteria (Fig. 1a,b) (Supplementary Note 1 and Supplementary Tables 1−5 provide more details). Rokubacteria is a recently proposed candidate phylum25, and its HpnP homologues have not been described thus far. Despite the sporadic occurrence of SC among bacteria, a recent comprehensive phylogenetic study suggests that SC was present in the individual common ancestors of three HpnP-containing phyla (Rokubacteria, Alphaproteobacteria and Cyanobacteria). This implies multiple events of SC gene loss within individual phyla, and HpnP seems to have followed a similar complex evolutionary history. It is currently not clear what selective pressure caused the loss or retention of the SC and HpnP genes.
a, Distribution in individual bacterial phyla; only phyla that harbour SC are shown. Light yellow colour indicates that only a small number of species possess the gene (below 10% of available genomes). Note that the orange colour does not necessarily mean the gene is ubiquitous in a phylum. Individual proteobacterial classes are treated as phyla in the main text. Abbreviations: FCB, Fibrobacteres–Chlorobi–Bacteroidetes group; PVC, Planctomycetes–Verrucomicrobia–Chlamydiae group. b, Distribution of SC and HpnP within the phyla Cyanobacteria and Alphaproteobacteria. The number of families within individual orders is shown in the second column, while the number of families that contain SC and HpnP is shown in the third and the fourth columns, respectively. Supplementary Tables 1−5 provide the details within individual families.
HpnP phylogenetic analysis
The evolutionary relationship of HpnP in 12 bacterial phyla was examined through phylogenetic analyses (Fig. 2 and Supplementary Figs. 1−3). HpnP forms a monophyletic group among multiple clades of uncharacterized HpnP-like proteins (HpnP clade; Fig. 2, insert). HpnP and HpnP-like proteins broadly retain three conserved domains: a B-12 binding domain, a radical SAM domain and a domain of unknown function (DUF4070). Vitamin B12 (cobalamin) dependency of HpnP was recently experimentally confirmed24, and our results suggest this cobalamin dependency is a general characteristic of HpnP homologues. Other distant homologues with a radical SAM domain do not retain all three conserved domains. Currently, methyltransferase activity is confirmed only for several proteins in the HpnP clade, and thus it is unknown if HpnP-like proteins are even involved in hopanoid (or more generally terpenoid) biosynthesis. Individual clades of HpnP and HpnP-like proteins display different taxonomical distributions and the tree topology within those clades generally does not follow the species tree at the phylum level. Some species were found to possess multiple HpnP homologies, in addition to HpnP. For instance, many cyanobacteria possess two HpnP homologues—HpnP and an uncharacterized HpnP-like protein. The latter was once thought to be HpnP26, but this was later corrected24. Those HpnP-like proteins serve as outgroup sequences in the present study.
The tree was generated using 135 representative sequences and 534 conserved sites. Filled black circles indicate that the node support is >85% for both maximum likelihood inference and Bayesian inference (node support is shown only for major clades). Filled grey circles indicate that the node support is above the same threshold for one of the two inferences. The presence/absence of the SC gene is indicated next to the tree. The insert displays major clades of HpnP homologues (HpnP and HpnP-like proteins) that retain three conserved domains; B-12 binding domain, radical SAM domain and DUF4070 domain. The scale bar represents 0.4 amino acid replacements per site per unit evolutionary time. Supplementary Figs. 1−3 provide the complete trees with the species annotation.
The deepest-branching HpnP homologues in the HpnP clade were obtained from Deltaproteobacteria (myxobacteria, in particular) that do not possess SC in their genomes (Fig. 2). This suggests that deltaproteobacterial HpnP homologues are not involved in hopanoid biosynthesis (Supplementary Note 2). We cannot exclude the possibility, however, that deltaproteobacteria containing HpnP homologues may acquire and methylate non-methylated hopanoid precursors from the environment. Except for Deltaproteobacteria, most bacteria that possess an early-diverging HpnP homologue also possess SC, and those bacteria presumably (although not experimentally confirmed) have the ability to produce 2-methylhopanoids. Currently, 2-methylhopanoid production has been confirmed only for the three late-branching phyla: Alphaproteobacteria, Cyanobacteria and Acidobacteria8,27,28. Thus, characterization of early-diverging HpnP homologues from other bacterial phyla, including Deltaproteobacteria, may provide important information about the functional evolution of radical SAM methyltransferases towards HpnP.
Focusing on HpnP proteins accompanied by SC, the tree topology does not replicate the known relationships between bacterial phyla and a complex history of horizontal gene transfer (HGT) is inferred (Fig. 2). However, Cyanobacteria, Rokubacteria and Alphaproteobacteria are well separated with robust nodal support by both maximum likelihood and Bayesian inferences (Fig. 2 and Supplementary Figs. 1 and 2), and no sign of recent HGT is observed. This suggests that the HGT events at the root of those sub-clades are ancient and the bacterial lineages that mediated those transfers (but were not necessarily producing 2-methylhopaonids themselves) are long extinct. Whereas HpnP sequences from Alphaproteobacteria and Cyanobacteria dominate our dataset, the evolutionary pattern seen in the two clades is distinct. Alphaproteobacterial sequences come from a sporadic number of sub-clades, suggesting HpnP emerged late in the group, while cyanobacterial sequences largely mirror the species tree, suggesting HpnP was inherited vertically in crown-group cyanobacteria. These observed patterns are described in more detail below.
Evolution of HpnP in Alphaproteobacteria
Unlike in previous studies that were based on more limited datasets16,18, alphaproteobacterial HpnP homologues do not form a monophyletic clade but instead cluster together with homologues from Acidobacteria and the newly proposed candidate phylum Eremiobacterota (Fig. 2). Hence, it is not clear if HpnP appeared only once in Alphaproteobacteria and was later horizontally transferred to the other phyla or if Alphaproteobacteria received HpnP through multiple HGT events from different sources. The two alphaproteobacterial clades are taxonomically distinct. One clade mostly consists of the family Methylobacteriaceae and some unclassified alphaproteobacteria, while the other principally contains Beijerinckiaceae and Nitrobacteraceae (Extended Data Fig. 1). These three alphaproteobacterial families belong to the same order Hyphomicrobiales (formerly Rhizobiales), while HpnP is nearly absent in other families (17 families in total; Supplementary Tables 2 and 3). Further, the observed HpnP tree topology is not consistent with the currently inferred species tree of Hyphomicrobiales (Extended Data Fig. 1), and HpnP is nearly absent from other alphaproteobacterial orders (Fig. 1b). Thus, we infer that HpnP was horizontally transferred to Alphaproteobacteria only after the divergence of Hyphomicrobiales, possibly multiple times independently.
Evolution of HpnP in Cyanobacteria
In contrast to Alphaproteobacteria, the HpnP branching order in Cyanobacteria broadly matches the species tree, despite the overall distribution of HpnP being sporadic at the species level (Fig. 3 and Supplementary Table 4). For instance, HpnP is found in two early-branching taxa (Gloeobacter and Gloeomargarita), and HpnP homologues from these taxa also branch early in the cyanobacterial HpnP tree. Here we examined the evolutionary history of cyanobacterial HpnP through reconciliation analyses of its tree topology. The reconciliation between the cyanobacterial HpnP tree and the species tree suggests that early-branching orders of the HpnP tree are well consistent with the species tree, while multiple transfer events of the HpnP gene seem to have occurred in individual sub-clades (Fig. 3 and Supplementary Figs. 4 and 5). The frequency of HGT is estimated to be no higher than ~15% (13 out of 86 branches) within HpnP-containing cyanobacterial species. Some putative transfer events are likely to be artefacts due to an inaccurate tree topology with low node supports and the heterotachy of some sub-clades in the HpnP tree (for example, weak node supports for the lineage that splits from Neosynechococcus; Fig. 3). The absence of HpnP in the majority of cyanobacteria is attributed to gene loss that seems to have occurred multiple times independently in major lineages (Extended Data Fig. 2 and Supplementary Note 3 for detailed discussions). Our results suggest that HpnP was present in the last common ancestor of Cyanobacteria and was subsequently vertically inherited, with multiple gene loss events and potentially some transfers within the phylum. SC has similarly been inferred to have been present in the last common ancestor of Cyanobacteria and vertically inherited19. However, this does not necessarily mean that SC and HpnP co-evolved, because no synteny is observed between the SC and HpnP genes. The ability to produce C-2 methylated hopanoids was thus probably present in the common ancestor of crown-group cyanobacteria, which contrasts with the here inferred late origin of alphaproteobacterial 2-methylhopanoid production (Supplementary Note 4 provides comparison with previous studies).
A species tree that contained 44 representative cyanobacterial species (Supplementary Fig. 4) was compared to the HpnP tree through reconciliation analyses (Supplementary Fig. 5 for the comparison result and HGT inference by the reconciliation analysis software Notung). Solid lines in the HpnP tree indicate that they are consistent with the species tree. Dashed lines indicate that they are not consistent with the species tree, and thus the presence of HGT is inferred by Notung. Filled black circles indicate that the node support is >85% for both maximum likelihood inference and Bayesian inference (node support is shown only for major clades). Filled grey circles indicate that the node support is above the same threshold for one of the two inferences. The scale bar represents 0.1 amino acid replacements per site per unit evolutionary time.
2-Methylhopanoids as aerobiosis biomarkers
The distribution of HpnP is nearly exclusive to obligate and facultative aerobes, even though the HpnP catalysis itself does not require molecular oxygen. HpnP-containing species constitute a subset of SC-containing bacteria, which are also mostly aerobes, although SC is additionally found in several anaerobic lineages (for example, Brocadiales, Desulfovibrionales)29,30. Hence, fossilized 2-methylhopanoids (2-methylhopanes) in the geological record are probable markers for aerobic bacteria and thus aerobic environments. This is consistent with an environmental survey that associated HpnP with microaerobic environments17. HpnP most likely evolved in, or was acquired by, stem-group cyanobacteria after the split from non-photosynthetic lineages (for example, Melainabacteria, Margulisbacteria; HpnP absent). In this framework, the analysis of geological 2-methylhopane records as aerobiosis markers potentially enables us to address the timing of incipient oxygen production before the onset of the GOE at ~2.4 billion years ago (Ga) as was originally proposed8, regardless of the phylogenetic identity of the biological host, if thermally well-preserved sedimentary sequences should ever be found22.
Source of fossilized 2-methylhopanoids
Understanding the evolutionary timeline of HpnP in individual phyla, in particular Cyanobacteria, Alphaproteobacteria and Rokubacteria, allows constraining the source of 2-methylhopanoids at different geological times. HpnP in additional bacteria suggests that Cyanobacteria was not the only lineage capable of producing 2-methylhopanoids. However, HpnP appears only sporadically in those additional lineages, possibly reflecting relatively recent HGT events or limited microbial sampling. Similarly, the presence of these proteins at the base of the HpnP tree could represent long branch attraction of proteins that are dissimilar from the better sampled clades (Cyanobacteria and Alphaproteobacteria)—perhaps representing a divergent function—or they could represent ancestry from an extinct lineage with an ancestral form of HpnP that was not necessarily involved in 2-methylhopanoid production, as implied for deltaproteobacterial HpnP homologues. We currently do not have enough data to adjudicate between these competing interpretations.
Nevertheless, accepting these limitations, we can conclude that 2-methylhopanoid biosynthesis existed in Cyanobacteria before it appeared within Alphaproteobacteria. The evolutionary history of Rokubacteria is not constrained, and it remains unclear if any members actually produce geologically relevant amounts of 2-methylhopanoids. Rokubacteria are neither primary producers, nor have they been detected in marine settings. Also, rokubacterial HpnP seems confined to several late-branching clades, although the exact timing of HpnP acquisition is not clear (Extended Data Fig. 3 and Supplementary Figs. 7−9). Hence, it appears unlikely that Rokubacteria substantially contributed to the Precambrian marine biomarker record. The same applies to other sporadic occurrences of HpnP discussed above. In contrast, cyanobacteria are generally accepted as important primary producers that substantially contributed to organic carbon burial throughout the mid-Proterozoic3,31. Also, our genome data reveal that HpnP is in fact distributed much more widely in marine cyanobacteria than previously known23 (Supplementary Table 5 and Supplementary Note 5 for more details). Therefore, cyanobacteria are the most likely source of fossilized 2-methylhopanoids before the evolution of HpnP-containing Alphaproteobacteria (Hyphomicrobiales).
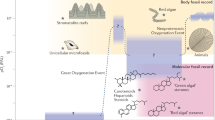
Constraining the emergence of HpnP-containing Hyphomicrobiales then provides a means to determine geological periods and formations with (nearly) exclusively cyanobacterial signatures. The evolutionary timeline of HpnP in Alphaproteobacteria was estimated using recently published phylogenomic analyses of Hyphomicrobiales (Fig. 4a)32. This published dated phylogeny suggests that Hyphomicrobiales emerged around 1.5 Ga (node number 4 in Fig. 4a), while the emergence of the stem groups of three major HpnP-containing families occurred only after 750 Ma (number 5 in Fig. 4a; nodes a, b and c). The phylogenetic relationship between those families in the HpnP tree is not consistent with the species tree of Hyphomicrobiales, as described above (Extended Data Fig. 1). Thus, the HpnP gene was probably horizontally transferred to those three families after 750 Ma. In contrast, HpnP is widespread within the individual three families, suggesting the presence of HpnP in the common ancestors of each of those individual families. The divergence of those three families is estimated to have occurred after 500 Ma (Fig. 4a; three triangles in red)32. Therefore, the emergence of HpnP in Alphaproteobacteria can be estimated to having occurred between the emergence of the stem groups and the divergence of the crown groups of three HpnP-containing families, somewhere between 750 and 500 Ma (node number 5; Fig. 4a). In turn, fossilized 2-methylhopanoids in marine sedimentary rocks deposited before 750 Ma probably indicate cyanobacterial sources.
a, Dated phylogeny of Alphaproteobacteria and the emergence of HpnP in the phylum. Both the tree topology and the node dates were adapted from a recent phylogenomic study (Supplementary Fig. 10)32. Numbers in the tree indicate major evolutionary events for the two phyla Cyanobacteria and Alphaproteobacteria: (1) divergence of crown-group cyanobacteria; (2) divergence of Alphaproteobacteria from Beta- and Gammaproteobacteria; (3) divergence of crown-group Alphaproteobacteria; (4) divergence of Hyphomicrobiales; (5) divergence of HpnP-containing Hyphomicrobiales families—Beijerinckiaceae (node a), Methylobacteriaceae (node b) and Nitrobacteraceae (node c). Nodes a, b and c indicate the emergence of three HpnP-containing families in Hyphomicrobiales. Node bars denote the 95% highest posterior density interval of posterior dates. Supplementary Fig. 10 provides the dated phylogeny with the species annotation. b, 2-Methylhopane index (2-MHI, % C31 2-methylhopane / (C31 2-methylhopane + C30 αβ-hopane)) throughout Earth’s history. The data contain newly analysed values from this study (open circles) and from the literature (open squares). Each circle and square represent the average of all 2-MHI values from individual geological formations or published studies (Supplementary Table 7). The underlying pale green bar diagram provides averages binned for geological time units (Supplementary Table 7). Blue-green and light-blue arrows indicate two geological periods when marine primary productivity was dominated by Cyanobacteria and algae, respectively. Abbreviations: GOE, Great Oxidation Event; Ma, million years ago. Figure adapted from ref. 32 under a Creative Commons license CC BY 4.0.
Non-contaminated 2-methylhopane record in the Precambrian
The record of the 2-methylhopane index (2-MHI) published thus far is generally characterized by high values in Precambrian rocks and oils (up to 19%), relative to those in younger organic matter, and an elevated proportion of cyanobacterial primary production was thus inferred for the early Earth8,14. However, the syngeneity (analytes having the same age as the host rock) of 2-methylhopanes in Precambrian samples has never been adequately and systematically assessed. By now, it is widely recognized that even traces of contamination can pose a serious problem for organic-lean Precambrian samples, as was previously scrutinized for Archaean biomarker studies22. Hence, we re-investigated Precambrian rocks using rigorous slice-extraction and interior–exterior experiments, allowing us to separately study non-contaminated sample interiors and contamination-prone sample exteriors (surfaces)22,33. Our analyses revealed that high 2-MHI values are restricted to sample exteriors—up to 17% in the 1.64 Ga Barney Creek Formation that hosts the oldest known 2-MHI record33 (Supplementary Note 6 and Supplementary Table 6)—whereas non-contaminated interiors exhibit 2-MHI values with an average of 1.1% (n = 42) and a maximum of only 2.4% (Extended Data Fig. 4). Abiotic hopanoid methylation at the position C-2 is highly unlikely (Supplementary Note 7 and Supplementary Fig. 12). As our results highlight the need for a systematic re-evaluation of Precambrian 2-methylhopane abundances, we compiled a new non-contaminated 2-MHI record based on newly analysed rock samples from the Phanerozoic to the Paleoproterozoic that underwent syngeneity assessment (n = 161), combined with previously published 2-MHI values, excluding any potentially contaminated samples from the pre-Ediacaran period (n = 507) (Supplementary Table 7).
In contrast to previous findings, the revised 2-MHI record exhibits consistently low relative 2-methylhopane abundances until the end of the Snowball Earth glaciations around 635 Ma (2-MHI < 2.4%; n = 93; average = 0.96%) (Fig. 4b). An initial increase to 5.4% is observed in the early Ediacaran ( ~ 620 Ma) and followed by multiple episodes of large fluctuations. While the 2-MHI in the Ediacaran and Phanerozoic can be extremely high for individual formations (up to 24%), the averaged values over geological time units remain moderate at ~5%.
Ecological implications of 2-methylhopanes
Comparatively low 2-MHI values (~1%) in the mid-Proterozoic represent the average contribution of cyanobacterial 2-methylhopanoids to mid-Proterozoic sediments and provide a baseline for gauging the activity of cyanobacteria, even though the 2-methylhopanoid production by marine cyanobacteria seems to be confined to nearshore environments34,35 (Supplementary Table 5 and Supplementary Note 8 for detailed discussions). The ‘rise of algae’ in the Cryogenian that broke the billion-year incumbency of cyanobacteria was attributed to a changing nutrient regime, which was triggered by massive glaciogenic inputs of phosphorus into oceans and allowed eukaryotic algae to permanently outcompete cyanobacteria3. Ecological shifts throughout the Ediacaran period—diminished abundances of microbial mats36,37 and the expansion of HpnP-free cyanobacteria as major primary producers in pelagic regions (Extended Data Fig. 2)—should have curtailed net cyanobacterial 2-methylhopanoid productions and preservations. This draws particular attention to the unexpected Ediacaran increase of the 2-MHI that probably reflects the emergence of non-cyanobacterial 2-methylhopanoid sources that may have played an important ecological role in Ediacaran oceans (Fig. 4b). Combining the biomarker record with time-calibrated genetic analyses points to the emergence of this lipid biosynthetic capacity in Alphaproteobacteria as an explanation (500–750 Ma; Fig. 4a). This inference is consistent with recent studies that proposed Alphaproteobacteria as the major source of Phanerozoic 2-methylhopanes23,24 and also the dominance of alphaproterobacterial HpnP genes in many modern marine metagenomes17 (Supplementary Notes 4 and 5). Hence, it is likely that the Ediacaran increase of the 2-MHI reflects the expansion of HpnP-containing Alphaproteobacteria.
We hypothesize that this dynamic shift in 2-methylhopanoid producers and the rise of algae proceeded in concert. It is well known that eukaryotes depend on symbiotic microbes for nutrient acquisition and have co-evolved with those symbionts, as observed for algae, plants and animals38,39. For instance, eukaryotes cannot biosynthesize vitamin B12 (cobalamin) de novo, although the majority of green algae require this nutrient for the biosynthesis of the essential amino acid methionine (Supplementary Note9)40. Dissolved oceanic vitamin B12 concentrations are generally too low to sustain the growth of algae, which thus rely on the uptake of microbially produced vitamin B12(ref.40). Vitamin B12 dependency evolved in several algal lineages independently during the course of evolution, through developing mutualistic relationships with microbial donors and retaining an efficient vitamin B12-dependent methionine synthase40. In modern environments, dominant vitamin B12 contributors to algae are Alphaproteobacteria and Gammaproteobacteria41,42 that utilize an oxygen-dependent pathway for vitamin B12 biosynthesis41, whose presence is predicted for many HpnP-containing Alphaproteobacteria (Supplementary Table 3). Hence, an expansion of vitamin B12-producing Alphaproteobacteria in mutualistic relationship with algae during the Ediacaran may be one factor tying the steep rise of 2-MHI values to the rise of algae (Supplementary Note 9 for more details). The elevated 2-MHI may additionally reflect an increased reworking of newly available algal biomass in increasingly oxygenated marine environments by heterotrophic Alphaproteobacteria. These hypotheses are complementary to the previously proposed relationship between 2-methylhopanoid production and the nitrogen cycle during Cretaceous oceanic anoxic events (Supplementary Note 4)23,24. In view of enhanced alphaproteobacterial 2-methylhopanoid production going back to the Ediacaran, it is likely that HpnP-containing Alphaproteobacteria were responsible for modulating the 2-MHI throughout the Phanerozoic.
Conclusions
The once suggested association of 2-methylhopanoids with cyanobacteria—a highly important proxy tool for our understanding of Earth system evolution and oxygen dynamics in the geological past—has been constantly debated. Both Cyanobacteria and Alphaproteobacteria can biosynthesize 2-methylhopanoids, and thus sedimentary 2-methylhopanes are not a diagnostic biomarker for a single taxonomic group. We show that such ambiguities can be refined by adding a genetic perspective. The phylogenetic tree topology of HpnP in Cyanobacteria suggests that HpnP was probably present in the common ancestor of crown-group cyanobacteria, whereas according to molecular clock analyses alphaproteobacterial lineages acquired HpnP after 750 Ma. This finding re-establishes the utility of 2-methylhopanes as a biomarker for cyanobacteria in pre-Ediacaran rocks, enabling us to measure the importance of HpnP-containing oxygenic cyanobacteria in the geological past. By contrast, 2-methylhopanes in post-Cryogenian oceans reflect additional signals from heterotrophic 2-methylhopanoid producers—Alphaproteobacteria. The synchronization between the 2-MHI increase during the Ediacaran and the ecological expansion of HpnP-containing Alphaproteobacteria and eukaryotic algae may not be coincidental, involving a vitamin B12-based mutualistic relationship between Alphaproteobacteria and algae and enhanced reworking of algal biomass by Alphaproteobacteria in increasingly oxygenated marine environments. Our study demonstrates the strength of combining the geological record of fossil hydrocarbon biomarkers with genetic analyses to gain insights into ancient ecosystems and provides an important precedent for refining our understanding of biomarker utility throughout Earth’s history.
Methods
Bioinformatics analyses
HpnP dataset construction
Representative sequences for hopanoid biosynthesis enzymes were identified from UniProt (https://www.uniprot.org/); HpnP (B3QHD1), SC (P33247). These protein sequences were utilized as seeds to identify homologous sequences in all three domains of life. Sequences were retrieved from GenBank (http://www.ncbi.nlm.nih.gov/), using BLASTp and PSI-Blast43,44, with the cut-off threshold of <1 × 10−5. Taxonomically redundant sequences were excluded. Protein domain identification was performed using HMMER v3.3.1 (ref. 45) with the PFAM-A database (http://ftp.ebi.ac.uk/pub/databases/Pfam/current_release/Pfam-A.hmm.gz).
Phylogenetic analysis
Sequences were aligned using Muscle v3.8.31 (ref. 46). Phylogenetic trees were constructed by maximum likelihood inference using IQ-TREE v2.1.06 (ref. 47) and by Bayesian inference using MrBayes v3.2.6 (ref. 48). Substitution models were selected using ModelFinder in IQ-TREE−LG matrix with empirical frequencies estimated from the data (F) and the FreeRate model for rate heterogeneity across sites (R). For maximum likelihood inference, branch support was obtained by Ultrafast bootstrap in IQ-TREE. For Bayesian inference, two Markov chain Monte Carlo chains were run for at least 1,000,000 generations until the maximum discrepancy between chains was <0.05. A consensus tree was generated from two chains using a burn-in of 10% of sampled points. In initial analyses, several protein sequences were found to display high sensitivity to sequence alignments and instable positioning in generated trees (accession numbers PYX91820 and HBG07613). These sequences were excluded from the subsequent analyses.
Cyanobacterial species tree construction and gene tree/species tree reconciliation
The list of cyanobacterial species with HpnP proteins was used to create a species tree. The species names were converted into taxon IDs using the NCBI Taxonomy name/id Status Report Page (https://www.ncbi.nlm.nih.gov/Taxonomy/TaxIdentifier/tax_identifier.cgi). These IDs were used with the research command line utility49 to download all proteins for each taxon in NCBI’s Identical Protein Group (IGP) database. Single-copy orthologous proteins were identified in these IGP datasets using OrthoFinder v2.5.4 (ref. 50). OrthoFinder identified 34 single-copy orthologue groups, which were individually aligned using MUSCLE46 and then concatenated together into a supermatrix using FASconCAT-G51. The species tree was generated from this supermatrix using IQ-TREE, including details of each protein’s beginning and end in the supermatrix alignment. We allowed IQ-TREE to determine the best model of amino acid evolution for each protein in the supermatrix individually before performing maximum likelihood inference. Once the species tree was generated, we compared how the topology of the cyanobacterial HpnP gene tree matched with the species tree using NOTUNG v2.9 tree reconciliation (DTL model)52.
Rokubacteria species tree construction and gene tree/species tree reconciliation
The phylum currently contains only metagenomic samples, and thus the same species tree construction as performed for cyanobacteria was not possible. Instead, a species tree was constructed by concatenating three ribosomal proteins L2, L3 and L4 (Supplementary Table 1), using IQ-TREE. The best substitution model was also determined by IQ-TREE. The topology of the rokubacterial HpnP tree was compared with the generated species tree using NOTUNG v2.9 (DTL model).
Biomarker analyses
Selection of previous biomarker studies
Previous studies that are included in our dataset are summarized in Supplementary Table 7 (black colour). Phanerozoic samples are generally organic rich, and thus the influence of trace contamination overprint is probably negligible in most cases. Moreover, a much higher density of available datapoints minimizes the risk of interpreting outliers and unrepresentative samples. Therefore, samples were selected to uniformly cover the whole Phanerozoic period without considering the syngeneity of observed biomarkers. In contrast, Precambrian samples are mostly organic lean and thus are easily overprinted by trace contamination as shown for samples from the 1.64 Ga McArthur Basin (Supplementary Note 6). To minimize uncertainty due to contamination, Precambrian samples in our dataset consist mostly of our newly analysed samples, except for Ediacaran oil samples that are highly organic rich and difficult to substantially adulterate through contamination overprint.
Biomarker extraction and gas chromatography-mass spectroscopy measurement
Samples that were newly analysed in this study are summarized in Supplementary Table 7 (highlighted in red). Biomarkers were extracted using interior–exterior and slice-extraction experiments as previously described22,53. For the interior–exterior experiments, rock samples were cut into two portions using a diamond saw—the exterior (exposed surface; approximately 3 mm thickness) and the interior (inner core). Slice-extraction experiments are an extension of the interior–exterior experiments, and rocks were cut into more than two slices to analyse the millimetre-scale distribution of biomarkers. Each slice was crushed independently with a stainless-steel puck mill and bitumen extracted with a Dionex Accelerated Solvent Extractor (ASE 200) using dichloromethane (DCM) for GR7 samples33 and 90:10% DCM:methanol for LV09001 samples. Saturated fractions were eluted with 1.5 dead volumes n-hexane on a microcolumn of annealed (300 °C, 12 h) and dry-packed silica gel. D4-C29 αααR-ethylcholestane (D4; Chiron Laboratories AS) was added to the saturate fraction before gas chromatography-mass spectroscopy (GC–MS) analyses with an Agilent 6890 gas chromatograph equipped with a 60 m DB-5 MS capillary column (0.25 mm i.d., 0.25 µm film thickness; Agilent Technologies) coupled to a Micromass Autospec Premier double sector mass spectrometer (Waters Corporation). Hopanes were analysed with M+ (412 for C30 hopanes)→ m/z 191 and methylhopanes with M+→ m/z 205 multiple reaction monitoring transitions. 2-Methylhopane peak assignment was based on the retention time and the comparison with the Australian Geological Survey Organisation standard. Using a DB-5 MS capillary column, C31 2-methylhopane elutes just before C30 αβ hopane on the chromatogram.
Pyrolysis
Aliquots (1 mg) of diplopterol (courtesy of P. Adam and P. Schaeffer, University of Strasbourg), 5α(H)-cholestanol (≥95%, Sigma-Aldrich) or cholesterol (≥99%, Sigma-Aldrich) were transferred to glass tubes flame sealed at one end (Duran, 8 mm diameter, 1 mm wall thickness) dissolved in DCM. After DCM evaporation, ~20 mg active carbon (Sigma-Aldrich, DARCO, 100 mesh particle size, CAS: 7440-44-0) was added, tubes were evacuated to ≤300 mTorr and flame sealed with a gas torch. After pyrolysis in an oven at 300 °C for 24 h, tubes were cooled to room temperature, cracked open and the active carbon was transferred with sequential solvent rinses of n-hexane, DCM and methanol onto a small silica plug in a 4 ml solid phase extraction glass tube and subsequently extracted with about 10 ml n-hexane, 10 ml DCM and at least 4 ml methanol. Once solvents were evaporated under a stream of N2, the pyrolysate was applied onto a silica gel microcolumn (about 500 mg in a glasswool-plugged Pasteur pipette), and the saturated hydrocarbon fraction was eluted with 1.5 dead volumes n-hexane followed by GC–MS analysis. Pyrolysates were analysed on a Thermo Quantum XLS Ultra triple-quadrupole MS coupled to a Thermo Trace GC Ultra fitted with a VF-1 MS column (40 m, 0.15 mm i.d., 0.15 μm film thickness).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or Supplementary Information. Additional and raw data and used sample material related to this paper may be requested from the authors.
Code availability
The data and code used to execute cyanobacterial species tree constructions are available at https://github.com/David-Gold/2022_HpnP.
References
Dahl, T. W. et al. Devonian rise in atmospheric oxygen correlated to the radiations of terrestrial plants and large predatory fish. Proc. Natl Acad. Sci. USA 107, 17911–17915 (2010).
Canfield, D. E., Poulton, S. W. & Narbonne, G. M. Late-Neoproterozoic deep-ocean oxygenation and the rise of animal life. Science 315, 92–95 (2007).
Brocks, J. J. et al. The rise of algae in Cryogenian oceans and the emergence of animals. Nature 548, 578–581 (2017).
Hallmann, C. & Summons, R. E. in Treatise on Geochemistry 2nd edn (eds Holland, H. D. & Turekian, K. K.) 139–155 (Elsevier, 2014).
Luo, G., Yang, H., Algeo, T. J., Hallmann, C. & Xie, S. Lipid biomarkers for the reconstruction of deep-time environmental conditions. Earth Sci. Rev. 189, 99–124 (2019).
Sáenz, J. P. et al. Hopanoids as functional analogues of cholesterol in bacterial membranes. Proc. Natl Acad. Sci. USA 112, 11971–11976 (2015).
Belin, B. J. et al. Hopanoid lipids: from membranes to plant–bacteria interactions. Nat. Rev. Microbiol. 16, 304–315 (2018).
Summons, R. E., Jahnke, L. L., Hope, J. M. & Logan, G. A. 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature 400, 554–557 (1999).
Kuypers, M. M. M., van Breugel, Y., Schouten, S., Erba, E. & Damsté, J. S. S. N2-fixing cyanobacteria supplied nutrient N for Cretaceous oceanic anoxic events. Geology 32, 853–856 (2004).
Jia, C. et al. Microbial response to limited nutrients in shallow water immediately after the end-Permian mass extinction. Geobiology 10, 60–71 (2012).
Zehr, J. P. & Capone, D. G. Changing perspectives in marine nitrogen fixation. Science 368, eaay9514 (2020).
Matys, E. D. et al. Bacteriohopanepolyols across environmental gradients in Lake Vanda, Antarctica. Geobiology 17, 308–319 (2019).
Doughty, D. M., Hunter, R. C., Summons, R. E. & Newman, D. K. 2-Methylhopanoids are maximally produced in akinetes of Nostoc punctiforme: geobiological implications. Geobiology 7, 524–532 (2009).
Knoll, A. H., Summons, R. E., Waldbauer, J. R. & Zumberge, J. E. in Evolution of Primary Producers in the Sea (eds Falkowski, P. G. & Knoll, A. H.) 133–163 (Academic Press, 2007).
Xie, S., Pancost, R. D., Yin, H., Wang, H. & Evershed, R. P. Two episodes of microbial change coupled with Permo/Triassic faunal mass extinction. Nature 434, 494–497 (2005).
Welander, P. V., Coleman, M. L., Sessions, A. L., Summons, R. E. & Newman, D. K. Identification of a methylase required for 2-methylhopanoid production and implications for the interpretation of sedimentary hopanes. Proc. Natl Acad. Sci. USA 107, 8537–8542 (2010).
Ricci, J. N. et al. Diverse capacity for 2-methylhopanoid production correlates with a specific ecological niche. ISME J. 8, 675–684 (2014).
Ricci, J. N., Michel, A. J. & Newman, D. K. Phylogenetic analysis of HpnP reveals the origin of 2-methylhopanoid production in Alphaproteobacteria. Geobiology 13, 267–277 (2015).
Santana-Molina, C., Rivas-Marin, E., Rojas, A. M. & Devos, D. P. Origin and evolution of polycyclic triterpene synthesis. Mol. Biol. Evol. 37, 1925–1941 (2020).
Gaucher, E. A. et al. The planetary biology of cytochrome P450 aromatases. BMC Biol. 2, 19 (2004).
Hoshino, Y. & Gaucher, E. A. On the origin of isoprenoid biosynthesis. Mol. Biol. Evol. 35, 2185–2197 (2018).
French, K. L. et al. Reappraisal of hydrocarbon biomarkers in Archean rocks. Proc. Natl Acad. Sci. USA 112, 5915–5920 (2015).
Naafs, B. D. A., Bianchini, G., Monteiro, F. M. & Sánchez-Baracaldo, P. The occurrence of 2-methylhopanoids in modern bacteria and the geological record. Geobiology 20, 41–59 (2022).
Elling, F. J. et al. Vitamin B12-dependent biosynthesis ties amplified 2-methylhopanoid production during oceanic anoxic events to nitrification. Proc. Natl Acad. Sci. USA 117, 32996–33004 (2020).
Becraft, E. D. et al. Rokubacteria: genomic giants among the uncultured bacterial phyla. Front. Microbiol. 8, 2264 (2017).
Cornejo-Castillo, F. M. & Zehr, J. P. Hopanoid lipids may facilitate aerobic nitrogen fixation in the ocean. Proc. Natl Acad. Sci. USA 116, 18269–18271 (2019).
Rashby, S. E., Sessions, A. L., Summons, R. E. & Newman, D. K. Biosynthesis of 2-methylbacteriohopanepolyols by an anoxygenic phototroph. Proc. Natl Acad. Sci. USA 104, 15099–15104 (2007).
Damsté, J. S. S., Rijpstra, W. I. C., Dedysh, S. N., Foesel, B. U. & Villanueva, L. Pheno- and genotyping of hopanoid production in Acidobacteria. Front. Microbiol. 8, 968 (2017).
Blumenberg, M. et al. Biosynthesis of hopanoids by sulfate-reducing bacteria (genus Desulfovibrio). Environ. Microbiol. 8, 1220–1227 (2006).
Niftrik, L. V. & Jetten, M. S. M. Anaerobic ammonium-oxidizing bacteria: unique microorganisms with exceptional properties. Microbiol. Mol. Biol. Rev. 76, 585–596 (2012).
Gueneli, N. et al. 1.1-billion-year-old porphyrins establish a marine ecosystem dominated by bacterial primary producers. Proc. Natl Acad. Sci. USA 115, E6978–E6986 (2018).
Wang, S., Meade, A., Lam, H.-M. & Luo, H. Evolutionary timeline and genomic plasticity underlying the lifestyle diversity in rhizobiales. mSystems 5, e00438–00420 (2020).
Brocks, J. J. et al. Biomarker evidence for green and purple sulphur bacteria in a stratified Palaeoproterozoic sea. Nature 437, 866–870 (2005).
Allen, M. A., Neilan, B. A., Burns, B. P., Jahnke, L. L. & Summons, R. E. Lipid biomarkers in Hamelin pool microbial mats and stromatolites. Org. Geochem. 41, 1207–1218 (2010).
Garby, T. J., Walter, M. R., Larkum, A. W. D. & Neilan, B. A. Diversity of cyanobacterial biomarker genes from the stromatolites of Shark Bay, Western Australia. Environ. Microbiol. 15, 1464–1475 (2013).
Gehling, J. G. Microbial mats in terminal Proterozoic siliciclastics; Ediacaran death masks. Palaios 14, 40–57 (1999).
Pawlowska, M. M., Butterfield, N. J. & Brocks, J. J. Lipid taphonomy in the Proterozoic and the effect of microbial mats on biomarker preservation. Geology 41, 103–106 (2013).
Zilber-Rosenberg, I. & Rosenberg, E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol. Rev. 32, 723–735 (2008).
Kazamia, E., Helliwell, K. E., Purton, S. & Smith, A. G. How mutualisms arise in phytoplankton communities: building eco-evolutionary principles for aquatic microbes. Ecol. Lett. 19, 810–822 (2016).
Croft, M. T., Lawrence, A. D., Raux-Deery, E., Warren, M. J. & Smith, A. G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438, 90–93 (2005).
Shelton, A. N. et al. Uneven distribution of cobamide biosynthesis and dependence in bacteria predicted by comparative genomics. ISME J. 13, 789–804 (2018).
Heal, K. R. et al. Two distinct pools of B12 analogs reveal community interdependencies in the ocean. Proc. Natl Acad. Sci. USA 114, 364–369 (2017).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Eddy, S. R. Accelerated profile HMM searches. PLoS Comput. Biol. 7, e1002195 (2011).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Minh, B. Q. et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 1530–1534 (2020).
Huelsenbeck, J. P. & Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 (2001).
Entrez programming utilities help. National Center for Biotechnology Information https://www.ncbi.nlm.nih.gov/books/NBK25501/ (2010).
Emms, D. M. & Kelly, S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20, 238 (2019).
Kück, P. & Longo, G. C. FASconCAT-G: extensive functions for multiple sequence alignment preparations concerning phylogenetic studies. Front. Zool. 11, 81 (2014).
Durand, D., Halldórsson, B. V. & Vernot, B. A hybrid micro–macroevolutionary approach to gene tree reconstruction. J. Comput. Biol. 13, 320–335 (2006).
Brocks, J. J. Millimeter-scale concentration gradients of hydrocarbons in Archean shales: live-oil escape or fingerprint of contamination? Geochim. Cosmochim. Acta 75, 3196–3213 (2011).
Sánchez-Baracaldo, P. Origin of marine planktonic cyanobacteria. Sci. Rep. 5, 17418 (2015).
Hirose, Y. et al. Diverse chromatic acclimation processes regulating phycoerythrocyanin and rod-shaped phycobilisome in cyanobacteria. Mol. Plant 12, 715–725 (2019).
Fournier, G. P. et al. The Archean origin of oxygenic photosynthesis and extant cyanobacterial lineages. Proc. R. Soc. B 288, 20210675 (2021).
Acknowledgements
We thank D. Edwards and the organic geochemistry team at Geoscience Australia for oil samples from the National Collection and the Geological Survey of Western Australia, the Northern Territory Geological Survey, T. Gallagher, E. Grosjean, K. Kirsimae, A. Lapland, L. Marynowski, M. Moczydłowska, S. Porter, N. Sheldon, E. Sperling and S. Zhang for rock specimens and extracts. We also thank P. Adam and P. Schaeffer (University of Strasbourg) for providing the diplopterol educt employed in the pyrolysis experiments and R. Tarozo (Max Planck Institute for Biogeochemistry) and J. Hope (The Australian National University) for assistance with GC–MS. This work was supported by DFG Priority programme 2237 and the Helmholtz Society to C.H.; Agouron geobiology postdoctoral fellowship to Y.H.; postdoctoral fellowship provided by the Central Research and Development Fund of the University of Bremen to B.J.N.; National Science Foundation grant 2044871 to D.A.G.; Australian Research Council grants DP160100607, DP170100556 and DP200100004 to J.J.B.; and National Institutes of Health grant R01AR069137, Human Frontier Science Program grant RGP0041, National Science Foundation grant 2032315 and Department of Defense grant MURI W911NF-16-1-0372 to E.A.G.
Funding
Open access funding provided by Helmholtz-Zentrum Potsdam Deutsches GeoForschungsZentrum - GFZ.
Author information
Authors and Affiliations
Contributions
Y.H. and B.J.N. conceived of the work. Y.H., E.A.G. and D.A.G. performed genetic data collection and analyses. B.J.N., G.V., L.M.v.M., C.B. C.H. and J.J.B. performed biomarker data collection and analyses. Y.H. and B.J.N. wrote the original draft. All authors participated in data interpretation, edited the draft and agreed to the published version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Evolutionary relationship of alphaproteobacterial HpnP proteins.
Three major HpnP-containing families are shown in the two alphaproteobacterial sub-clades within the HpnP tree (Methylobacteriaceae, Nitrobacteraceae and Beijerinckiaceae). The inset indicates the species relationship between the three Hyphomicrobiales families32. See Supplementary Fig. 1 for the complete tree with the species annotation.
Extended Data Fig. 2 Evolution of the extant lineages of marine planktonic cyanobacteria and its relationship to HpnP gene loss.
HpnP was likely lost long before the emergence of extant planktonic marine species. The dated species tree is adapted from a recent molecular clock study (see Supplementary Fig. 6 for the original figure with the species annotation)54. Presence/absence of HpnP in individual sub-clades was inferred from Supplementary Table 5 and recently-published phylogenomic studies of Cyanobacteria55,56. Inference for the vertical inheritance of the HpnP gene (solid line) is based on Fig. 3. Several representative HpnP-containing species are shown next to the corresponding clades. Asterisks indicate that the species is closely related to the corresponding clade, but is not included in the dataset in the cited study. Light blue circles indicate the possible timing of HpnP gene loss in the lineages that include modern marine planktonic species. It is noted that the timing of HpnP gene loss is possibly even earlier, depending on the actual HpnP evolutionary history in Cyanobacteria (dashed lines; vertical inheritance vs. horizontal transfer). Figure adapted from ref. 54 under a Creative Commons license CC BY 4.0.
Extended Data Fig. 3 Distribution of HpnP in Rokubacteria.
A species tree that contained 56 representative rokubacterial species (Supplementary Fig. 7) was compared to the HpnP tree (Supplementary Fig. 8) through Notung reconciliation analyses (Supplementary Fig. 9). Solid lines in the HpnP tree indicate that they are consistent with the species tree. Dashed lines indicate that they are not consistent with the species tree and thus the presence of HGT is inferred by Notung. Filled black circles indicate that the node support is >85% (node support is shown only for major clades). The scale bar represents 0.1 amino acid replacements per site per unit evolutionary time.
Extended Data Fig. 4 Stratigraphic trend of the 2-MHI.
2-MHI values were calculated for the Paleoproterozoic LV09001 drill core from the McArthur Basin in Northern Australia ( ~ 1.64 Ga) (see also Supplementary Note 6). Representative chromatograms for non-methylated hopanes (C30 diahopanes, C30 & C31 hopanes) and methylhopanes (2α and 3β) are shown (478.2 m depth). C31 2-methylhopane elutes just prior to C30 αβ-hopane on a DB-5 MS capillary column. Chromatograms are annotated by Metastable Reaction Monitoring (MRM) precursor → products pairs. Percentages provide relative heights of the largest peaks in the chromatograms.
Supplementary information
Supplementary Information
Supplementary Notes 1−9, Figs. 1−12, References (Supplementary Table 7 references included) and captions for Tables 1–7.
Supplementary Data 1
Maximum likelihood HpnP tree data for Supplementary Fig. 1.
Supplementary Data 2
Bayesian HpnP tree data for Supplementary Fig. 2.
Supplementary Data 3
Maximum likelihood HpnP-like protein tree data for Supplementary Fig. 3.
Supplementary Data 4
Multiple sequence alignment data for Supplementary Fig. 1.
Supplementary Data 5
Multiple sequence alignment data for Supplementary Fig. 3.
Supplementary Tables
Supplementary Tables 1–7.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hoshino, Y., Nettersheim, B.J., Gold, D.A. et al. Genetics re-establish the utility of 2-methylhopanes as cyanobacterial biomarkers before 750 million years ago. Nat Ecol Evol 7, 2045–2054 (2023). https://doi.org/10.1038/s41559-023-02223-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-023-02223-5
- Springer Nature Limited