Abstract
Sweat bees have repeatedly gained and lost eusociality, a transition from individual to group reproduction. Here we generate chromosome-length genome assemblies for 17 species and identify genomic signatures of evolutionary trade-offs associated with transitions between social and solitary living. Both young genes and regulatory regions show enrichment for these molecular patterns. We also identify loci that show evidence of complementary signals of positive and relaxed selection linked specifically to the convergent gains and losses of eusociality in sweat bees. This includes two pleiotropic proteins that bind and transport juvenile hormone (JH)—a key regulator of insect development and reproduction. We find that one of these proteins is primarily expressed in subperineurial glial cells that form the insect blood–brain barrier and that brain levels of JH vary by sociality. Our findings are consistent with a role of JH in modulating social behaviour and suggest that eusocial evolution was facilitated by alteration of the proteins that bind and transport JH, revealing how an ancestral developmental hormone may have been co-opted during one of life’s major transitions. More broadly, our results highlight how evolutionary trade-offs have structured the molecular basis of eusociality in these bees and demonstrate how both directional selection and release from constraint can shape trait evolution.





Similar content being viewed by others
Data availability
Raw sequencing data are available at NCBI under the following accession numbers: PRJNA613468, PRJNA629833, PRJNA718331 and PRJNA512907 (Hi-C). Hi-C data and genome assemblies are also available at www.dnazoo.org. Genomes and browsers can be accessed at beenomes.princeton.edu and on NCBI: PRJNA613468. Please address inquiries or material requests to skocher@princeton.edu.
Code availability
Repository with code is on GitHub at https://github.com/kocherlab/HalictidCompGen.
References
Kocher, S. D. & Paxton, R. J. Comparative methods offer powerful insights into social evolution in bees. Apidologie 45, 289–305 (2014).
Schwarz, M. P., Richards, M. H. & Danforth, B. N. Changing paradigms in insect social evolution: insights from halictine and allodapine bees. Annu. Rev. Entomol. 52, 127–150 (2007).
Kocher, S. D. et al. The genetic basis of a social polymorphism in halictid bees. Nat. Commun. 9, 4338 (2018).
Wcislo, W. T. & Danforth, B. N. Secondarily solitary: the evolutionary loss of social behavior. Trends Ecol. Evol. 12, 468–474 (1997).
Gibbs, J., Brady, S. G., Kanda, K. & Danforth, B. N. Phylogeny of halictine bees supports a shared origin of eusociality for Halictus and Lasioglossum (Apoidea: Anthophila: Halictidae). Mol. Phylogenet. Evol. 65, 926–939 (2012).
Danforth, B. N., Conway, L. & Ji, S. Phylogeny of eusocial Lasioglossum reveals multiple losses of eusociality within a primitively eusocial clade of bees (Hymenoptera: Halictidae). Syst. Biol. 52, 23–36 (2003).
Batra, S. W. T. Nests and social behavior of halictine bees of India (Hymenoptera: Halictidae). Indian J. Entomol. 28, 375–393 (1966).
Michener, C. D. The Social Behavior of the Bees: A Comparative Study (Belknap Press of Harvard University Press, 1974).
Plateaux-Quénu, C. Un nouveau type de société d’Insectes: Halictus marginatus Brullé (Hym., Apoidea). Ann. Biol. 35, 325–455 (1959).
Michener, C. D. Comparative social behavior of bees. Annu. Rev. Entomol. 14, 299–342 (1969).
Kapheim, K. M. et al. Draft genome assembly and population genetics of an agricultural pollinator, the solitary alkali bee (Halictidae: Nomia melanderi). G3 9, 625–634 (2019).
Kapheim, K. M. et al. Genomic signatures of evolutionary transitions from solitary to group living. Science 348, 1139–1143 (2015).
Kapheim, K. M. et al. Developmental plasticity shapes social traits and selection in a facultatively eusocial bee. Proc. Natl Acad. Sci. USA 117, 13615–13625 (2020).
Johnson, B. R. Taxonomically restricted genes are fundamental to biology and evolution. Front. Genet. 9, 407 (2018).
Jasper, W. C. et al. Large-scale coding sequence change underlies the evolution of postdevelopmental novelty in honey bees. Mol. Biol. Evol. 32, 334–346 (2015).
Johnson, B. R. & Linksvayer, T. A. Deconstructing the superorganism: social physiology, groundplans, and sociogenomics. Q. Rev. Biol. 85, 57–79 (2010).
Feldmeyer, B., Elsner, D. & Foitzik, S. Gene expression patterns associated with caste and reproductive status in ants: worker-specific genes are more derived than queen-specific ones. Mol. Ecol. 23, 151–161 (2014).
Ferreira, P. G. et al. Transcriptome analyses of primitively eusocial wasps reveal novel insights into the evolution of sociality and the origin of alternative phenotypes. Genome Biol. 14, R20 (2013).
Johnson, B. R. & Tsutsui, N. D. Taxonomically restricted genes are associated with the evolution of sociality in the honey bee. BMC Genomics 12, 164 (2011).
Simola, D. F. et al. Social insect genomes exhibit dramatic evolution in gene composition and regulation while preserving regulatory features linked to sociality. Genome Res. 23, 1235–1247 (2013).
Warner, M. R., Qiu, L., Holmes, M. J., Mikheyev, A. S. & Linksvayer, T. A. Convergent eusocial evolution is based on a shared reproductive groundplan plus lineage-specific plastic genes. Nat. Commun. 10, 2651 (2019).
Molodtsova, D., Harpur, B. A., Kent, C. F., Seevananthan, K. & Zayed, A. Pleiotropy constrains the evolution of protein but not regulatory sequences in a transcription regulatory network influencing complex social behaviors. Front. Genet. 5, 431 (2014).
Søvik, E., Bloch, G. & Ben-Shahar, Y. Function and evolution of microRNAs in eusocial Hymenoptera. Front. Genet. 6, 193 (2015).
Engelmann, F. & Mala, J. The interactions between juvenile hormone (JH), lipophorin, vitellogenin, and JH esterases in two cockroach species. Insect Biochem. Mol. Biol. 30, 793–803 (2000).
de Kort, C. A. D. & Koopmanschap, A. B. Molecular characteristics of lipophorin, the juvenile hormone-binding protein in the hemolymph of the Colorado potato beetle. Arch. Insect Biochem. Physiol. 5, 255–269 (1987).
Sevala, V. L., Bachmann, J. A. S. & Schal, C. Lipophorin: a hemolymph juvenile hormone binding protein in the german cockroach, Blattella germanica. Insect Biochem. Mol. Biol. 27, 663–670 (1997).
Martins, J. R., Nunes, F. M. F., Cristino, A. S., Simões, Z. L. P. & Bitondi, M. M. G. The four hexamerin genes in the honey bee: structure, molecular evolution and function deduced from expression patterns in queens, workers and drones. BMC Mol. Biol. 11, 23 (2010).
Ismail, S. M. & Gillott, C. Identification, characterization, and developmental profile of a high molecular weight, juvenile hormone-binding protein in the hemolymph of the migratory grasshopper, Melanoplus sanguinipes. Arch. Insect Biochem. Physiol. 29, 415–430 (1995).
Daneman, R. & Barres, B. A. The blood-brain barrier–lessons from moody flies. Cell 123, 9–12 (2005).
Stork, T. et al. Organization and function of the blood-brain barrier in Drosophila. J. Neurosci. 28, 587–597 (2008).
Nene, V. et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316, 1718–1723 (2007).
Dudchenko, O. et al. De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science 356, 92–95 (2017).
Ashby, R., Forêt, S., Searle, I. & Maleszka, R. MicroRNAs in honey bee caste determination. Sci. Rep. 6, 18794 (2016).
Kapheim, K. M. et al. Brain microRNAs among social and solitary bees. R. Soc. Open Sci. 7, 200517 (2020).
Collins, D. H. et al. MicroRNAs associated with caste determination and differentiation in a primitively eusocial insect. Sci. Rep. 7, 45674 (2017).
Behura, S. K. & Whitfield, C. W. Correlated expression patterns of microRNA genes with age-dependent behavioural changes in honeybee. Insect Mol. Biol. 19, 431–439 (2010).
Liu, F. et al. Next-generation small RNA sequencing for microRNAs profiling in Apis mellifera: comparison between nurses and foragers. Insect Mol. Biol. 21, 297–303 (2012).
Greenberg, J. K. et al. Behavioral plasticity in honey bees is associated with differences in brain microRNA transcriptome. Genes Brain Behav. 11, 660–670 (2012).
Nunes, F. M. F., Ihle, K. E., Mutti, N. S., Simões, Z. L. P. & Amdam, G. V. The gene vitellogenin affects microRNA regulation in honey bee (Apis mellifera) fat body and brain. J. Exp. Biol. 216, 3724–3732 (2013).
Bendena, W. G., Hui, J. H. L., Chin-Sang, I. & Tobe, S. S. Neuropeptide and microRNA regulators of juvenile hormone production. Gen. Comp. Endocrinol. 295, 113507 (2020).
Simão, F. A., Waterhouse, R. M., Ioannidis, P., Kriventseva, E. V. & Zdobnov, E. M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212 (2015).
Armstrong, J. et al. Progressive Cactus is a multiple-genome aligner for the thousand-genome era. Nature 587, 246–251 (2020).
Chen, S., Krinsky, B. H. & Long, M. New genes as drivers of phenotypic evolution. Nat. Rev. Genet. 14, 645–660 (2013).
Sinha, S., Liang, Y. & Siggia, E. Stubb: a program for discovery and analysis of cis-regulatory modules. Nucleic Acids Res. 34, W555–W559 (2006).
Lowe, C. B. et al. Three periods of regulatory innovation during vertebrate evolution. Science 333, 1019–1024 (2011).
Miura, K., Oda, M., Makita, S. & Chinzei, Y. Characterization of the Drosophila Methoprene-tolerant gene product: juvenile hormone binding and ligand-dependent gene regulation. FEBS J. 272, 1169–1178 (2005).
Charles, J.-P. et al. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl Acad. Sci. USA 108, 21128–21133 (2011).
Li, M., Mead, E. A. & Zhu, J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc. Natl Acad. Sci. USA 108, 638–643 (2011).
Zhang, Z., Xu, J., Sheng, Z., Sui, Y. & Palli, S. R. Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, Methoprene tolerant. J. Biol. Chem. 286, 8437–8447 (2011).
Otto, N. et al. The sulfite oxidase Shopper controls neuronal activity by regulating glutamate homeostasis in Drosophila ensheathing glia. Nat. Commun. 9, 3514 (2018).
Quinn, P. M. J., Moreira, P. I., Ambrósio, A. F. & Alves, C. H. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol. Commun. 8, 189 (2020).
Wittwer, B. et al. Solitary bees reduce investment in communication compared with their social relatives. Proc. Natl Acad. Sci. USA 114, 6569–6574 (2017).
Lahti, D. C. et al. Relaxed selection in the wild. Trends Ecol. Evol. 24, 487–496 (2009).
Wertheim, J. O., Murrell, B., Smith, M. D., Kosakovsky Pond, S. L. & Scheffler, K. RELAX: detecting relaxed selection in a phylogenetic framework. Mol. Biol. Evol. 32, 820–832 (2015).
Martin, R. M. & Cardoso, M. C. Chromatin condensation modulates access and binding of nuclear proteins. FASEB J. 24, 1066–1072 (2010).
Wyatt, C. D. R. et al. Genetic toolkit for sociality predicts castes across the spectrum of social complexity in wasps. Preprint at bioRxiv https://doi.org/10.1101/2020.12.08.407056 (2020).
Wang, M., Zhao, Y. & Zhang, B. Efficient test and visualization of multi-set intersections. Sci. Rep. 5, 16923 (2015).
Loiseau, P., Davies, T., Williams, L. S., Mishima, M. & Palacios, I. M. Drosophila PAT1 is required for Kinesin-1 to transport cargo and to maximize its motility. Development 137, 2763–2772 (2010).
Hidayat, P. & Goodman, W. G. Juvenile hormone and hemolymph juvenile hormone binding protein titers and their interaction in the hemolymph of fourth stadium Manduca sexta. Insect Biochem. Mol. Biol. 24, 709–715 (1994).
Hartfelder, K. & Emlen, D. J. in Insect Endocrinology (ed. Gilbert, L. I.) 464–522 (Academic Press, 2012).
Burmester, T. Evolution and function of the insect hexamerins. Eur. J. Entomol. 96, 213–226 (1999).
Smolenaars, M. M. W., Madsen, O., Rodenburg, K. W. & Van der Horst, D. J. Molecular diversity and evolution of the large lipid transfer protein superfamily. J. Lipid Res. 48, 489–502 (2007).
Telfer, W. H. & Kunkel, J. G. The function and evolution of insect storage hexamers. Annu. Rev. Entomol. 36, 205–228 (1991).
Fan, Y., Schal, C., Vargo, E. L. & Bagnères, A.-G. Characterization of termite lipophorin and its involvement in hydrocarbon transport. J. Insect Physiol. 50, 609–620 (2004).
Gu, X., Quilici, D., Juarez, P., Blomquist, G. J. & Schal, C. Biosynthesis of hydrocarbons and contact sex pheromone and their transport by lipophorin in females of the German cockroach (Blattella germanica). J. Insect Physiol. 41, 257–267 (1995).
Fan, Y., Chase, J., Sevala, V. L. & Schal, C. Lipophorin-facilitated hydrocarbon uptake by oocytes in the German cockroach Blattella germanica (L.). J. Exp. Biol. 205, 781–790 (2002).
Schal, C. et al. Tissue distribution and lipophorin transport of hydrocarbons and sex pheromones in the house fly, Musca domestica. J. Insect Sci. 1, 12 (2001).
Zhou, X., Oi, F. M. & Scharf, M. E. Social exploitation of hexamerin: RNAi reveals a major caste-regulatory factor in termites. Proc. Natl Acad. Sci. USA 103, 4499–4504 (2006).
Scharf, M. E., Buckspan, C. E., Grzymala, T. L. & Zhou, X. Regulation of polyphenic caste differentiation in the termite Reticulitermes flavipes by interaction of intrinsic and extrinsic factors. J. Exp. Biol. 210, 4390–4398 (2007).
Hunt, J. H. et al. A diapause pathway underlies the gyne phenotype in Polistes wasps, revealing an evolutionary route to caste-containing insect societies. Proc. Natl Acad. Sci. USA 104, 14020–14025 (2007).
Hawkings, C., Calkins, T. L., Pietrantonio, P. V. & Tamborindeguy, C. Caste-based differential transcriptional expression of hexamerins in response to a juvenile hormone analog in the red imported fire ant (Solenopsis invicta). PLoS ONE 14, e0216800 (2019).
Hunt, J. H. et al. Differential gene expression and protein abundance evince ontogenetic bias toward castes in a primitively eusocial wasp. PLoS ONE 5, e10674 (2010).
Zhou, X., Tarver, M. R. & Scharf, M. E. Hexamerin-based regulation of juvenile hormone-dependent gene expression underlies phenotypic plasticity in a social insect. Development 134, 601–610 (2007).
Murrell, B. et al. Detecting individual sites subject to episodic diversifying selection. PLoS Genet. 8, e1002764 (2012).
Hartfelder, K. Insect juvenile hormone: from “status quo” to high society. Braz. J. Med. Biol. Res. 33, 157–177 (2000).
Smith, A. R., Kapheim, K. M., Pérez-Ortega, B., Brent, C. S. & Wcislo, W. T. Juvenile hormone levels reflect social opportunities in the facultatively eusocial sweat bee Megalopta genalis (Hymenoptera: Halictidae). Horm. Behav. 63, 1–4 (2013).
Tibbetts, E. A. & Izzo, A. S. Endocrine mediated phenotypic plasticity: condition-dependent effects of juvenile hormone on dominance and fertility of wasp queens. Horm. Behav. 56, 527–531 (2009).
Frederik Nijhout, H. Insect Hormones (Princeton Univ. Press, 1998).
Bloch, G., Wheeler, D. E. & Robinson, G. E. in Hormones, Brain and Behavior (eds Pfaff, D. W. et al.) 195–235 (Academic Press, 2002).
West-Eberhard, M. J. in Natural History and Evolution of Paper Wasps (eds Mary Jane West-Eberhard, M. J. & Turillazzi, S.) 290–317 (Oxford Univ. Press, 1996).
Brankatschk, M. & Eaton, S. Lipoprotein particles cross the blood-brain barrier in Drosophila. J. Neurosci. 30, 10441–10447 (2010).
Pandey, A. & Bloch, G. Juvenile hormone and ecdysteroids as major regulators of brain and behavior in bees. Curr. Opin. Insect Sci. 12, 26–37 (2015).
Glastad, K. M. et al. Epigenetic regulator CoREST controls social behavior in ants. Mol. Cell 77, 338–351.e6 (2020).
Rubin, B. E. R., Jones, B. M., Hunt, B. G. & Kocher, S. D. Rate variation in conserved noncoding DNA reveals regulatory pathways associated with social evolution. Phil. Trans. R. Soc. B 374, 20180247 (2019).
Tong, C., Avilés, L., Rayor, L.S., Mikheyev, A. S. & Linksvayer, T. A. Genomic signatures of recent convergent transitions to social life in spiders. Nat. Commun. 13, 6967 (2022).
Nijhout, H. F. & Wheeler, D. E. Juvenile hormone and the physiological basis of insect polymorphisms. Q. Rev. Biol. 57, 109–133 (1982).
Nijhout, H. F. & Reed, M. C. A mathematical model for the regulation of juvenile hormone titers. J. Insect Physiol. 54, 255–264 (2008).
West-Eberhard, M. J. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 20, 249–278 (1989).
Ju, L. et al. Hormonal gatekeeping via the blood brain barrier governs behavior. Preprint at bioRxiv https://doi.org/10.1101/2022.12.01.518733 (2022).
Corbitt, T. S. & Hardie, J. Juvenile hormone effects on polymorphism in the pea aphid, Acyrthosiphon pisum. Entomol. Exp. Appl. 38, 131–135 (1985).
Zera, A. J. & Denno, R. F. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 42, 207–230 (1997).
Bell, W. J. Factors controlling initiation of vitellogenesis in a primitively social bee, Lasioglossum zephyrum (Hymenoptera: Halictidae). Insectes Soc. 20, 253–260 (1973).
Shpigler, H. Y. et al. Juvenile hormone regulates brain-reproduction tradeoff in bumble bees but not in honey bees. Horm. Behav. 126, 104844 (2020).
Pandey, A., Motro, U. & Bloch, G. Juvenile hormone interacts with multiple factors to modulate aggression and dominance in groups of orphan bumble bee (Bombus terrestris) workers. Horm. Behav. 117, 104602 (2020).
Amdam, G. V., Csondes, A., Fondrk, M. K. & Page, R. E. Jr. Complex social behaviour derived from maternal reproductive traits. Nature 439, 76–78 (2006).
Amdam, G. V., Norberg, K., Fondrk, M. K. & Page, R. E. Jr. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc. Natl Acad. Sci. USA 101, 11350–11355 (2004).
Page, R. E., Scheiner, R., Erber, J. & Amdam, G. V. The development and evolution of division of labor and foraging specialization in a social insect (Apis mellifera L.). Curr. Top. Dev. Biol. 74, 253–286 (2006).
Turillazzi, S. & West-Eberhard, M. J. Natural History and Evolution of Paper Wasps (Oxford Univ. Press, 1996).
West-Eberhard, M. J. Flexible strategy and social evolution. In Animal Societies. Theories and Facts (eds Ito, Y., Brown, J. L. and Kikkawa, J.) 35–51 (Japan Scientific Societies Press, Ltd., Tokyo, 1987).
West-Eberhard, M. J. Developmental Plasticity and Evolution (Oxford Univ. Press, 2003).
Toth, A. L. & Robinson, G. E. Evo-devo and the evolution of social behavior. Trends Genet. 23, 334–341 (2007).
Toth, A. L. et al. Brain transcriptomic analysis in paper wasps identifies genes associated with behaviour across social insect lineages. Proc. Biol. Sci. 277, 2139–2148 (2010).
Berens, A. J., Hunt, J. H. & Toth, A. L. Comparative transcriptomics of convergent evolution: different genes but conserved pathways underlie caste phenotypes across lineages of eusocial insects. Mol. Biol. Evol. 32, 690–703 (2015).
Rittschof, C. C. & Robinson, G. E. Behavioral genetic toolkits: toward the evolutionary origins of complex phenotypes. Curr. Top. Dev. Biol. 119, 157–204 (2016).
Qiu, B. et al. Towards reconstructing the ancestral brain gene-network regulating caste differentiation in ants. Nat. Ecol. Evol. 2, 1782–1791 (2018).
Feldmeyer, B. et al. Evidence for a conserved queen-worker genetic toolkit across slave-making ants and their ant hosts. Mol. Ecol. 31, 4991–5004 (2022).
Weisenfeld, N. I., Kumar, V., Shah, P., Church, D. M. & Jaffe, D. B. Direct determination of diploid genome sequences. Genome Res. 27, 757–767 (2017).
Waterhouse, R. M. et al. BUSCO applications from quality assessments to gene prediction and phylogenomics. Mol. Biol. Evol. 35, 543–548 (2018).
Seppey, M., Manni, M. & Zdobnov, E. M. BUSCO: assessing genome assembly and annotation completeness. Methods Mol. Biol. 1962, 227–245 (2019).
Jackman, S. D. et al. ABySS 2.0: resource-efficient assembly of large genomes using a Bloom filter. Genome Res. 27, 768–777 (2017).
Burton, J. N. et al. Chromosome-scale scaffolding of de novo genome assemblies based on chromatin interactions. Nat. Biotechnol. 31, 1119–1125 (2013).
Dudchenko, O. et al. The Juicebox Assembly Tools module facilitates de novo assembly of mammalian genomes with chromosome-length scaffolds for under $1000. Preprint at https://www.biorxiv.org/content/10.1101/254797v1 (2018).
Hoff, K. J., Lange, S., Lomsadze, A., Borodovsky, M. & Stanke, M. BRAKER1: unsupervised RNA-seq-based genome annotation with GeneMark-ET and AUGUSTUS. Bioinformatics 32, 767–769 (2016).
Hoff, K. J., Lomsadze, A., Borodovsky, M. & Stanke, M. in Gene Prediction: Methods and Protocols (ed. Kollmar, M.) 65–95 (Springer, 2019).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019).
Holt, C. & Yandell, M. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinformatics 12, 491 (2011).
Cantarel, B. L. et al. MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 18, 188–196 (2008).
Haas, B. J. et al. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic Acids Res. 31, 5654–5666 (2003).
Haas, B. J., Zeng, Q., Pearson, M. D., Cuomo, C. A. & Wortman, J. R. Approaches to fungal genome annotation. Mycology 2, 118–141 (2011).
Haas, B. J. et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 8, 1494–1512 (2013).
Zdobnov, E. M. et al. OrthoDB v9.1: cataloging evolutionary and functional annotations for animal, fungal, plant, archaeal, bacterial and viral orthologs. Nucleic Acids Res. 45, D744–D749 (2017).
Emms, D. M. & Kelly, S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 16, 157 (2015).
Emms, D. M. & Kelly, S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20, 238 (2019).
Bryant, D. M. et al. A tissue-mapped axolotl de novo transcriptome enables identification of limb regeneration factors. Cell Rep. 18, 762–776 (2017).
Klopfenstein, D. V. et al. GOATOOLS: a Python library for gene ontology analyses. Sci. Rep. 8, 10872 (2018).
Wang, K. & Nishida, H. REGULATOR: a database of metazoan transcription factors and maternal factors for developmental studies. BMC Bioinformatics 16, 114 (2015).
Domazet-Lošo, T., Brajković, J. & Tautz, D. A phylostratigraphy approach to uncover the genomic history of major adaptations in metazoan lineages. Trends Genet. 23, 533–539 (2007).
Drost, H.-G., Gabel, A., Grosse, I. & Quint, M. Evidence for active maintenance of phylotranscriptomic hourglass patterns in animal and plant embryogenesis. Mol. Biol. Evol. 32, 1221–1231 (2015).
Misof, B. et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767 (2014).
Branstetter, M. G. et al. Phylogenomic insights into the evolution of stinging wasps and the origins of ants and bees. Curr. Biol. 27, 1019–1025 (2017).
Dohrmann, M. & Wörheide, G. Dating early animal evolution using phylogenomic data. Sci. Rep. 7, 3599 (2017).
Bradley, R. K. et al. Fast statistical alignment. PLoS Comput. Biol. 5, e1000392 (2009).
Sackton, T. B. et al. Convergent regulatory evolution and loss of flight in paleognathous birds. Science 364, 74–78 (2019).
Smith, M. D. et al. Less is more: an adaptive branch-site random effects model for efficient detection of episodic diversifying selection. Mol. Biol. Evol. 32, 1342–1353 (2015).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Yanai, I. et al. Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics 21, 650–659 (2005).
Pennell, M. W. et al. geiger v2.0: an expanded suite of methods for fitting macroevolutionary models to phylogenetic trees. Bioinformatics 30, 2216–2218 (2014).
Friedländer, M. R., Mackowiak, S. D., Li, N., Chen, W. & Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 40, 37–52 (2012).
Kozomara, A. & Griffiths-Jones, S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 42, D68–D73 (2014).
Enright, A. J. et al. MicroRNA targets in Drosophila. Genome Biol. 5, R1 (2003).
Krüger, J. & Rehmsmeier, M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 34, W451–W454 (2006).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Finak, G. et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 16, 278 (2015).
Paten, B. et al. Cactus: algorithms for genome multiple sequence alignment. Genome Res. 21, 1512–1528 (2011).
Hubisz, M. J., Pollard, K. S. & Siepel, A. PHAST and RPHAST: phylogenetic analysis with space/time models. Brief. Bioinform. 12, 41–51 (2011).
Siepel, A. et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 15, 1034–1050 (2005).
Capella-Gutiérrez, S., Silla-Martínez, J. M. & Gabaldón, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Yang, Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 13, 555–556 (1997).
Yang, Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 (2007).
Stamatakis, A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 (2006).
Wang, L. et al. Peak annotation and verification engine (PAVE) for untargeted LC-MS metabolomics. Anal. Chem. 91, 1838–1846 (2019).
Pahlke, S., Seid, M. A., Jaumann, S. & Smith, A. The loss of sociality is accompanied by reduced neural investment in mushroom body volume in the sweat bee Augochlora pura (Hymenoptera: Halictidae). Ann. Entomol. Soc. Am. 114, 637–642 (2021).
Le Guilloux, V., Schmidtke, P. & Tuffery, P. Fpocket: an open source platform for ligand pocket detection. BMC Bioinformatics 10, 168 (2009).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015).
Kelley, L. A. & Sternberg, M. J. E. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4, 363–371 (2009).
Edgar, R. C. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5, 113 (2004).
Acknowledgements
We thank our many colleagues who contributed samples and field support to this dataset, including: J. Gibbs, S. Droege, R. Jeanson, J. Milam, J. Straka, M. Podolak, J. Cane and M. Hagadorn; M. Richards, C. Plateaux-Quenu, J. Gibbs and L. Packer for discussion and insights on halictid life history and behaviour; T. Sackton, R. Corbett-Detig, N. Clark, A. Siepel and X. Xue for providing discussion and advice on data analysis; W. Tong for the bee drawings and M. Sheehan for assistance with RNA library preparation; and J. Rabinowitz and lab for support and access to LC–MS equipment. This work was supported by NSF-DEB1754476 awarded to S.D.K. and B.G.H., NIH 1DP2GM137424-01 to S.D.K., USDA NIFA postdoctoral fellowship 2018-67012-28085 to B.E.R.R., DFG PA632/9 to R.J.P., a Smithsonian Global Genome Initiative award GGI-Peer-2016-100 to W.T.W. and C.J.K., a Smithsonian Institution Competitive Grants Program for Biogenomics (W.T.W., K.M.K., B.M.J.), a Smithsonian Tropical Research Institute fellowship to C.J.K., and a gift from Jennifer and Greg Johnson to W.T.W. M.F.O. was supported by Vicerrectoría de Investigación, UCR, project B7287. E.L.A. was supported by an NSF Physics Frontiers Center Award (PHY1427654), the Welch Foundation (Q-1866), a USDA Agriculture and Food Research Initiative Grant (2017-05741), and an NIH Encyclopedia of DNA Elements Mapping Center Award (UM1HG009375). Sampling permit details: S.D.K., E.S.W. and M.F.O. (R-055-2017-OT-CONAGEBIO), S.D.K. (P526P-15-04026) and R.J.P. (Belfast City Council, Parks and Leisure Dept).
Author information
Authors and Affiliations
Contributions
B.M.J., B.E.R.R. and S.D.K. designed the study. B.M.J., B.E.R.R. and S.D.K. wrote the initial draft of the manuscript. All authors revised and commented on the manuscript. B.M.J., B.E.R.R., E.S.W., B.M.J., S.D.K., M.F.O., K.M.K., R.J.P., W.T.W., C.J.K. and K.S.O. collected samples. B.E.R.R., C.J.K., Z.Y.W. and N.V.D. generated genomic libraries. O.D., P.A.A., M.P., A.D.O. and D.W. performed Hi-C sequencing and genome assembly. B.E.R.R., L.R.P. and A.E.W. conducted genome annotation/alignment. B.M.J., B.E.R.R., S.D.K., O.D., K.M.K., B.G.H., J.S., F.V. and I.M.T. conducted genomic analyses. A.E.W. curated the database. W.L., E.S.W., S.R.J., K.G., S.M.D., C.J.K., Z.Y.W., M.J.M., K.S.O. and N.V.D. performed laboratory experiments. E.L.A. supervised Hi-C sequencing and genome assembly. S.D.K. provided overall project supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Guojie Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
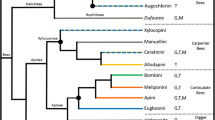
Extended Data Fig. 1 Typical life cycle for solitary and eusocial sweat bees.
In a typical solitary species, reproductive females produce a single brood that is a mix of males and females. However, some solitary species are multivoltine and can produce multiple reproductive broods in a year. In typical eusocial halictid species, females produce two broods: first workers, then reproductives. At the end of the season, females mate and overwinter as adults.
Extended Data Fig. 2 Genome assembly statistics.
Nineteen genomes are included in this comparative dataset. 15 de novo assemblies were generated using a combination of 10x genomics and/or Hi-C, and 2 previously-published genomes (N. melanderi11, L. albipes3) were improved by scaffolding with Hi-C data. M. genalis13 and D. novaeangliae12 were used as-is. (a) Genome assembly lengths ranged from ~300 to 420 Mb. (b) Scaffold N50s for each species following Hi-C scaffolding, and (c) GC content was consistent across species. (d) RepeatModeler was used to characterize different types of repeats in the halictid assemblies. (e) Numbers of genes (in thousands) for each species following individual annotation. (f) Conserved non-exonic elements (CNEEs) were called using phastCons on a progressive Cactus alignment. (g) microRNAs were also characterized using brain tissue from available species and from34. For some species, fresh tissue was not available (N/A).
Extended Data Fig. 3 Human-mouse chromosomal dotplot.
Dotplots comparing the chromosomes of two mammalian species, human and mouse, separated by comparable evolutionary distance to the bees examined in this study (~80MY to common ancestor). The vertical and horizontal lines outline the boundaries of chromosomes in respective species, and the numbers on the axes mark the relevant chromosome name and orientation, with ‘-’ in front of the chromosome name representing a reverse complement to the chromosome sequence as reported in the assembly. See Extended Data Fig. 4 for more details. The human-mouse one-to-one alignments file was downloaded from https://github.com/mcfrith/last-genome-alignments.
Extended Data Fig. 4 Pairwise chromosomal dotplots for halictid species.
Dotplots showing alignment of chromosome-length scaffolds from 16 bee assemblies against the N. melanderi (NMEL) chromosome-length genome assembly. The NMEL reference (generated as part of this study) is shown on the y-axis. The x-axis shows the chromosome-length scaffolds in the respective bee assemblies that have been ordered and oriented to best match the NMEL chromosomes in order to facilitate comparison. The vertical and horizontal lines outline the boundaries of chromosomes in respective species, and the numbers on the axes mark the relevant chromosome name and orientation, with ‘-’ in front of the chromosome name representing a reverse complement to the chromosome sequence as reported in the assembly. Each dot represents the position of a 1000 bp syntenic stretch between the two genomes identified by Progressive Cactus alignments. The colour of the dots reflects the orientation of individual alignments with respect to NMEL (red indicates collinearity, whereas blue indicates inverted orientation). The dotplots illustrate that, with the exception of a few species, highly conserved regions belonging to the same chromosome in one species tend to lie on the same chromosome in other bee species, even though their relative position within a chromosome may change dramatically. This rearrangement pattern accounts for the characteristic appearance of the dotplots with large diffuse blocks of scrambled chromosome-to-chromosome alignments appearing along the diagonal. The pattern is visibly different from those characteristic of mammalian chromosome evolution (for example, see Extended Data Fig. 3). The few exceptions are species with multiple fissions (L. marginatum, L. albipes) and fusions (Augochlora pura, L. vierecki, L. pauxillum, L. malachurum) events. In the fission species, the syntenic regions that belonged to two different chromosomes in one bee species tend to belong to different chromosomes after the fission. The analysis of the fusion species suggests that the regions corresponding to separate chromosomes in the closely related species (and likely the ancestral species) remain separate in the new genome, possibly corresponding to the two chromosome arms. The alignments have been extracted from the hal file using the cactus hal2maf utility with the following parameters: –maxRefGap 500 –maxBlockLen 1000 –refGenome NMEL.
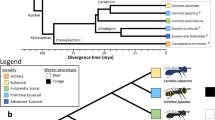
Extended Data Fig. 5 Relationship between selection and gene age associated with eusocial origins, maintenance, and reversions to solitary life histories.
Gene age ranges from the oldest Bilaterian group (Age=1) to the youngest, halictid-specific taxonomically restricted genes (Age=9). The relaxation panel demonstrates that there is a greater proportion of young genes experiencing relaxed selection when eusociality is lost (HyPhy RELAX, FDR < 0.1; Pearson’s r = 0.869, p = 0.002); we find no significant association with relaxation on extant, eusocial branches (Pearson correlation, r = −0.044, p = 0.91). Next, we looked at the sets of genes that show intensification of selection pressures (HyPhy RELAX, FDR < 0.1), but neither of these sets showed any significant association with gene age (Eusocial: Pearson correlation, r = 0.244, p = 0.492; Solitary: Pearson correlation, r = 0.627, p = 0.071). Finally, we looked at genes that showed evidence of positive selection (HyPhy aBSREL, FDR < 0.05). We found no relationship between gene age and the proportion of genes with evidence of positive selection on at least 1 branch representing the origins of eusociality (Positive, gains: Pearson correlation, r = 0.156, p = 0.688). Likewise, there was no relationship between gene age and the proportion of genes with evidence for positive selection (HyPhy aBSREL, FDR < 0.05) on at least 1 loss branch in the Halictini and on 1 loss branch in the Augochlorini (Positive, losses: Pearson correlation, r = 0.403, p = 0.283). Shading represents the 95% confidence intervals.
Extended Data Fig. 6 Evidence of positive selection in domains of Hex110 and ApoLpp.
Both Hex110 (a) and ApoLpp (b) show evidence for domain-specific positive selection associated with the origins of eusociality. Predicted binding pockets by PHYRE are shown with pink rectangles, glycosylation sites in orange squares. Pink hexagons denote MEME-identified sites that also had a mutational effect score > =1 for Hex110 and >1 for ApoLpp. In both JHBPs, MEME identifies sites in functional regions of the protein, including the receptor binding domain and predicted binding pocket for ApoLpp as well as in all three Hemocyanin domains (associated with storage functions in these proteins) and in the predicted binding pocket of Hex110. Moreover, for both proteins, we also find region-specific, faster rates of evolution on eusocial branches compared to non-eusocial outgroups in this phylogeny: the predicted binding pocket for ApoLpp and all Hemocyanin domains for Hex110. Taken together, these results suggest that positive selection shaped protein function as eusociality emerged in this group of bees.
Extended Data Fig. 7 Apolipoprotein has experienced pervasive positive selection throughout the insects.
HyPhy aBSREL was used to identify branches with evidence of positive selection (highlighted in blue). Arrows indicate the direction of significant rate shifts detected on relevant branches. The only branch tested that did now show a significant rate shift is indicated by an ‘o’.
Extended Data Fig. 8 JH III is present in multiple bumblebee tissue types, including the brain.
LC-MS was used to measure JH III levels in dissected brain tissue and hemolymph from worker bumblebees (Koppert). Tissues were dissected in PBS. Negative control=fresh PBS, PBS control=3uL of buffer collected following brain dissections from each sample. RCC = retrocerebral complex. The RCC synthesizes JH and immediately releases it, it is not known to store JH78. Hemolymph (3uL) was collected by centrifuging thorax tissue from each sample. Ovaries and brains were dissected in PBS and the entire organ was used for JH III quantification. In all samples, we find detectable levels of JH III in the hemolymph, brain, and ovaries. N = 5 for all sample/tissue types except negative control, where n = 1. Note that because all samples were generated by extraction in a constant volume of buffer from the total input tissue, estimated amounts are not directly comparable across different tissue types. Boxes show median, 25th and 75th percentiles. Whiskers show minimum and maximum values without outliers.
Extended Data Fig. 9 Stable Isotope Tracing of Juvenile Hormone (JH).
Absolute quantification of JH III in the brain (a) and bodies (d) of bumblebees treated with acetone or JH III-D3. Absolute quantification of labelled JH III in the brains (b) and bodies (e) of bees treated with acetone or JH III-D3. Labelled JH III-D3 levels are high after 24 hours, but decay significantly by 72 hours. (c) Labelled JH III-D3 applied to abdomens of bumblebees is detectable in brains 24 h later (acetone is control). n = 3 tissues/condition. (f) JH III-D3 accounts for nearly all the total JH III in bee bodies after 24 hours, indicating that the labelled compound is well-absorbed by the bee. n = 3 bumblebee workers for each experimental condition. Boxes show median, 25th and 75th percentiles. Whiskers show minimum and maximum values.
Extended Data Fig. 10 JH III and JH III-D3 quantification.
(a) Chemical structure of JH III in positive ionization. JH III can exist in two forms of equal m/z and produces one fragment that retains the D3 label. (b) Parent and fragment m/z values for unlabelled and labelled JH III. (c) Chromatograph of unlabelled JH III. (d) Chromatograph of labelled JH III showing similar retention time to unlabelled JH III. (e) Mass spectra of unlabelled JH III showing the expected masses based on B. (f) Mass spectra of a 50/50 mix of labelled and unlabelled JH III showing the expected masses based on B. (g) Standard curve demonstrating a range of detection of JH III. (h) Chromatograph of unlabelled JH III in the brains of bees painted with acetone (grey dotted line) or with JH III-D3 (purple solid line). (i) Chromatograph of labelled JH III in the brains of bees painted with acetone (grey dotted line) or with JH III-D3 (purple solid line). (j) Mass spectra of JH III in bee brains painted with acetone showing the expected masses based on B. (k) Mass spectra of JH III in bee brains painted with JH III-D3 showing the expected masses based on B. (l) Absolute quantification of JH III in the brains of bees painted with acetone or JH III-D3. (m) Absolute quantification of JH III-D3 in the brains of bees painted with acetone or JH III-D3. n = 3 replicates for each panel. Boxes show median and 25th and 75th percentiles. Whiskers show minimum and maximum values.
Supplementary information
Supplementary Information
Supplementary Methods, Text, Legends for Tables 1–15 and References.
Supplementary Table
Supplementary Tables 1–15.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jones, B.M., Rubin, B.E.R., Dudchenko, O. et al. Convergent and complementary selection shaped gains and losses of eusociality in sweat bees. Nat Ecol Evol 7, 557–569 (2023). https://doi.org/10.1038/s41559-023-02001-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-023-02001-3
- Springer Nature Limited
This article is cited by
-
Exciting times for evolutionary biology
Nature Ecology & Evolution (2024)