Abstract
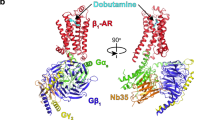
There is considerable uncertainty about the mechanism by which the β2-adrenergic receptor (β2AR) is activated. Here we use molecular metadynamics computations to predict the mechanism by which an agonist induces the activation of the β2AR and its cognate Gs protein. We found that binding agonist alone to the inactive β2AR does not break the ionic lock and hence does not drive the β2AR towards the activated conformation. However, we found that attaching the inactive Gs protein to the agonist-bound inactive β2AR (containing the ionic lock) leads to partial insertion of Gαs-α5 into the core of β2AR, which breaks the ionic lock, leading to activation of the Gs protein coupled to β2AR. Upon activation, the Gαs protein undergoes a remarkable opening of the GDP binding pocket, making the GDP available for exchange or release. Concomitantly, Gαs-α5 undergoes a remarkable expansion in the β2AR cytoplasmic region after the ionic lock is broken, inducing TM6 to displace outward by ~5 Å from TM3.







Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published Article (and its Supplementary Information files). We deposited (https://figshare.com/articles/dataset/free_energy_files/22217674) the metadynamics protocol and associate free-energy results along with the initial and final protein complex structures. Source data are provided with this paper.
Code availability
GROMACS (https://www.gromacs.org/) and Plumed (https://www.plumed.org/) are all available as open source.
References
Lefkowitz, R. J. Seven transmembrane receptors: something old, something new. Acta Physiol. 190, 9–19 (2007).
Hauser, A. S., Attwood, M. M., Rask-Andersen, M., Schiöth, H. B. & Gloriam, D. E. Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 16, 829–842 (2017).
Overington, J. P., Al-Lazikani, B. & Hopkins, A. L. How many drug targets are there?. Nat. Rev. Drug Discov. 5, 993–996 (2006).
Fredriksson, R., Lagerström, M. C., Lundin, L.-G. & Schiöth, H. B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups and fingerprints. Mol. Pharmacol. 63, 1256–1272 (2003).
Lefkowitz, R. J. The superfamily of heptahelical receptors. Nat. Cell Biol. 2, E133–E136 (2000).
Ross, E. M., Maguire, M. E., Sturgill, T. W., Biltonen, R. L. & Gilman, A. G. Relationship between the β-adrenergic receptor and adenylate cyclase. J. Biol. Chem. 252, 5761–5775 (1977).
De Lean, A., Stadel, J. M. & Lefkowitz, R. J. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled β-adrenergic receptor. J. Biol. Chem. 255, 7108–7117 (1980).
MacGregor, D. A., Prielipp, R. C., Butterworth, J. F. IV, James, R. L. & Royster, R. L. Relative efficacy and potency of β-adrenoceptor agonists for generating cAMP in human lymphocytes. Chest 109, 194–200 (1996).
Clark, A. J. The reaction between acetyl choline and muscle cells. J. Physiol. 61, 530–546 (1926).
Karlin, A. On the application of ‘a plausible model’ of allosteric proteins to the receptor for acetylcholine. J. Theor. Biol. 16, 306–320 (1967).
Nygaard, R. et al. The dynamic process of β2-adrenergic receptor activation. Cell 152, 532–542 (2013).
Manglik, A. et al. Structural insights into the dynamic process of β2-adrenergic receptor signaling. Cell 161, 1101–1111 (2015).
Rosenbaum, D. M. et al. Structure and function of an irreversible agonist-β2 adrenoceptor complex. Nature 469, 236–240 (2011).
Gregorio, G. G. et al. Single-molecule analysis of ligand efficacy in β2 AR–G-protein activation. Nature 547, 68–73 (2017).
Lerch, M. T. et al. Viewing rare conformations of the β2 adrenergic receptor with pressure-resolved DEER spectroscopy. Proc. Natl Acad. Sci. USA 117, 31824–31831 (2020).
Dror, R. O. et al. Activation mechanism of the β2-adrenergic receptor. Proc. Natl Acad. Sci. USA 108, 18684–18689 (2011).
Vilardaga, J.-P., Bünemann, M., Krasel, C., Castro, M. & Lohse, M. J. Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat. Biotechnol. 21, 807–812 (2003).
Barducci, A., Bussi, G. & Parrinello, M. Well-tempered metadynamics: a smoothly converging and tunable free-energy method. Phys. Rev. Lett. 100, 020603 (2008).
Cherezov, V. et al. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318, 1258–1265 (2007).
Rasmussen, S. G. et al. Crystal structure of the β2 adrenergic receptor–Gs protein complex. Nature 477, 549–555 (2011).
Ballesteros, J. A. & Weinstein, H. in Methods in Neurosciences Vol. 25 (ed Sealfon, S. C.), 366–428 (Elsevier, 1995).
Pándy-Szekeres, G. et al. GPCRdb in 2018: adding GPCR structure models and ligands. Nucleic Acids Res. 46, D440–D446 (2017).
Kobilka, B. K. G protein coupled receptor structure and activation. Biochim. Biophys. Acta 1768, 794–807 (2007).
Ballesteros, J. A. et al. Activation of the β2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem. 276, 29171–29177 (2001).
Yao, X. et al. Coupling ligand structure to specific conformational switches in the β2-adrenoceptor. Nat. Chem. Biol. 2, 417–422 (2006).
Liu, X. et al. Structural insights into the process of GPCR-G protein complex formation. Cell 177, 1243–1251 (2019).
Sprang, S. R. G protein mechanisms: insights from structural analysis. Annu. Rev. Biochem. 66, 639–678 (1997).
Oldham, W. M. & Hamm, H. E. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 9, 60–71 (2008).
Liu, R. et al. Palmitoylation regulates intracellular trafficking of β2 adrenergic receptor/arrestin/phosphodiesterase 4D complexes in cardiomyocytes. PLoS ONE 7, e42658 (2012).
Palczewski, K. et al. Crystal structure of rhodopsin: AG protein-coupled receptor. Science 289, 739–745 (2000).
Branduardi, D., Bussi, G. & Parrinello, M. Metadynamics with adaptive Gaussians. J. Chem. Theory Comput. 8, 2247–2254 (2012).
Dror, R. O. et al. Identification of two distinct inactive conformations of the β2-adrenergic receptor reconciles structural and biochemical observations. Proc. Natl Acad. Sci. USA 106, 4689–4694 (2009).
Eswar, N., Eramian, D., Webb, B., Shen, M.-Y. & Sali, A. in Structural Proteomics 145–159 (Springer, 2008).
Hilger, D. et al. Structural insights into differences in G protein activation by family A and family B GPCRs. Science 369, eaba3373 (2020).
Oldham, W. M., Van Eps, N., Preininger, A. M., Hubbell, W. L. & Hamm, H. E. Mechanism of the receptor-catalyzed activation of heterotrimeric G proteins. Nat. Struct. Mol. Biol. 13, 772–777 (2006).
Onrust, R. et al. Receptor and βγ binding sites in the α subunit of the retinal G protein transducin. Science 275, 381–384 (1997).
DeMars, G., Fanelli, F. & Puett, D. The extreme C-terminal region of Gαs differentially couples to the luteinizing hormone and β2-adrenergic receptors. Mol. Endocrinol. 25, 1416–1430 (2011).
Markby, D. W., Onrust, R. & Bourne, H. R. Separate GTP binding and GTPase activating domains of a Gα subunit. Science 262, 1895–1901 (1993).
Carpenter, B., Nehmé, R., Warne, T., Leslie, A. G. & Tate, C. G. Structure of the adenosine A2A receptor bound to an engineered G protein. Nature 536, 104–107 (2016).
Dror, R. O. et al. Structural basis for nucleotide exchange in heterotrimeric G proteins. Science 348, 1361–1365 (2015).
Mafi, A., Kim, S.-K., Chou, K. C., Güthrie, B. & Goddard, W. A. III Predicted structure of fully activated Tas1R3/1R3′ homodimer bound to G protein and natural sugars: structural insights into G protein activation by a Class C sweet taste homodimer with natural sugars. J. Am. Chem. Soc. 143, 16824–16838 (2021).
Kwon, Y. et al. Dimerization of β2-adrenergic receptor is responsible for the constitutive activity subjected to inverse agonism. Cell Chem. Biol. 29, 1532–1540 (2022).
Mafi, A., Kim, S.-K. & Goddard, W. A. The atomistic level structure for the activated human κ-opioid receptor bound to the full Gi protein and the MP1104 agonist. Proc. Natl Acad. Sci. USA 117, 5836–5843 (2020).
Mafi, A., Kim, S.-K. & Goddard, W. A. Mechanism of β-arrestin recruitment by the μ-opioid G protein-coupled receptor. Proc. Natl Acad. Sci. USA 117, 16346–16355 (2020).
Zhang, Y. et al. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 546, 248–253 (2017).
Liang, Y.-L. et al. Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor–Gs complex. Nature 555, 121–125 (2018).
García-Nafría, J., Lee, Y., Bai, X., Carpenter, B. & Tate, C. G. Cryo-EM structure of the adenosine A2A receptor coupled to an engineered heterotrimeric G protein. eLife 7, e35946 (2018).
Hu, Q. & Shokat, K. M. Disease-causing mutations in the G protein Gαs subvert the roles of GDP and GTP. Cell 173, 1254–1264 (2018).
Guex, N. & Peitsch, M. C. SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997).
Griffith, A. R. DarwinDock and GAG-Dock: Methods and Applications for Small Molecule Docking (California Institute of Technology, 2017).
Mayo, S. L., Olafson, B. D. & Goddard, W. A. DREIDING: a generic force field for molecular simulations. J. Phys. Chem. 94, 8897–8909 (1990).
Tak Kam, V. W. & Goddard, W. A. III Flat-bottom strategy for improved accuracy in protein side-chain placements. J. Chem. Theory Comput. 4, 2160–2169 (2008).
Ring, A. M. et al. Adrenaline-activated structure of β2-adrenoceptor stabilized by an engineered nanobody. Nature 502, 575–579 (2013).
Warne, T. et al. The structural basis for agonist and partial agonist action on a β1-adrenergic receptor. Nature 469, 241–244 (2011).
Wacker, D. et al. Conserved binding mode of human β2 adrenergic receptor inverse agonists and antagonist revealed by X-ray crystallography. J. Am. Chem. Soc. 132, 11443–11445 (2010).
Needleman, S. B. & Wunsch, C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J. Mol. Biol. 48, 443–453 (1970).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Raniolo, S. & Limongelli, V. Ligand binding free-energy calculations with funnel metadynamics. Nat. Protoc. 15, 2837–2866 (2020).
Maier, J. A. et al. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015).
Wang, J., Wolf, R. M., Caldwell, J. W., Kollman, P. A. & Case, D. A. Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 (2004).
da Silva, A. W. S. & Vranken, W. F. ACPYPE-AnteChamber PYthon Parser interfacE. BMC Res. Notes 5, 367 (2012).
Wang, J., Wang, W., Kollman, P. A. & Case, D. A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 25, 247–260 (2006).
Jakalian, A., Jack, D. B. & Bayly, C. I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J. Comput. Chem. 23, 1623–1641 (2002).
Dickson, C. J. et al. Lipid14: the amber lipid force field. J. Chem. Theory Comput. 10, 865–879 (2014).
Lindorff-Larsen, K. et al. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 78, 1950–1958 (2010).
Meagher, K. L., Redman, L. T. & Carlson, H. A. Development of polyphosphate parameters for use with the AMBER force field. J. Comput. Chem. 24, 1016–1025 (2003).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Khoury, G. A., Thompson, J. P., Smadbeck, J., Kieslich, C. A. & Floudas, C. A. Forcefield_PTM: ab initio charge and AMBER forcefield parameters for frequently occurring post-translational modifications. J. Chem. Theory Comput. 9, 5653–5674 (2013).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 52, 7182–7190 (1981).
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Miyamoto, S. & Kollman, P. A. Settle: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 13, 952–962 (1992).
Hess, B. P-LINCS: a parallel linear constraint solver for molecular simulation. J. Chem. Theory Comput. 4, 116–122 (2008).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1, 19–25 (2015).
Pronk, S. et al. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29, 845–854 (2013).
Tribello, G. A., Bonomi, M., Branduardi, D., Camilloni, C. & Bussi, G. PLUMED 2: new feathers for an old bird. Comput. Phys. Commun. 185, 604–613 (2014).
Acknowledgements
A.M., S.-K.K. and W.A.G. acknowledge support from the NIH (R01HL155532 and R35HL150807).
Author information
Authors and Affiliations
Contributions
W.A.G. and A.M. designed the project. A.M. carried out all calculations. A.M. and S.-K.K. prepared all figures and tables and the Supplementary Information. A.M. wrote the manuscript with W.A.G. and S.-K.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Irina Tikhonova and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
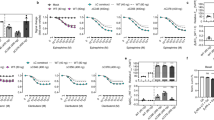
Extended Data Fig. 1 Agonists alone do not activate β2AR.
(a) The optimized inverse agonist (ICI118551)-bound β2AR, featuring the ionic lock in the cytoplasmic region. (b) ICI118551-bound β2AR: MetaMD free energy of the distance between TM3 [the center of mass of Cα for residues 129-137] and TM6 [the center of mass of Cα for residues 266-277]. (c) inverse agonist: ICI118551: MetaMD free energy of the strength for the ionic lock between R1313.50(CZ)- E2686.30(CD). The weighted averages and the standard deviations were calculated for the reported ΔGif within the converged period. The metaMD free energies were reweighted31 for estimation of the error. We used block averaging (n = 951 blocks) to report the standard deviation. (d) The free energy of breaking the ionic lock in the presence of (inverse agonist and agonists) and in the absence of ligands. Comparison of our optimized ICI118551 (inverse-agonist)-bound β2AR with (e) epinephrine-bound β2AR and (f) BI167107-bound β2AR. All RMSDs were calculated for the backbone atoms on the TM domain of β2AR.
Extended Data Fig. 2 Agonist binding is the main driver for activation of the β2AR-Gs protein complex.
The optimized (a) unliganded-β2AR (apo); (c) carazolol-bound -β2AR (inverse agonist); and (e) ICI118551-bound -β2AR (inverse agonist) coupled to inactive Gs protein-bound GDP. (b), (d) & (f) MetaMD free energy versus the distance between the α-helical (AH) [the center of mass of Cαs for the residues 69-204] and Ras-like [the center of mass of Cαs for the residues 223-241, 250-285, and 294-358] subdomains. The weighted averages and the standard deviations were calculated for the reported ΔGif within the converged period. The metaMD free energies were reweighted31 for estimating the error. We used block averaging (n = 951 blocks) to report the standard deviation for each data point.
Supplementary information
Supplementary Information
Supplementary Figs. 1–20, Tables 1–5 and Videos 1–3.
Supplementary Video 1
MetaMD simulation of β2AR-bound BI167107 structure, starting from the activated structure (PDB 3SN6), but Gs protein was eliminated.
Supplementary Video 2
MetaMD simulation of β2AR-bound BI167107-GS-GDP structure from the intracellular view, showing a remarkable expansion in the cytoplasmic cavity of β2AR.
Supplementary Video 3
MetaMD simulation of β2AR-bound BI167107-GS-GDP structure, showing a remarkable GS protein opening, making GDP water exposed.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig./Table 1
Statistical source data.
Source Data Extended Data Fig./Table 2
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mafi, A., Kim, SK. & Goddard, W.A. The dynamics of agonist-β2-adrenergic receptor activation induced by binding of GDP-bound Gs protein. Nat. Chem. 15, 1127–1137 (2023). https://doi.org/10.1038/s41557-023-01238-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01238-6
- Springer Nature Limited