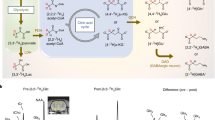
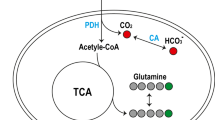
Abstract
Impaired glucose metabolism in the brain has been linked to several neurological disorders. Positron emission tomography and carbon-13 magnetic resonance spectroscopic imaging (MRSI) can be used to quantify the metabolism of glucose, but these methods involve exposure to radiation, cannot quantify downstream metabolism, or have poor spatial resolution. Deuterium MRSI (2H-MRSI) is a non-invasive and safe alternative for the quantification of the metabolism of 2H-labelled substrates such as glucose and their downstream metabolic products, yet it can only measure a limited number of deuterated compounds and requires specialized hardware. Here we show that proton MRSI (1H-MRSI) at 7 T has higher sensitivity, chemical specificity and spatiotemporal resolution than 2H-MRSI. We used 1H-MRSI in five volunteers to differentiate glutamate, glutamine, γ-aminobutyric acid and glucose deuterated at specific molecular positions, and to simultaneously map deuterated and non-deuterated metabolites. 1H-MRSI, which is amenable to clinically available magnetic-resonance hardware, may facilitate the study of glucose metabolism in the brain and its potential roles in neurological disorders.







Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Source data for Figs. 1, 4 and 6 and for Supplementary Figs. 1–3 and 5 are provided with this paper. The raw data acquired in the study are too large to be publicly shared, yet they are available for research purposes from the corresponding authors on reasonable request. The data generated by post-processing methods (that is, metabolite maps, MR spectra and outcomes of their quantification in the LCModel) are available at https://doi.org/10.5281/zenodo.5705959. The shared data are in the minc, niifti and MRSpa data formats. A priori information (‘the basis sets’) needed for MRS/MRSI data quantification in the LCModel is also available via the same link.
Code availability
The custom code for the time-course analysis using linear and exponential fits was performed using custom-made Python code (v3.10) available at https://github.com/MRSI-HFMR-GroupVienna/DeuteriumToProtonExchangeMRS.
References
Kim, M. et al. What do we know about dynamic glucose-enhanced (DGE) MRI and how close is it to the clinics? Horizon 2020 GLINT consortium report. MAGMA 35, 87–104 (2022).
de Graaf, R. A., Mason, G. F., Patel, A. B., Behar, K. L. & Rothman, D. L. In vivo 1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed. 16, 339–357 (2003).
Ruhm, L. et al. Deuterium metabolic imaging in the human brain at 9.4 Tesla with high spatial and temporal resolution. Neuroimage https://doi.org/10.1016/j.neuroimage.2021.118639 (2021).
Rich, L. J. et al. 1H magnetic resonance spectroscopy of 2H-to-1H exchange quantifies the dynamics of cellular metabolism in vivo. Nat. Biomed. Eng. 4, 335–342 (2020).
van Zijl, P. C. M. & Brindle, K. M. Spectroscopic measurements of metabolic fluxes. Nat. Biomed. Eng. 4, 254–256 (2020).
Zhu, X.-H., Lu, M. & Chen, W. Quantitative imaging of brain energy metabolisms and neuroenergetics using in vivo X-nuclear 2H, 17O and 31P MRS at ultra-high field. J. Magn. Reson. 292, 155–170 (2018).
Koppenol, W. H., Bounds, P. L. & Dang, C. V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 11, 325–337 (2011).
Norat, P. et al. Mitochondrial dysfunction in neurological disorders: exploring mitochondrial transplantation. NPJ Regen. Med. https://doi.org/10.1038/s41536-020-00107-x (2020).
Manji, H. et al. Impaired mitochondrial function in psychiatric disorders. Nat. Rev. Neurosci. https://doi.org/10.1038/nrn3229 (2012).
Hahn, A. et al. Quantification of task-specific glucose metabolism with constant infusion of 18F-FDG. J. Nucl. Med. 57, 1933–1940 (2016).
Rischka, L. et al. Reliability of task-specific neuronal activation assessed with functional PET, ASL and BOLD imaging. J. Cereb. Blood Flow Metab. https://doi.org/10.1177/0271678x211020589 (2021).
Hesketh, R. L. et al. Magnetic resonance imaging is more sensitive than PET for detecting treatment-induced cell death-dependent changes in glycolysis. Cancer Res. 79, 3557–3569 (2019).
Rothman, D. L. et al. in Encyclopedia of Biological Chemistry 3rd edn 688–700 (Elsevier, 2021).
Shulman, R. G., Rothman, D. L., Behar, K. L. & Hyder, F. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci. 27, 489–495 (2004).
Wang, Z. J. et al. Hyperpolarized 13C MRI: state of the art and future directions. Radiology https://doi.org/10.1148/radiol.2019182391 (2019).
Rischka, L. et al. Reduced task durations in functional PET imaging with [18F]FDG approaching that of functional MRI. Neuroimage 181, 323–330 (2018).
Stiernman, L. J. et al. Dissociations between glucose metabolism and blood oxygenation in the human default mode network revealed by simultaneous PET-fMRI. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2021913118 (2021).
Terpstra, M. et al. Test-retest reproducibility of neurochemical profiles with short-echo, single-voxel MR spectroscopy at 3T and 7T. Magn. Reson. Med. 76, 1083–1091 (2016).
Hingerl, L. et al. Clinical high-resolution 3D-MR spectroscopic imaging of the human brain at 7 T. Invest. Radiol. 55, 239–248 (2020).
Bednarik, P. et al. Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla. J. Cereb. Blood Flow Metab. 35, 601–610 (2015).
Seuwen, A., Schroeter, A., Grandjean, J., Schlegel, F. & Rudin, M. Functional spectroscopic imaging reveals specificity of glutamate response in mouse brain to peripheral sensory stimulation. Sci. Rep. 9, 10563 (2019).
Scherer, T., Sakamoto, K. & Buettner, C. Brain insulin signalling in metabolic homeostasis and disease. Nat. Rev. Endocrinol. https://doi.org/10.1038/s41574-021-00498-x (2021).
Gruetter, R. et al. Localized in vivo 13C NMR spectroscopy of the brain. NMR Biomed. 16, 313–338 (2003).
Mason, G. F. et al. A comparison of 13C NMR measurements of the rates of glutamine synthesis and the tricarboxylic acid cycle during oral and intravenous administration of [1-13C]glucose. Brain Res. Protoc. 10, 181–190 (2003).
Iozzo, P. & Guzzardi, M. A. Imaging of brain glucose uptake by PET in obesity and cognitive dysfunction: life-course perspective. Endocr. Connect. 8, R169–R183 (2019).
Kuehn, B. M. In Alzheimer research, glucose metabolism moves to center stage. JAMA 323, 297–299 (2020).
Bednařík, P. et al. Neurochemical responses to chromatic and achromatic stimuli in the human visual cortex. J. Cereb. Blood Flow Metab. 38, 347–359 (2018).
De Feyter, H. M. et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci. Adv. 4, eaat7314 (2018).
Lu, M., Zhu, X.-H., Zhang, Y., Mateescu, G. & Chen, W. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. J. Cereb. Blood Flow Metab. 37, 3518–3530 (2017).
Tiwari, V., An, Z., Wang, Y. & Choi, C. Distinction of the GABA 2.29 ppm resonance using triple refocusing at 3 T in vivo. Magn. Reson. Med. 80, 1307–1319 (2018).
de Graaf, R. A., Thomas, M. A., Behar, K. L. & De Feyter, H. M. Characterization of kinetic isotope effects and label loss in deuterium-based isotopic labeling studies. ACS Chem. Neurosci. 12, 234–243 (2021).
Veltien, A. et al. Simultaneous recording of the uptake and conversion of glucose and choline in tumors by deuterium metabolic imaging. Cancers 13, 4034 (2021).
Plecko, B. et al. Oral β-hydroxybutyrate supplementation in two patients with hyperinsulinemic hypoglycemia: monitoring of β-hydroxybutyrate levels in blood and cerebrospinal fluid, and in the brain by in vivo magnetic resonance spectroscopy. Pediatr. Res. https://doi.org/10.1203/00006450-200208000-00025 (2002).
Scafidi, S., Jernberg, J., Fiskum, G. & McKenna, M. C. Metabolism of exogenous [2,4-13C]β-hydroxybutyrate following traumatic brain injury in 21–22-day-old rats: an ex vivo NMR study. Metabolites. 12, 710 (2022).
Craft, S. et al. The ketogenic diet as a potential prevention or therapeutic strategy for AD. Alzheimer’s Dement. https://doi.org/10.1002/alz.038148 (2020).
Wright, J. N., Saneto, R. P. & Friedman, S. D. Hydroxybutyrate detection with proton MR spectroscopy in children with drug-resistant epilepsy on the ketogenic diet. Am. J. Neuroradiol. 39, 1336–1340 (2018).
Lebon, V. et al. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J. Neurosci. https://doi.org/10.1523/jneurosci.22-05-01523.2002 (2002).
Ross, J. M. et al. High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1008189107 (2010).
Liguori, C. et al. CSF lactate levels, τ proteins, cognitive decline: a dynamic relationship in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry https://doi.org/10.1136/jnnp-2014-308577 (2015).
Hingerl, L. et al. Density-weighted concentric circle trajectories for high resolution brain magnetic resonance spectroscopic imaging at 7T. Magn. Reson. Med. https://doi.org/10.1002/mrm.26987 (2018).
Cember, A. T. J. et al. Integrating 1H MRS and deuterium labeled glucose for mapping the dynamics of neural metabolism in humans. Neuroimage 251, 118977 (2022).
Maudsley, A. A. et al. Advanced magnetic resonance spectroscopic neuroimaging: experts’ consensus recommendations. NMR Biomed. https://doi.org/10.1002/nbm.4309 (2021).
Wilson, M. et al. Methodological consensus on clinical proton MRS of the brain: review and recommendations. Magn. Reson. Med. 82, 527–550 (2019).
Hyder, F., Fulbright, R. K., Shulman, R. G. & Rothman, D. L. Glutamatergic function in the resting awake human brain is supported by uniformly high oxidative energy. J. Cereb. Blood Flow Metab. 33, 339–347 (2013).
Hyder, F. & Rothman, D. L. Quantitative fMRI and oxidative neuroenergetics. NeuroImage https://doi.org/10.1016/j.neuroimage.2012.04.027 (2012).
Yu, Y., Herman, P., Rothman, D. L., Agarwal, D. & Hyder, F. Evaluating the gray and white matter energy budgets of human brain function. J. Cereb. Blood Flow Metab. https://doi.org/10.1177/0271678x17708691 (2018).
Pan, J. W. et al. Spectroscopic imaging of glutamate C4 turnover in human brain. Magn. Reson. Med. 44, 673–679 (2000).
de Graaf, R. A., Mason, G. F., Patel, A. B., Behar, K. L. & Rothman, D. L. In vivo 1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed. 16, 339–357 (2003).
Moreno, A., Blüml, S., Hwang, J. H. & Ross, B. D. Alternative 1-13C glucose infusion protocols for clinical 13C MRS examinations of the brain. Magn. Reson. Med. https://doi.org/10.1002/mrm.1158 (2001).
Sundar, L. K. S. et al. Towards quantitative [18F]FDG-PET/MRI of the brain: automated MR-driven calculation of an image-derived input function for the non-invasive determination of cerebral glucose metabolic rates. J. Cereb. Blood Flow Metab. 39, 1516–1530 (2019).
Shiyam Sundar, L. K. et al. Fully integrated PET/MR imaging for the assessment of the relationship between functional connectivity and glucose metabolic rate. Front. Neurosci. 14, 252 (2020).
Andronesi, O. C. et al. Motion correction methods for MRS: experts’ consensus recommendations. NMR Biomed. 34, e4364 (2021).
Dikaios, N., Arridge, S., Hamy, V., Punwani, S. & Atkinson, D. Direct parametric reconstruction from undersampled (k, t)-space data in dynamic contrast enhanced MRI. Med. Image Anal. https://doi.org/10.1016/j.media.2014.05.001 (2014).
Knutsson, L., Xu, X., van Zijl, P. C. M. & Chan, K. W. Y. Imaging of sugar‐based contrast agents using their hydroxyl proton exchange properties. NMR Biomed. https://doi.org/10.1002/nbm.4784 (2022).
Mason, G. F. et al. Simultaneous determination of the rates of the TCA cycle, glucose utilization, α-ketoglutarate/glutamate exchange, and glutamine synthesis in human brain by NMR. J. Cereb. Blood Flow. Metab. https://doi.org/10.1038/jcbfm.1995.2 (1995).
Ross, B., Lin, A., Harris, K., Bhattacharya, P. & Schweinsburg, B. Clinical experience with 13C MRS in vivo. NMR Biomed. 16, 358–369 (2003).
Moser, P. et al. Intra‐session and inter‐subject variability of 3D‐FID‐MRSI using single‐echo volumetric EPI navigators at 3T. Magn. Reson. Med. 83, 1920–1929 (2020).
DiNuzzo, M. et al. Perception is associated with the brain’s metabolic response to sensory stimulation. eLife https://doi.org/10.7554/eLife.71016 (2022).
Dou, W. et al. Automatic voxel positioning for MRS at 7 T. MAGMA https://doi.org/10.1007/s10334-014-0469-9 (2015).
Gruetter, R. & Tkac, I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn. Reson. Med. 43, 319–323 (2000).
Oz, G. & Tkac, I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: validation in the cerebellum and brainstem. Magn. Reson. Med. 65, 901–910 (2011).
Bednarik, P. et al. Effect of ketamine on human neurochemistry in posterior cingulate cortex: a pilot magnetic resonance spectroscopy study at 3 Tesla. Front. Neurosci. https://doi.org/10.3389/fnins.2021.609485 (2021).
Tkac, I., Starcuk, Z., Choi, I. Y. & Gruetter, R. In vivo 1H- NMR spectroscopy of rat brain at 1 ms echo time. Magn. Reson. Med. 41, 649–656 (1999).
Ridler, T. W. & Calvard, S. Picture thresholding using an iterative selection method. IEEE Trans. Syst. Man Cybern. 8, 630–632 (1978).
Strasser, B. et al. Coil combination of multichannel MRSI data at 7 T: MUSICAL. NMR Biomed. 26, 1796–1805 (2013).
Moser, P. et al. Non-Cartesian GRAPPA and coil combination using interleaved calibration data – application to concentric-ring MRSI of the human brain at 7 T. Magn. Reson. Med. https://doi.org/10.1002/mrm.27822 (2019).
Maudsley, A. A. et al. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magn. Reson. Med. https://doi.org/10.1002/mrm.21875 (2009).
Považan, M. et al. Mapping of brain macromolecules and their use for spectral processing of 1H-MRSI data with an ultra-short acquisition delay at 7 T. Neuroimage 121, 126–135 (2015).
Gröhn, H. et al. Influence of repetitive transcranial magnetic stimulation on human neurochemistry and functional connectivity: a pilot MRI/MRS study at 7 T. Front. Neurosci. https://doi.org/10.3389/fnins.2019.01260 (2019).
Oz, G. et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology 270, 658–679 (2014).
Kreis, R. The trouble with quality filtering based on relative Cramer-Rao lower bounds. Magn. Reson. Med. 75, 15–18 (2016).
Hangel, G. et al. Inter-subject stability and regional concentration estimates of 3D-FID-MRSI in the human brain at 7 T. NMR Biomed. https://doi.org/10.1002/nbm.4596 (2021).
Acknowledgements
We thank P. Bolan of the Center for Magnetic Resonance Research, University of Minnesota, and C. Rogers, University of Cambridge, for providing a tool to store and apply 7 T B0-shims for the 7 T MR scanner; V. Mlynarik for helpful discussions; and the study participants whose help is greatly appreciated. P.B. was supported by the European Union’s Horizon 2020 research and innovation programme under a Marie Skłodowska-Curie grant (agreement no. 846793), and by a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation (no. 27238). A.S. received funding from the European Union’s Horizon 2020 research and from an innovation programme under a Marie Skłodowska-Curie grant (agreement no. 794986). The authors acknowledge support from the Austrian Science Fund (FWF) (grants P 30701 and KLI 718 to W.B., I 6037 to B.S., KLI 782 to T.S., and KLI 646 to G.H.). W.B. acknowledges the support of the following NIH grant: R01EB031787. D.K.D. acknowledges support from the following National Institutes of Health grants: BTRC P41 EB027061 and P30 NS076408
Author information
Authors and Affiliations
Contributions
P.B., A.S. and W.B. wrote the manuscript draft. P.B., D.G. and L.H. acquired the data. P.B., D.G., F.N., L.H., D.K.D., B.S., B.S-D., G.H. and A.S. processed the data. P.B., W.B., T.S. and R.L. conceptualized the study design. P.B., A.S., W.B. and R.L. obtained funding. M.K. and S.T. contributed to data interpretation. P.B. and W.B are guarantors of the integrity of the entire study. All authors edited and approved the submitted version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
R. Lanzenberger received travel grants and/or conference speaker honoraria within the past three years from Bruker BioSpin MR and Heel, and has served as a consultant for Ono Pharmaceutical. He also received investigator-initiated research funding from Siemens Healthcare regarding clinical research using PET/MR and is a shareholder of the start-up company BM Health GmbH since 2019. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Kevin Brindle, Ferdia Gallagher and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and table.
Data
Source data for the Supplementary figures.
Source data
Source Data for Fig. 1
MRS data quantified with the LCModel.
Source Data for Fig. 4
MRS data quantified with the LCModel, including timing.
Source Data for Fig. 6
MRS data quantified with the LCModel, including timing.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bednarik, P., Goranovic, D., Svatkova, A. et al. 1H magnetic resonance spectroscopic imaging of deuterated glucose and of neurotransmitter metabolism at 7 T in the human brain. Nat. Biomed. Eng 7, 1001–1013 (2023). https://doi.org/10.1038/s41551-023-01035-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-023-01035-z
- Springer Nature Limited
This article is cited by
-
Repeatability of deuterium metabolic imaging of healthy volunteers at 3 T
European Radiology Experimental (2024)
-
Advances and prospects in deuterium metabolic imaging (DMI): a systematic review of in vivo studies
European Radiology Experimental (2024)
-
MUW researcher of the month
Wiener klinische Wochenschrift (2024)