Abstract
Current poultry vaccines against influenza A viruses target the globular head region of the hemagglutinin (HA1), providing limited protection against antigenically divergent strains. Experimental subunit vaccines based on the conserved ectodomain of the matrix protein 2 (M2e) induce cross-reactive antibody responses, but fail to fully prevent virus shedding after low pathogenic avian influenza (LPAI) virus challenge, and are ineffective against highly pathogenic avian influenza (HPAI) viruses. This study assessed the benefits of combining nanoparticles bearing three tandem M2e repeats (NR-3M2e nanorings or NF-3M2e nanofilaments) with an HA1 subunit vaccine in protecting chickens against a heterologous HPAI H5N1 virus challenge. Chickens vaccinated with the combined formulations developed M2e and HA1-specific antibodies, were fully protected from clinical disease and mortality, and showed no histopathological lesions or virus shedding, unlike those given only HA1, NR-3M2e, or NF-3M2e. Thus, the combined vaccine formulations provided complete cross-protection against HPAI H5N1 virus, and prevented environmental virus shedding, crucial for controlling avian influenza outbreaks.
Similar content being viewed by others
Introduction
Influenza A viruses (IAV), whose natural reservoirs are wild aquatic birds, infect a wide range of species including domestic birds and mammals such as pigs and humans1. IAV are divided into subtypes based on two proteins on the surface of the virus: hemagglutinin (HA) and neuraminidase (NA). There are 18 different HA subtypes and 11 different NA subtypes (H1 through H18 and N1 through N11, respectively). H17N10 and H18N11 are restricted to bats. Generally, avian IAV are classified into low pathogenicity avian influenza (LPAI) or high pathogenic avian influenza (HPAI) viruses on the basis of their pathobiological effects in gallinaceous species2. In chickens, infections with LPAI viruses are usually asymptomatic or cause low to mild clinical signs associated with viral replication in the respiratory, digestive, and/or reproductive systems2. Nevertheless, in the case of comorbidities, clinical signs and lesions can be more prominent in affected birds. In addition, some Asian LPAI H9N2 viruses can cause more severe clinical symptoms and even mortality in chickens2. HPAI viruses replicate in multiple tissues leading to systemic disease with damages in multiple visceral organs, nervous and cardiovascular systems causing high morbidity and mortality2. HPAI H5 viruses derived from the Gs/GD lineage, which has evolved worldwide into diverse clades, subclades and reassortant viruses (“H5Nx viruses”), cause important economic losses to the poultry industry. In addition, they are considered as a serious threat to public health worldwide through their zoonotic and pandemic potential.
Vaccination, when implemented with good farm biosecurity measures, can be an effective approach to control IAV infections in poultry and to reduce the economic impacts. Although vaccination of poultry against IAV varies between countries, licensed vaccines include whole inactivated viruses (WIV) emulsified in oil adjuvants and live recombinant viral vectors with HA-encoding gene inserts3. These vaccines generate variable levels of neutralizing antibodies directed towards the globular head domain of HA (HA1), which can prevent viral entry into permissive host cells and subsequent virus replication. While antigenic mismatch between vaccine strains and circulating IAV reduces vaccine efficacy, antigenically updated vaccines can provide improved protection against the clinical disease and virus shedding3,4,5,6,7,8,9. However, antigenic drift resulting from mutations, mainly in the HA gene and virus reassortment events that occur periodically in circulating IAV have a deleterious impact on vaccine efficacy in the field3.
Owing to the high genetic and antigenic variability of IAV, current research focuses on developing subunit vaccines which contain “universal” viral antigens, including a conserved sequence located in the ectodomain of the matrix protein 2 (M2), termed M2e10. M2 protein is sparsely expressed at the surface of the virus, but is abundant on the surface of IAV-infected cells. The protection conferred by M2e-based vaccines is associated with the production of M2e-specific antibodies that can eliminate infected cells mainly through Fc-mediated effector mechanisms, although M2e-specific T-cell responses may also contribute to eliminating virus-infected cells10. While vaccination trials in mouse or ferret models have demonstrated that M2e is a promising antigen candidate for inducing broad protection against a range of IAV, M2e-based vaccines were not protective in pigs11,12,13, and results in chickens were lukewarm so far10,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32. Vaccine formulations incorporating single14,28,32 or multiple15,16,17,18,19,20,22,23,29,30,31,32 M2e antigens alone or combined with other conserved epitopes, such as those located in the stalk domain of HA (HA2)28,29, were unable to protect chickens against mortality associated with HPAI viruses28,29,30,31, nor to prevent virus shedding upon experimental infections with LPAI14,15,16,17,18,19,20,22,23 or HPAI28,29,30,31,32 viruses. While subunit vaccines based solely on M2e did not provide complete protection against IAV, two recent studies indicated that the supplementation of H5N1 WIV vaccines with M2e conferred clinical protection and impeded virus shedding after heterologous HPAI H5 virus infection in chickens31,33. Nonetheless, the WIV and challenge virus strains used in these studies belonged to the same second-order clade. Moreover, the ability of these supplemented vaccines to prevent virus replication in different organs was not demonstrated in any of these studies.
We previously developed two innovative nanostructures with inherent immune adjuvant property, namely nanorings (NRs) and nanofilaments (NFs), that showed high efficiency as vaccine delivery platforms for the M2e antigen in mice. NRs are composed of recombinant nucleoprotein (N) of the respiratory syncytial virus (RSV), which self-assembles into homogenous rings of about 15 nm in diameter when expressed in Escherichia coli (E. coli)34,35,36. NFs, on the other hand, are composed of recombinant curli-specific gene A (CsgA) protein, a major extracellular matrix component contributing to biofilm formation in numerous enteric bacteria37. Recombinant CsgA monomers produced in E. coli spontaneously self-assemble in vitro into cross-β-sheet quaternary structures forming long and unbranched fibrils with diameter ranging from 5 to 15 nm and length that can reach over 1 µm38,39. Thus, recombinant chimeric NRs and NFs were produced as vaccines against IAV by fusing three tandem repeats of the M2e peptide (3M2e) at the C-terminal end of the N sequence of RSV (NR-3M2e), and by linking the N-terminal domain of CsgA with 3M2e (NF-3M2e), respectively. Mice immunized with NR-3M2e or NF-3M2e developed potent M2e-specific humoral and cellular immune responses and survived a homologous H1N1 IAV challenge34,40,41. The efficacy of NR-3M2e and NF-3M2e in anti-IAV vaccination in chickens has not been demonstrated yet. In addition, the ability of enhancing the cross-protective potential of subunit vaccines by combining HA1 and M2e remains unexplored in chickens. In this study, we evaluated the benefit of supplementing NR-3M2e or NF-3M2e with an HA1 subunit vaccine against a genetically divergent HPAI H5N1 virus challenge in specific pathogen-free (SPF) chickens.
Results
Biochemical and structural characterization of NR-3M2e and NF-3M2e
In this study, we evaluated the effect of the supplementation of nanoparticles incorporating M2e antigens with an HA1 subunit vaccine in the cross-protection against HPAI H5N1 virus challenge in chickens. To this end, chimeric NR-3M2e and NF-3M2e were produced and their (supra)structural characteristics were validated. The purified NR-3M2e and NF-3M2e preparations appeared as a single band at the predicted molecular weight by SDS-PAGE and Coomassie blue staining (Fig. 1A, D). Western blotting using specific antibodies validated the identity and antigenicity of preparation (Fig. 1B, E). NR-3M2e exhibited ring-like morphological features by transmission electron microscopy (TEM) (Fig. 1C), and dynamic light scattering (DLS) analysis indicated that the preparation was mainly composed of a homogenous population with a hydrodynamic radius of around 20 nm (Supplementary Fig. 1).
Recombinant NR-3M2e were produced and purified as described in “Methods” section, and then analyzed by (A) SDS-PAGE followed by Coomassie blue staining, (B) Western blot assays with (B, left panel) N-specific polyclonal antibodies and (B, right panel) M2-specific monoclonal antibody, and (C) transmission electron microscopy after negative staining. Recombinant NF-3M2e were produced and purified as described in “Methods” section, and then analyzed by (D) SDS-PAGE followed by Coomassie blue staining and (E) Western blot assays with M2-specific monoclonal antibody, and (F) transmission electron microscopy after negative staining.
The self-assembly and morphology of purified NF-3M2e were confirmed by circular dichroism (CD) spectroscopy, fluorescence spectroscopy and TEM. CD indicated that NF-3M2e displayed a characteristic minimum at ~218 nm and maximum at ~197 nm, corresponding to a β-sheet-rich secondary structure (Supplementary Fig. 2)42. Thioflavin T (ThT) fluorescence further confirmed the presence of the cross-β-sheet quaternary structure, whereas 8-anilino-1- naphthalenesulfonic acid (ANS) revealed the formation of accessible hydrophobic pockets upon nanofilament formation (Supplementary Fig. 2C). TEM (Fig. 1F) analyses of NF-3M2e revealed the formation of a dense network of fibrillar assemblies with widths in the order of 2.5–5 nm. These observations supported the typical morphological heterogeneities of CsgA, in accordance with previous studies39,43,44,45.
The co-administration of NR-3M2e or NF-3M2e with HA1 protein triggered M2e-and HA1-specific antibody responses in chickens
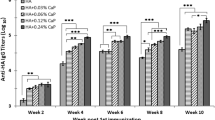
Firstly, we evaluated the immunogenicity of our vaccine formulations. Chickens received three intramuscular administrations, at 2-week intervals, of NR-3M2e or NF-3M2e admixed with HA1 protein, or each single component alone. Vaccine antigens were emulsified with Emulsigen®-P, an oil-in-water adjuvant used in veterinary vaccination46. The HA1-specific antibody responses were assessed by an hemagglutination inhibition (HI) assay (Fig. 2) and competitive enzyme-linked immunosorbent assay (ELISA) (Fig. 3A). Chickens immunized with HA1 protein exhibited significant HI titers as soon as day 28 post-primary vaccination in comparison with non-vaccinated chickens, and the highest titers were observed after the second boost (Fig. 2). A high intragroup variability was observed, and the administration of a second boost was necessary to maximize the number of seroconverted chickens (1/15 and 11/15 chickens presenting HI titers ≥ 16 at day 28 and day 42 post-primary vaccination, respectively). Chickens immunized with NR-3M2e or NF-3M2e admixed with HA1 exhibited significant HI titers after the second boost in comparison with non-vaccinated chickens, with a high intragroup variability (6/15 and 10/14 chickens presenting HI titers ≥ 16 at day 42 post-primary vaccination with [NR-3M2e + HA1] and [NF-3M2e + HA1], respectively). The co-administration of NF-3M2e with HA1 did not have a major impact on HI titers in comparison with the administration of HA1 protein alone (Fig. 2). By contrast, chickens co-immunized with NR-3M2e and HA1 exhibited lower HI titers in comparison with chickens immunized with HA1 alone, even after the second boost. The competitive ELISA confirmed that chickens immunized with HA1, [NR-3M2e + HA1] or [NF-3M2e + HA1] developed significant HA1-specific antibody responses at day 42 post-primary vaccination (Fig. 3A). Chickens immunized with NR-3M2e or NF-3M2e administered alone or in combination with HA1 developed significant M2e-specific IgG at day 42 post-primary vaccination, and there was no difference in the magnitude of the response whether HA1 was incorporated in the formulation or not (Fig. 3B). In conclusion, chickens immunized with HA1 protein in combination with NR-3M2e or NF-3M2e developed significant HA1- and M2e-specific antibody responses, and the co-administration of the two IAV antigens did not notably hinder the induction of the response directed against each of the antigens.
Chickens received at 2-week intervals three intramuscular administrations of HA1 protein in combination with NR-3M2e or NF-3M2e, or each single component alone. Chickens receiving PBS were included as a negative control group. All preparations were admixed with Emulsigen®-P. Serum HI titers were determined on days 14, 28 and 42 post-primary vaccination using four HA units of the A/turkey/Ontario/6213/1966 virus. Data from each chicken are presented, including the geometric mean with 95% confidence interval. The number of chickens with HI titers ≥16 is indicated on the top of the graph. *statistically significant difference (P < 0.05) in comparison to PBS group (at the same day post-primary vaccination). ¤statistically significant difference (P < 0.05) between the indicated groups.
A Chickens received at 2-week intervals three intramuscular administrations of HA1 protein alone or in combination with NR-3M2e or NF-3M2e. All preparations were admixed with Emulsigen®-P. The serum HA1-specific antibody response was analyzed by competitive ELISA on days 14, 28 and 42 post-primary vaccination (ppv) using HA1 protein from A/turkey/Ontario/6213/1966 as coating antigen. Data are presented as arithmetic means with SEM. “% inhibition” represents (ODchicken serum + Mab/ODMab)*100. The dotted bar represents the positivity threshold (40%). B Chickens received at 2-week intervals three intramuscular administrations of NR-3M2e or NF-3M2e alone or in combination with recombinant HA1 protein. Chickens receiving HA1 or PBS were included as control groups. All preparations were admixed with Emulsigen®-P. Serum M2e-specific antibody response was analyzed by indirect ELISA on day 42 post-primary vaccination. Data are presented as arithmetic means with SEM. *, statistically significant difference (P < 0.05) in comparison to PBS and HA1 groups.
The supplementation of NR-3M2e or NF-3M2e with HA1 protein enhanced the clinical and virological protection of chickens against heterologous HPAI H5N1 virus challenge
Two weeks after the third immunization, chickens were experimentally infected with a heterologous HPAI H5N1 virus and monitored daily for mortality and clinical signs for up to 21 days post-challenge (dpc) (Fig. 4and Supplementary Table 1). The challenge virus belonged to clade 2.3.4.4c, whose HA gene was derived from the Eurasian Gs/GD-like H5N1 lineage47. The HA1 vaccine protein was derived from a North American lineage LPAI H5N1 virus, and shared only 81% identity in amino acid sequence with the HA1 sequence of the challenge virus, allowing to evaluate the broad-protective potential of our vaccine formulations. Unvaccinated chickens (PBS group) and chickens immunized with NR-3M2e or NF-3M2e started to die from 2 to 3 dpc, with 100% mortality by 4 dpc (NF-3M2e and NR-3M2e) or 7 dpc (unvaccinated). Thirty-three percent of chickens (5/15) immunized with HA1 protein survived the challenge by 21 dpc, and no clinical signs were observed in the surviving chickens. Finally, all chickens immunized with HA1 protein in combination with NR-3M2e or NF-3M2e survived the infection, with no protracted clinical signs (Fig. 4 and Supplementary Table 1). The virus was detected in the oropharyngeal (Fig. 5A) and/or cloacal (Fig. 5B) swabs of all unvaccinated chickens, and in chickens immunized with NR-3M2e or NF-3M2e. Viral RNA was detected in the swabs of all dead chickens and all surviving chickens with the exception of three surviving chickens in the HA1 group (Supplementary Table 1). In sharp contrast, no viral RNA was detected in both cloacal and oropharyngeal swabs of birds immunized with [NR-3M2e + HA1] or [NF-3M2e + HA1] during the course of the experiment (Fig. 5A, B). Thus, the supplementation of NR-3M2e or NF-3M2e with HA1 protein was essential to completely prevent chickens from clinical disease, mortality, and virus shedding after a challenge with heterologous H5N1 virus.
Chickens received at 2-week intervals three intramuscular administrations of HA1 protein in combination with NF-3M2e or NR-3M2e, or each single component alone. Chickens receiving PBS were included as a control group. All preparations were admixed with Emulsigen®-P. Two weeks after the third immunization, all chickens were challenged with 2.3 × 102 PFU of heterologous HPAI H5N1 virus and monitored daily for mortality. The survival curves of infected chickens are expressed as the percentages of surviving chickens. The log-rank (Mantel-Cox) test was used to compare survival curves. *statistically significant difference (P < 0.05) in comparison to PBS group. ¤statistically significant difference (P < 0.05) between the indicated groups.
Chickens received at 2-week intervals three intramuscular administrations of HA1 protein in combination with NF-3M2e or NR-3M2e, or each single component alone. Chickens receiving PBS were included as a control group. All preparations were admixed with Emulsigen®-P. Two weeks after the third immunization, all chickens were challenged with 2.3 × 102 PFU of heterologous HPAI H5N1 virus. A Oropharyngeal and (B) cloacal swabs were collected on 3, 5, 7, 10, and 14 days post-challenge (dpc), and viral RNA copies were determined by qRT-PCR. Data from each chicken are presented, including the geometric mean with 95% confidence interval. The number of positive swabs/total swabs is indicated on the top of the graph. *, statistically significant difference (P < 0.05) in comparison to PBS group (at the same dpc).
The supplementation of NR-3M2e or NF-3M2e with HA1 protein induced protection against histological lesions after heterologous HPAI H5N1 virus challenge in chickens
The protective potential of the vaccine formulations to prevent lung, brain and heart tissue lesions associated with H5N1 virus challenge was evaluated by histological and immunohistochemical analyses from a dead chicken which received PBS (at 3 dpc), and from euthanized chickens which received NR-3M2e or NF-3M2e (at 3 dpc), HA1 (at 19 dpc), or [NR-3M2e + HA1] or [NF-3M2e + HA1] (at 21 dpc). Infections with HPAI H5N1 viruses usually result in moderate pulmonary lesions in chickens48. However, in this study, chickens in the PBS group exhibited obvious lung lesions, consisting of necrosis and hypercellularity (Fig. 6A). Mild increase in cellularity due to inflammatory cell infiltration was observed in the NR-3M2e (Fig. 6C) and NF-3M2e (Fig. 6E) groups. By contrast, no prominent lung lesion was noticeable in the HA1 (Fig. 6G), [NR-3M2e + HA1] (Fig. 6I) or [NF-3M2e + HA1] (Fig. 6K) groups. IAV antigen was extensively detected in scattered alveolar macrophages and necrotic focus in the PBS group, and in fewer endothelial cells and alveolar macrophages in the HA1 group (Fig. 6B, H). While staining for virus antigen was extensive and involved large number of alveolar macrophages in the lungs of chickens vaccinated with NR-3M2e (Fig. 6D), scanty and few viral antigen-laden macrophages appeared in the NF-3M2e group (Fig. 6F). No virus antigen was visible in the lungs of chickens immunized with either NR-3M2e or NF-3M2e supplemented with HA1 (Fig. 6J, L).
Chickens received at 2-week intervals three intramuscular administrations of NR-3M2e or NF-3M2e in combination with HA1 protein, or each single component alone. Chickens receiving PBS were included as a control group. All preparations were admixed with Emulsigen®-P. Two weeks after the third immunization, all chickens were challenged with 2.3 × 102 PFU of heterologous HPAI H5N1 virus. Lungs were collected at selected days post-challenge (dpc), and sections were stained with hematoxylin and eosin (HE) before histological analyis (left panels), or stained with anti-NP antibody before immunohistochemical (IHC) analysis (right panels). A Histological and (B) IHC analyses of the lung tissue of the PBS group at 3 dpc. The arrow in panel A indicates areas of necrosis and the arrow in panel B indicates influenza antigen-laden alveolar macrophages. C Histological and (D) IHC analyses of the lung tissue of the NR-3M2e group at 3 dpc. Arrow in panel D indicates influenza antigen-laden alveolar macrophages. E Histological and (F) IHC analyses of the lung tissue of the NF-3M2e group at 3 dpc. Arrow in panel F indicates influenza antigen-laden alveolar macrophages. G Histological and (H) IHC analyses of the lung tissue of the HA1 group at 19 dpc. Arrow in panel H indicates influenza antigen in endothelial and alveolar macrophages. I Histological and (J) IHC analyses of the lung tissue of the [NR-3M2e + HA1] group at 21 dpc. K Histological and (L) IHC analyses of the lung tissue of the [NF-3M2e + HA1] group at 21 dpc. Scale = 200 µm.
Histological lesions in the brain of the PBS group (Fig. 7A) were similar to that in the NR-3M2e (Fig. 7C), NF-3M2e (Fig. 7E), or HA1 (Fig. 7G) groups, and included areas of multifocal to focal necrotizing encephalitis with perivascular cuffing of mononuclear inflammatory cell infiltrates. By contrast, no microscopic brain lesion was observed in the group immunized with [NR-3M2e + HA1] (Fig. 7I). Although the extent of the virus antigen load varied between the groups, the virus was detected in the brain of the PBS (Fig. 7B), NR-3M2e (Fig. 7D), NF-3M2e (Fig. 7F), and HA1 (Fig. 7H) groups. No viral antigen was detected in the [NR-3M2e + HA1] group (Fig. 7J). Finally, whereas necrotic foci were observed in the heart tissue of chickens immunized with HA1 (Fig. 8G), no cardiac lesion was detected in the PBS group (Fig. 8A) or in chickens immunized with the M2e-based nanoparticles administered alone (Fig. 8C, E) or in combination with HA1 (Fig. 8I, K). Remarkable amounts of virus antigen were detected in the cardiac fibers in all groups of chickens (Fig. 8B, D, F, H) with the exception of chickens immunized with the supplemented vaccine formulations, which did not harbor any traces of virus antigen in the cardiac tissue (Fig. 8J, L). To conclude, the supplementation of NR-3M2e or NF-3M2e with HA1 protein prevented virus replication and subsequent pathological alterations in chicken lung, brain, and heart tissues after infection with a heterologous HPAI H5N1 virus.
Chickens received at 2-week intervals three intramuscular administrations of NR-3M2e or NF-3M2e in combination with HA1 protein, or each single component alone. Chickens receiving PBS were included as a control group. All preparations were admixed with Emulsigen®-P. Two weeks after the third immunization, all chickens were challenged with 2.3 × 102 PFU of heterologous HPAI H5N1 virus. Brains were collected at selected days post-challenge (dpc), and sections were stained with hematoxylin and eosin (HE) before histological analyis (left panels) or stained with anti-NP antibody before immunohistochemical (IHC) analysis (right panels). A Histological and (B) IHC analyses of the brain tissue of the PBS group at 3 dpc. The arrows in panel A and B indicate necrotic foci and influenza antigen in necrotic areas. C Histological and (D) IHC analyses of the brain tissue of the NR-3M2e group at 3 dpc. The arrows in panel C indicate multifocal necrosis and gliosis. The arrows in panel D indicate viral antigen localization in areas of necrotic foci. E Histological and (F) IHC analyses of the brain tissue of the NF-3M2e group at 3 dpc. Arrows in panel E indicate multifocal necrosis and gliosis. Arrows in panel F indicate viral antigen. G Histological and (H) IHC analyses of the brain tissue of the HA1 group at 19 dpc. Arrow in panel G indicates prominent perivascular cuffing of mononuclear inflammatory cells and scattered necrotic cells throughout the neutrophil. I Histological and (J) IHC analyses of the brain tissue of the [NR-3M2e + HA1] group at 21 dpc. The brain of the [NF-3M2e + HA1] group was not collected during necropsy. Scale = 200 µm.
Chickens received at 2-week intervals three intramuscular administrations of NR-3M2e or NF-3M2e in combination with HA1 protein, or each single component alone. Chickens receiving PBS were included as a control group. All preparations were admixed with Emulsigen®-P. Two weeks after the third immunization, all chickens were challenged with 2.3 × 102 PFU of heterologous HPAI H5N1 virus. Hearts were collected at selected days post-challenge (dpc), and sections were stained with hematoxylin and eosin (HE) before histological analyis (left panels) or stained with anti-NP antibody before immunohistochemical (IHC) analysis (right panels). A Histological and (B) IHC analyses of the heart tissue of the PBS group at 3 dpc. C Histological and (D) IHC analyses of the heart tissue of the NR-3M2e group at 3 dpc. E Histological and (F) IHC analyses of the heart tissue of the NF-3M2e group at 3 dpc. G Histological and (H) IHC analyses of the heart tissue of the HA1 group at 19 dpc. I Histological and (J) IHC analyses of the heart tissue of the [NR-3M2e + HA1] group at 21 dpc. K Histological and (L) IHC analyses of the heart tissue of the [NF-3M2e + HA1] group at 21 dpc. In the IHC panels, arrows indicate focal to extensive immunostaining for influenza virus antigen. Scale = 200 µm.
Discussion
Current anti-IAV vaccines are based on anti-HA mediated immunity, and vaccines must be regularly updated to follow the dynamics of the antigenic changes in the virus3. Besides, traditional WIV vaccines are produced in embryonated chicken eggs, a technology that is time-consuming and requires a large supply of SPF eggs3. In this study, we evaluated the efficacy of vaccine formulations incorporating the conserved M2e peptide and HA1 protein against an experimental challenge in chickens with HPAI H5N1 virus. The vaccine HA1 gene was derived from a strain antigenically divergent from the challenge strain.
M2e is an attractive vaccine candidate because its amino acid sequence is highly conserved among IAV49. Since M2e peptide is poorly immunogenic per se, several approaches have been developed to increase its immunogenicity, including its fusion to adjuvant sequences14,17,28,29,30, and its expression within nanoparticles16,18,22,31,50,51. In this study, we grafted M2e antigen to NRs and NFs, two nanoplatforms with inherent adjuvant activity. NRs are composed of 10-11 NRSV protomers entrapping random single-stranded bacterial RNA fragments of 70 to 77 bases35. Previous in vitro experiments using NRs fused to the green-fluorescent-protein (GFP) demonstrated that NRs were rapidly captured by mouse antigen-presenting cells (APCs) (including macrophage and dendritic cell lines), and the punctuated fluorescence observed by confocal microscopy suggested that internalized NRs were located in endosomal compartments52. NRs stimulated the expression of co-stimulatory molecule CD86 and the secretion of type I IFNs by mouse APCs in a MyD88-dependent pathway (Riffault S, unpublished results). Both the organized structure of NRs and encapsidated RNA fragments, which are natural ligands for pattern-recognition receptors such as Toll-like receptor (TLR) 7/8, may be involved in the immunostimulatory properties of NRs (Riffault S, unpublished results). Previous studies in mice demonstrated the genetic linking of H1N1 M2e or HA2 proteins to NRs increased the immunogenicity and protective potential of the antigens against a homologous H1N1 challenge34,40. On the other hand, CsgA fibrils engage the membrane heterodimeric TLR1/TLR2 and the cytosolic inflammasome NOD-like receptor pyrin 3 (NLRP3), inducing the activation and maturation of APCs and the production of IL-1β and IL-6 pro-inflammatory cytokines that are important for adaptative immune responses41,53. The present study demonstrated that chickens immunized with NF-3M2e or NR-3M2e exhibited robust M2e-specific antibody responses, and that NRs and NFs are effective scaffolds for the delivery of IAV antigens in vivo.
Although chickens vaccinated with HA1 protein developed significant levels of specific antibodies, almost 70% of the chickens died after the heterologous H5N1 virus challenge by the end of the experimental period of 21 days. Moreover, viral RNA was detected in the oropharynx and cloaca of two surviving chickens through the 14 day-sampling period. This result is in agreement with other studies where experimental H5 HA subunit vaccines, such as baculovirus-expressed HA54, virus-like particles (VLP) displaying HA antigens55, or commercial viral vector vaccines expressing HA56,57,58 alone, failed to confer full protection or to prevent virus shedding after challenge with HPAI H5 virus whose HA sequence shared less than 90% identity with the vaccine HA. Notably, the percentage of HA homology between the vaccine and challenge strain is not always an adequate indicator for protection, and key amino acids can dictate the protection offered by HA-based vaccines. Indeed, several studies reported that chickens vaccinated with H5 HA subunit vaccines were not completely protected from clinical disease and/or virus shedding after infection with HPAI H5 viruses whose HA sequence shared ≥ 98% identity with the vaccine sequence, or whose HA belonged to the same (sub)clade as the vaccine HA59,60,61,62,63,64,65. A multitude of other experimental factors could also influence the efficacy of HA subunit vaccines, including the vaccine dose59,66,67,68,69, the adjuvant used59,66,70,71,72,73,74, the route of vaccination75,76, the number67,68,77,78 of vaccine doses, the challenge virus dose79, the time interval between vaccination and challenge80, and the age of chickens68.
In this study, all chickens vaccinated with NR-3M2e or NF-3M2e died within 4 dpc, and substantial amounts of viral RNA was detected in the oropharyngeal and cloacal swabs, implying the lack of protection when the nanovaccines were used as standalone vaccines. The lack of protection observed for these vaccine groups correlated with the appearance of histological lesions and the detection of virus antigen in the lung, brain, and heart tissues. It is noteworthy that several M2e-based subunit vaccines have been tested in chickens against HPAI viruses25,28,30,31,32. Although these studies associated M2e peptides with various adjuvants or delivery systems, and used multiple immunization regimens to increase the immune response to M2e, strategies targeting M2e alone showed no or limited efficacy25,28,30,31,32. Compared to anti-HA antibodies that interfere with the binding of IAV to sialic acid receptors present on the surface of infected epithelial cells and block viral entry, anti-M2e antibodies do not exert neutralizing activity against the virus but, instead, can eliminate virus-infected cells via antibody-dependent cell-mediated and complement-dependent cytotoxicity mechanisms81,82,83. Thus, M2e-based vaccines do not confer sterilizing immunity, but rather alleviate or suppress the symptoms of the disease by reducing the viral spread. Although some reports indicated that M2e vaccines suppressed clinical signs23 or prevented lung lesions23 after LPAI virus challenge, these vaccines were inefficient to protect chickens against HPAI virus infections25,28,30,31,32.
In contrast to chickens immunized with HA1 protein or M2e nanovaccines alone, chickens vaccinated with formulations that combined HA1 and each of the nanovaccines ([NF-3M2e + HA1] or [NR-3M2e + HA1]) survived the challenge. Remarkably, they did not exhibit any outward clinical signs, tissue pathology or virus shedding. Different vaccine strategies combining IAV antigens have been explored in attempts to broaden the protection against HPAI viruses in chickens. For example, VLP incorporating multiple HA derived from various subtypes or H5 clades protected chickens against clinical disease after heterologous challenge with H5N2 and H7N3 viruses55, or H5N1 and H5N8 viruses61, respectively. Nonetheless, in all these studies, viral excretion was not completely suppressed55,61. Another strategy based on viral vectors co-expressing H5 HA protein and conserved IAV antigens such as NA84 or nucleoprotein (NP)85 did not improve the cross-protection against H5 viruses in comparison with the viral vectors expressing HA alone. Actually, only formulations consisting of H5N1 WIV admixed with M2e, either incorporated within VLP or expressed by recombinant baculovirus, abolished both clinical disease and virus shedding after challenge with HPAI virus belonging to the same second-order subclade as the WIV strain (clade 2.3)31,33. New subclades are designated when the average percentage pairwise nucleotide distances exceed 1.5% from existing clades or subclades, depending on their order. Thus, in these studies, the HA gene homology between the vaccine and the challenge virus strain was greater than 90%. In the present study, the percentage of homology between the vaccine and the challenge virus strain did not exceed 81%. Moreover, the vaccine virus strain was a LPAI strain while the challenge virus strain belonged to the H5Nx viral constellation.
To the best of our knowledge, the current study demonstrated for the first time the ability of a subunit vaccine formulation incorporating conserved M2e antigens and HA1 to provide a complete protection from disease and virus shedding after a challenge with highly divergent HPAI H5 virus in chickens. Such protection may be due to the neutralizing activity of HA1-specific antibodies as a first virus barrier, followed later on by the M2e-specific antibody- or T cell-mediated cytotoxic activities that would contribute to completely eliminate the virus and prevent virus dissemination within the host. Whatever the mechanism is, our study demonstrated that the supplementation of NR-3M2e or NF-3M2e with HA1 protein is an alternative strategy to enhance the cross-protection against avian influenza H5 viruses in chickens. In the commercial poultry settings, highly desirable and cost-effective vaccines are those that can offer relatively high protection from the first dose. In line with this approach, it would be very interesting to investigate in the future whether our combined vaccine formulations provide adequate protection even after reducing the number of vaccine doses.
Methods
Viruses and vaccine antigens
A/chicken/BC/FAV-002/2015 HPAI H5N1 (2.3.4.4c) virus [accession numbers: KP892988 to KP892995] isolated from a backyard layer chicken flock near Chilliwack, British Columbia (National Centre for Foreign Animal Disease [NCFAD], Winnipeg, Canada) was used in the heterologous challenge experiment47. The virus was inoculated via the allantoic route in 9-day-old SPF embryonated chicken eggs (Canadian Food Inspection Agency [CFIA] Fallowfield Laboratory, Ottawa, Canada) and incubated at 37°C for 48-72 h or until embryos died. The allantoic fluid was harvested and the virus titer was determined in Madin-Darby Canine Kidney (MDCK) cells and expressed as PFU/ml. The stock virus had a titer of 1.2 × 106 PFU/ml. The M2e sequence MSLLTEVETPTRTEWECRCSDSSD was derived from A/chicken/BC/FAV-002/2015 HPAI H5N1 virus. The two cysteine residues of the M2e sequence (amino acids 17 and 19) were substituted to two serine residues to avoid formation of a disulfide bond that could impair the assembly of the nanoparticles34. The recombinant HA1 protein was produced from North American lineage A/turkey/Ontario/6213/1966 LPAI H5N1 virus (GenBank accession number CY107858).
Production of HA1 protein
The expression and purification of soluble recombinant HA1 protein was performed as previously described86. Briefly, the codon-optimized HA1 gene for expression in insect cells was cloned into a pAB-beeTM-FH vector (AB Vector, San Diego, CA) through GenScript services (GenScript USA Inc, Piscataway, NJ). The vector was purified and co-transfected with linearized baculovirus vector DNA and ProFoldTM-ER1 (AB Vector) into Spodoptera frugiperda (Sf9) insect cells to generate recombinant baculovirus containing the HA1 gene. The baculovirus was plaque-purified and sequenced for HA1 gene validation. Sf9 cells were infected with the baculovirus, and incubated for 72 h at 27°C under agitation. Cells were pelleted and lysed with I-PER insect cell protein extraction reagent (Pierce Biotechnology, Rockford, IL). The supernatant was purified using Ni-NTA bead resin and the presence of the recombinant protein was confirmed by Western blotting (Supplementary Fig. 3) using mouse monoclonal antibody against HA1 (40).
Production of NR-3M2e
The pET-N-Sac plasmid was obtained from the pET-N plasmid, which contained the full-length coding sequence of the N protein derived from RSV Long strain (ATCC VR-26, GenBank accession number AY911262.1), by introducing a SacI restriction enzyme site in frame and at the C-terminal end of the N sequence34. To obtain the pET-N-3M2e plasmid containing three repetitions of the M2e sequence, the synthesized pUC57-3M2e plasmid (ProteoGenix, Schiltigheim, France) was used as a template to amplify the 3M2e sequence by PCR using the Phusion high-fidelity DNA polymerase (Thermo Fisher Scientific, Waltham, MA, USA) with gene-specific primers flanked with SacI/SacI (forward primer/reverse primer) sites. The PCR-amplified 3M2e sequence was then digested by SacI enzyme and inserted into the pET-N-Sac plasmid using the same restriction site. NR-3M2e were expressed and purified as previously described34,36,40. The identity and antigenicity of NR-3M2e were validated by SDS-PAGE followed by Western blot assays with anti-N (rabbit polyclonal antibody against N at a dilution of 1:3,000) and anti-M2e (mouse 14C2 monoclonal antibody against M2 (Santa Cruz Biotechnology, Dallas, TX) at a dilution of 1:3,000) antibodies. The structural integrity of the NRs was confirmed by DLS and TEM, as described previously40. The absence of endotoxins in the NR-3M2e vaccine was confirmed using the toxin sensor chromogenic LAL endotoxin assay (Pierce Biotechnology, Rockford, IL) with a sensitivity limit of 0.03 EU/ml.
Production of NF-3M2e
The pET-29a (+) plasmid containing three repetitions of the M2e sequence fused in frame at the N-terminal end of the E. coli CsgA sequence (GenBank accession number WP124055573) was provided by GenScript (Piscataway, NJ). To express NF-3M2e, E. coli NiCo21(DE3) competent cells (New England Biolabs Inc., Ipswich, MA, USA) transformed with the pET-3M2e-CsgA plasmid were grown at 37 °C under agitation (225 rpm) in LB medium containing 50 μg/ml of kanamycin. Protein expression was induced by adding 1 µM IPTG to mid-log-phase cells (OD600nm ~ 1) and subsequent incubation for 1 h at 37 °C. Cells were then harvested by centrifugation at 5000 × g for 15 min and stored at -80 °C. All purification steps were performed under denaturing conditions with 8 M guanidine hydrochloride (GdnHCl) to prevent uncontrolled aggregation, until the assembly was precisely initiated by switching from 8 M GdnHCl to PBS buffer. Thawed cell pellet was resuspended in 8 M GdnHCl, PBS pH 7.2 and sonicated on ice using six cycles of 20 s. Clarification of lysate was achieved by centrifugation at 30,000 × g for 30 min at 4 °C. The protein of interest was purified over a Ni-charged ProfinityTM metal ion affinity chromatography (IMAC) resin (Bio-Rad Laboratories, Inc., Hercules, CA, USA) in a gravity flow column equilibrated in 8 M GdnHCl, PBS pH 7.2. The column was washed with 8 M GdnHCl, PBS pH 7.2 supplemented with 12.5 mM imidazole, and protein was eluted using 250 mM imidazole in PBS pH 7.4. A final desalting step was performed using a Sephadex G-25 fine desalting column in PBS pH 7.4. The identity and antigenicity of NF-3M2e were validated by SDS-PAGE followed by Western blot assays with anti-M2 (mouse 14C2 monoclonal antibody against M2 (Santa Cruz Biotechnology, Dallas, TX) at a dilution of 1:3,000) antibody. The (supra)structural characterization of the NFs was validated by CD spectroscopy, fluorescence spectroscopy and TEM, as described below. Removal of endotoxins from the NF-3M2e vaccine was performed using Pierce endotoxin-removal spin columns and was confirmed using the toxin sensor chromogenic LAL endotoxin assay with a sensitivity limit of 0.03 EU/ml.
Circular dichroism spectroscopy
A Jasco J-815 CD (Jasco Inc., Easton, MD, USA) spectrometer was used to record Far-UV CD spectra from 190 to 260 nm at room temperature. The wavelength step was set at 0.5 nm with a scan rate of 20 nm × min-1. Each collected spectrum was background subtracted with protein-free buffer (PBS pH 7.4). The raw data were converted to mean residue ellipticity (MRE) as described elsewhere43,44.
Fluorescence spectroscopy
ThT (1 mM) was added to NF-3M2e sample to reach a final ThT concentration of 40 µM, and fluorescence emission was recorded by a PTI QuantaMasterTM (Horiba Ltd., Kyoto, Japan) spectrofluorometer from 450 nm to 550 nm at an excitation wavelength of 440 nm. The final concentration of ANS was 450 µM and the fluorescence emission was measured between 385 nm and 550 nm with a constant excitation at 370 nm. Fluorescence was measured in ultramicro 10 mm length quartz cells. Data are expressed as arithmetic means with the standard error of the mean ( ± SEM) of at least three individual experiments performed in triplicate.
Transmission electron microscopy
NF-3M2e samples at 0, 24 and 48 h of self-assembly were diluted in 20 mM Tris-HCl buffer (pH 7.4) before being applied to glow-discharged carbon films on 400 mesh copper grids. After adsorption, samples were negatively stained with 1.5% uranyl formate for 1 min and air-dried for 15 min. Images were recorded using a FEI Tecnai 12 BioTwin microscope as described elsewhere43,44. Electron micrographs of NR-3M2e were acquired using a CM12 TEM (Royal Philips Electronics, Amsterdam, Netherlands) at 80 kV excitation voltage. Samples at 0.05-1 mg/ml were applied onto an airglow- discharged carbon-coated 200-mesh copper grid and stained with a 2% uranyl acetate aqueous solution.
Dynamic light scattering
Size measurements with the Zetasizer Nano series (Malvern Panalytical, Malvern, UK) based on the principle of DLS were made at 20°C using a helium-neon laser wavelength of 633 nm and detection angle of 173°. The results were presented as size distribution by volume calculated from the Malvern software.
Animal experiments
All animal experiments were conducted after approval by the Canadian Science Centre for Human and Animal Health institutional guidelines for animal care (AUD# C-20-007). One-day-old SPF white Leghorn layer chickens (CFIA, Ottawa, Canada) were housed in heated BSL3 plus animal cubicles at the NCFAD on a floor system. Feed and water were ad libitum, and the animal cubicles were enriched with cupboards and porches. When chickens were 21-day-old, they were randomly assigned to six experimental groups (n = 14-15/group) and kept in a separate cubicle. The vaccine formulations consisted of HA1 protein (15 µg) admixed with NF-3M2e (50 µg) or with NR-3M2e (50 µg), or each single component administered alone. The last group served as unvaccinated PBS group. Before administration, vaccine antigens were mixed with Emulsigen®-P (MVP Adjuvants, Phibro Animal Health, NJ) for 2 h under gentle agitation. Three vaccinations were applied via the intramuscular route. The two booster vaccinations were applied two weeks apart. On days 14, 28, and 42 post-primary vaccination, sera were collected from each bird. Chickens were challenged at day 42 post-primary vaccination with 2.3 × 102 PFU of heterologous A/chicken/BC/FAV-002/2015 virus in 100 µl endotoxin-free PBS via the intranasal route (50 µl/nostril). They were observed daily for clinical signs and scored as follows: 1) clinically normal - chickens fed, drank and moved well; 2) mild clinical disease - chickens showed either one or a combination of the following clinical signs: depression, mouth breathing, diarrhea, mild neurological signs, wing drooping, facial edema - but moved freely, ate and drank; 3) severely sick - chickens showed any of the above clinical signs, were unable to move and drink - chickens euthanized and recorded as dead birds the next day; 4) dead - chickens died acutely with no overt clinical signs. The clinical scores of each chicken of each group are indicated in Supplementary Table 1. Moreover, oropharyngeal and cloacal swabs were collected on 3, 5, 7, 10 and 14 dpc. On 3 dpc (PBS, NF-3M2e and NR-3M2e groups), 19 dpc (HA1 group) and 21 dpc ([NR-3M2e + HA1] and [NF-3M2e + HA1] groups), chickens (n = 1 per group) were euthanized and tissue samples from brain, lung, and heart were collected and fixed in 10% neutral-buffered formalin for histopathological/immunohistochemistry assessments. For euthanasia of sick birds and at the end of the experiment, each bird was anesthetized with isoflurane (5% for induction and 2-3% for maintenance, delivered in 100% oxygen via a mask) using a Bain open anesthetic system (Benson Medical Industries INC., ON, Canada) set with a flow rate of 2–5 L/min. The birds were then euthanized by intracardiac injection of euthanyl (BIMEDA-MTC Animal Health INC., ON, Canada) at a dose of 40-60 mg/kg (Supplementary File 1).
Hemagglutination inhibition assay
The HI assay was performed according to WOAH manual standardized protocol87. Briefly, two-fold serially diluted serum samples from each chicken were incubated with four HA units of the A/turkey/Ontario/6213/1966 (H5N1) virus in a V-bottom plates (Costar, Cole-Parmer Canada, Quebec, Canada) and incubated for 30 min. Subsequently, 0.5% chicken red blood cells were added and incubated for another 30 min. The HI titers were scored as the reciprocal of the highest serum dilution resulting in 100% inhibition of chicken red blood cells from hemagglutination. When no HI was obtained, an HI titer of 2 was given for plotting.
Enzyme-linked immunosorbent assay
HA1-specific antibody response in serum samples was evaluated by competitive ELISA as described previously86 with modifications. Briefly, 96-well flat-bottom polystyrene plates (Nunc MaxiSorp™; Nunc, Roskilde, Denmark) were coated with recombinant HA1 protein in carbonate- bicarbonate buffer 0.1 M (pH 9.6). After blocking, diluted sera (1:10) were added to the plates right before the addition of F73-H5-3 monoclonal antibody (1:300)86, and subsequently incubated for 1 h at 37°C with agitation. Each serum was assayed in duplicate. Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:2000) (Jackson) was added and incubated at 37°C for 1 h with agitation. Tetramethylbenzidine (TMB) substrate was added, and plates were further incubated for 15 min at room temperature with shaking in the dark. Enzyme reaction was stopped with 2 M H2SO4, and absorbance was read at 450 nm with an ELISA plate reader. Results are expressed as percent inhibition [1-(ODchicken serum+Mab/ODMab)*100]. Values above 40% inhibition are considered positive.
The presence of M2e-specific antibodies in serum samples was evaluated by indirect ELISA using the same 96-well flat-bottom microtiter plates (Nunc MaxiSorp™) as above. The plates were coated with synthetic M2e peptide (200 ng/well in 0.1 M carbonate-bicarbonate buffer (pH 9.6)) overnight at 4°C. Plates were washed five times with PBS containing 0.05% Tween 20 (PBS-T), blocked in 0.5% BSA for 1 h at room temperature, and diluted sera (1:3200) were added and incubated at 37°C for 1 h. Negative control wells only contained PBS-T. Plates were washed and then incubated with HRP-conjugated donkey anti-chicken IgG (1:5000 in PBS-T) at 37°C for 1 h under agitation. TMB substrate was then added and plates were further incubated for 10 min at room temperature in the dark with gentle agitation. The enzymatic reaction was stopped with 1% 2 M H2SO4. The plates were read with an ELISA plate reader at an absorbance of 450 nm. The results were expressed as optical density (OD) values whose background (twice the OD450nm of negative wells) was subtracted.
Determination of viral RNA shedding by quantitative real-time polymerase chain reaction
Total RNA was extracted from the oropharyngeal and cloacal swabs using the MagMAX 1835 Nucleic Acid Isolation Kit (ThermoFisher Scientific, Waltham, MA, USA). The spiked Enteroviral armoured RNA (ARM-ENTERO; Asuragen) was used as an exogenous extraction and reaction control. Quantitative real-time polymerase chain reaction (qRT-PCR) targeting the matrix (M) gene of IAV was used to quantify viral RNA shedding as described previously88. Samples with Ct values > 38 were considered as negative as none of these samples yielded infectious viruses when inoculated via the allantoic route in 9-day-old SPF embryonated chicken eggs. A standard curve was generated from a 10-fold dilution series of an in vitro synthesized RNA template from the M gene of IAV to calculate M gene-specific RNA copy numbers in the samples based on the linear regression of the standard curve in LightCycler®480 System (Roche Diagnostics). The results were expressed as log10 viral RNA copy numbers/µl. The viral RNA copy numbers/µl of each chicken are indicated in Supplementary Table 1.
Histopathology and immunohistochemistry
Tissues were collected only when birds reached a humane endpoint because they were severely ill (not eating, drinking, moving) and due to neurological signs or at the end of the experiments. Tissues fixed in 10% neutral phosphate buffered formalin were sectioned at 5 µm and stained with haematoxylin and eosin for histopathologic analysis. For immunohistochemistry, paraffin tissue sections were quenched for 10 min in aqueous 3% H2O2 and then pretreated with proteinase K for 15 min for epitope retrieval. A mouse monoclonal antibody specific for influenza A NP (F26NP9)89 was applied on the sections at a dilution of 1:10,000 for 1 h. The microscopic sections were then visualized using a horseradish peroxidase labeled polymer, Envision®+ system (anti-mouse) (Dako, USA), and reacted with the chromogen diaminobenzidine (DAB). The sections were then counter stained with Gill’s hematoxylin. The virus antigen load and distribution in the tissue section were visualized under the microscope and the intensity of coloration (brown) in the tissues of the different groups were qualitatively evaluated.
Statistical data analysis
The log-rank (Mantel-Cox) test was used to compare the survival rates between groups. Otherwise, differences between the experimental groups were analyzed for significance using the Mann-Whitney rank sum test. All analyses were done using the Sigma Plot system v11.0 (Systat Software, San Jose, CA, USA). A P value < 0.05 was considered statistically significant.
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information file.
References
Swayne, D., Suarez, D. & Sims, L. Influenza in Diseases of Poultry (ed. Swayne, D. E.) 210-256 (John Wiley & Sons, Inc., 2020).
Pantin-Jackwood, M. J. & Swayne, D. E. Pathogenesis and pathobiology of avian influenza virus infection in birds. Rev. Sci. Tech. 28, 113–136 (2009).
Swayne, D. E. & Kapczynski, D. R. Vaccines And Vaccination For Avian Influenza In Poultry in Animal Influenza (ed Swayne, D. E.) 378-434 (John Wiley & Sons, Inc., 2017).
Suarez, D. L. & Pantin-Jackwood, M. J. Recombinant viral-vectored vaccines for the control of avian influenza in poultry. Vet. Microbiol. 206, 144–151 (2017).
Tian, G. et al. Protective efficacy in chickens, geese and ducks of an H5N1-inactivated vaccine developed by reverse genetics. Virology 341, 153–162 (2005).
van der Goot, J. A., Koch, G., de Jong, M. C. & van Boven, M. Quantification of the effect of vaccination on transmission of avian influenza (H7N7) in chickens. Proc. Natl. Acad. Sci. USA. 102, 18141–18146 (2005).
Ge, J. et al. Newcastle disease virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous H5N1 avian influenza viruses. J. Virol. 81, 150–158 (2007).
Bublot, M. et al. Efficacy of a fowlpox-vectored avian influenza H5 vaccine against Asian H5N1 highly pathogenic avian influenza virus challenge. Avian Dis 51, 498–500 (2007).
Gardin, Y. et al. Experimental and field results regarding immunity induced by a recombinant turkey herpesvirus H5 vector vaccine against H5N1 and other H5 highly pathogenic avian influenza virus challenges. Avian Dis 60, 232–237 (2016).
Kolpe, A., Schepens, B., Fiers, W. & Saelens, X. M2-based influenza vaccines: recent advances and clinical potential. Expert Rev. Vaccines 16, 123–136 (2017).
Opriessnig, T. et al. An experimental universal swine influenza A virus (IAV) vaccine candidate based on the M2 ectodomain (M2e) peptide does not provide protection against H1N1 IAV challenge in pigs. Vaccine 42, 220–228 (2024).
Heinen, P. P., Rijsewijk, F. A., de Boer-Luijtze, E. A. & Bianchi, A. T. J. Vaccination of pigs with a DNA construct expressing an influenza virus M2-nucleoprotein fusion protein exacerbates disease after challenge with influenza A virus. J. Gen. Virol. 83, 1851–1859 (2002).
Bernelin-Cottet, C. et al. A universal influenza vaccine can lead to disease exacerbation or viral control depending on delivery strategies. Front. Immunol. 7, 641 (2016).
Zhang, Z. et al. Fusion to chicken C3d enhances the immunogenicity of the M2 protein of avian influenza virus. Virol. J. 7, 89 (2010).
Hajam, I. A., Kim, J. & Lee, J. H. Intranasally administered polyethylenimine adjuvanted influenza M2 ectodomain induces partial protection against H9N2 influenza A virus infection in chickens. Vet. Immunol. Immunopathol. 209, 78–83 (2019).
Babapoor, S. et al. A novel vaccine using nanoparticle platform to present immunogenic M2e against avian influenza infection. Influenza Res. Treat. 2011, 126794 (2011).
Dabaghian, M. et al. Vaccination with recombinant 4 x M2e.HSP70c fusion protein as a universal vaccine candidate enhances both humoral and cell-mediated immune responses and decreases viral shedding against experimental challenge of H9N2 influenza in chickens. Vet. Microbiol. 174, 116–126 (2014).
Elaish, M. et al. Immunogenicity and protective efficacy of the norovirus P particle-M2e chimeric vaccine in chickens. Vaccine 33, 4901–4909 (2015).
Elaish, M. et al. Supplementation of inactivated influenza vaccine with norovirus P particle-M2e chimeric vaccine enhances protection against heterologous virus challenge in chickens. PLoS ONE 12, e0171174 (2017).
Elaish, M. et al. Protective immunity against influenza virus challenge by norovirus P particle-M2e and HA2-AtCYN vaccines in chickens. Vaccine 37, 6454–6462 (2019).
Hajam, I. A., Senevirathne, A., Hewawaduge, C., Kim, J. & Lee, J. H. Intranasally administered protein coated chitosan nanoparticles encapsulating influenza H9N2 HA2 and M2e mRNA molecules elicit protective immunity against avian influenza viruses in chickens. Vet. Res. 51, 37 (2020).
Tang, Y. et al. Chimaeric VP2 proteins from infectious bursal disease virus containing the N-terminal M2e of H9 subtype avian influenza virus induce neutralizing antibody responses to both viruses. Avian Pathol. 42, 260–267 (2013).
Hajam, I. A., Kim, J. & Lee, J. H. Salmonella Gallinarum delivering M2eCD40L in protein and DNA formats acts as a bivalent vaccine against fowl typhoid and H9N2 infection in chickens. Vet. Res. 49, 99 (2018).
Kim, J. H., Hajam, I. A. & Lee, J. H. Oral immunization with a novel attenuated Salmonella Typhimurium encoding influenza HA, M2e and NA antigens protects chickens against H7N9 infection. Vet. Res. 49, 12 (2018).
Layton, S. L. et al. Vaccination of chickens with recombinant Salmonella expressing M2e and CD154 epitopes increases protection and decreases viral shedding after low pathogenic avian influenza challenge. Poult. Sci. 88, 2244–2252 (2009).
Yang, W. T. et al. Lactobacillus plantarum displaying conserved M2e and HA2 fusion antigens induces protection against influenza virus challenge. Appl. Microbiol. Biotechnol. 102, 5077–5088 (2018).
Hajam, I. A. et al. Oral immunization with an attenuated Salmonella Gallinarum encoding the H9N2 haemagglutinin and M2 ectodomain induces protective immune responses against H9N2 infection in chickens. Avian Pathol. 49, 486–495 (2020).
Kalaiyarasu, S. et al. Elicitation of highly pathogenic avian influenza H5N1 M2e and HA2-specific humoral and cell-mediated immune response in chicken following immunization with recombinant M2e-HA2 fusion orotein. Front. Vet. Sci. 7, 571999 (2020).
Li, J. et al. Nanoparticle vaccine for avian influenza virus: a challenge study against highly pathogenic H5N2 subtype. J. Virol. Antivir. Res. 7, 1 (2018).
Reese, K. A. et al. A novel lactococcal vaccine expressing a peptide from the M2 antigen of H5N2 highly pathogenic avian influenza A virus prolongs survival of vaccinated chickens. Vet. Med. Int. 2013, 316926 (2013).
Song, B. M. et al. Supplemented vaccination with tandem repeat M2e virus-like particles enhances protection against homologous and heterologous HPAI H5 viruses in chickens. Vaccine 34, 678–686 (2016).
Zhang, X. et al. Vaccination with different M2e epitope densities confers partial protection against H5N1 influenza A virus challenge in chickens. Intervirology 54, 290–299 (2011).
Zhang, Z. et al. Coimmunization with recombinant epitope-expressing baculovirus enhances protective effects of inactivated H5N1 vaccine against heterologous virus. Vet. Microbiol. 203, 143–148 (2017).
Herve, P. L. et al. A novel subnucleocapsid nanoplatform for mucosal vaccination against influenza virus that targets the ectodomain of matrix protein 2. J. Virol. 88, 325–338 (2014).
Tawar, R. G. et al. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science 326, 1279–1283 (2009).
Tran, T. L. et al. The nine C-terminal amino acids of the respiratory syncytial virus protein P are necessary and sufficient for binding to ribonucleoprotein complexes in which six ribonucleotides are contacted per N protein protomer. J. Gen. Virol. 88, 196–206 (2007).
Barnhart, M. M. & Chapman, M. R. Curli biogenesis and function. Annu. Rev. Microbiol. 60, 131–147 (2006).
Wang, X., Hammer, N. D. & Chapman, M. R. The molecular basis of functional bacterial amyloid polymerization and nucleation. J. Biol. Chem. 283, 21530–21539 (2008).
Wang, X., Smith, D. R., Jones, J. W. & Chapman, M. R. In vitro polymerization of a functional Escherichia coli amyloid protein. J. Biol. Chem. 282, 3713–3719 (2007).
Calzas, C. et al. Immunogenicity and protective potential of mucosal vaccine formulations based on conserved epitopes of influenza A viruses fused to an innovative ring nanoplatform in mice and chickens. Front. Immunol. 12, 772550 (2021).
Lamontagne, F. et al. Engineered curli nanofilaments as a self-adjuvanted antigen delivery platform. Adv. Healthc. Mater. 12, e2300224 (2023).
Groenning, M. Binding mode of thioflavin T and other molecular probes in the context of amyloid fibrils-current status. J. Chem. Biol. 3, 1–18 (2010).
Nguyen, P. T. et al. Identification of transmissible proteotoxic oligomer-like fibrils that expand conformational diversity of amyloid assemblies. Commun. Biol. 4, 939 (2021).
Zottig, X. et al. Guiding the morphology of amyloid assemblies by electrostatic capping: from polymorphic twisted fibrils to uniform nanorods. Small 15, e1901806 (2019).
Dueholm, M. S. et al. Fibrillation of the major curli subunit CsgA under a wide range of conditions implies a robust design of aggregation. Biochemistry 50, 8281–8290 (2011).
Abolnik, C., O’Kennedy, M., Murphy, M. & Wandrag, D. Efficacy of a plant-produced clade 2.3.4.4 H5 influenza virus-like particle vaccine in layer hens. Vet. Vaccine 1, 100001 (2022).
Berhane, Y. et al. Pathobiological characterization of a novel reassortant highly pathogenic H5N1 virus isolated in British Columbia, Canada, 2015. Sci. Rep. 6, 23380 (2016).
Kwon, Y. K. & Swayne, D. E. Different routes of inoculation impact infectivity and pathogenesis of H5N1 high pathogenicity avian influenza virus infection in chickens and domestic ducks. Avian Dis. 54, 1260–1269 (2010).
Hasan, N. H., Ignjatovic, J., Peaston, A. & Hemmatzadeh, F. Avian influenza virus and DIVA strategies. Viral Immunol. 29, 198–211 (2016).
Zottig, X. et al. Self-assembled peptide nanorod vaccine confers protection against influenza A virus. Biomaterials 269, 120672 (2021).
Bricha, S. et al. Synthetic multicomponent nanovaccines based on the molecular co-assembly of beta-peptides protect against influenza A virus. ACS Infect. Dis. 9, 1232–1244 (2023).
Roux, X. et al. Sub-nucleocapsid nanoparticles: a nasal vaccine against respiratory syncytial virus. PLoS ONE 3, e1766 (2008).
Tukel, C. et al. Toll-like receptors 1 and 2 cooperatively mediate immune responses to curli, a common amyloid from enterobacterial biofilms. Cell. Microbiol. 12, 1495–1505 (2010).
Swayne, D. E., Perdue, M. L., Beck, J. R., Garcia, M. & Suarez, D. L. Vaccines protect chickens against H5 highly pathogenic avian influenza in the face of genetic changes in field viruses over multiple years. Vet. Microbiol. 74, 165–172 (2000).
Pushko, P. et al. Virus-like particles displaying H5, H7, H9 hemagglutinins and N1 neuraminidase elicit protective immunity to heterologous avian influenza viruses in chickens. Virology 501, 176–182 (2017).
Kapczynski, D. R. et al. Vaccine protection of chickens against antigenically diverse H5 highly pathogenic avian influenza isolates with a live HVT vector vaccine expressing the influenza hemagglutinin gene derived from a clade 2.2 avian influenza virus. Vaccine 33, 1197–1205 (2015).
Swayne, D. E., Garcia, M., Beck, J. R., Kinney, N. & Suarez, D. L. Protection against diverse highly pathogenic H5 avian influenza viruses in chickens immunized with a recombinant fowlpox vaccine containing an H5 avian influenza hemagglutinin gene insert. Vaccine 18, 1088–1095 (2000).
Kapczynski, D. R. et al. Homologous and heterologous antigenic matched vaccines containing different H5 hemagglutinins provide variable protection of chickens from the 2014 U.S. H5N8 and H5N2 clade 2.3.4.4 highly pathogenic avian influenza viruses. Vaccine 35, 6345–6353 (2017).
Crawford, J. et al. Baculovirus-derived hemagglutinin vaccines protect against lethal influenza infections by avian H5 and H7 subtypes. Vaccine 17, 2265–2274 (1999).
Swayne, D. E., Beck, J. R., Perdue, M. L. & Beard, C. W. Efficacy of vaccines in chickens against highly pathogenic Hong Kong H5N1 avian influenza. Avian Dis. 45, 355–365 (2001).
Kapczynski, D. R. et al. Vaccination with virus-like particles containing H5 antigens from three H5N1 clades protects chickens from H5N1 and H5N8 influenza viruses. Vaccine 34, 1575–1581 (2016).
Veits, J. et al. Protective efficacy of several vaccines against highly pathogenic H5N1 avian influenza virus under experimental conditions. Vaccine 26, 1688–1696 (2008).
Rauw, F. et al. Further evidence of antigenic drift and protective efficacy afforded by a recombinant HVT-H5 vaccine against challenge with two antigenically divergent Egyptian clade 2.2.1 HPAI H5N1 strains. Vaccine 29, 2590–2600 (2011).
Lardinois, A. et al. Potency of a recombinant NDV-H5 vaccine against various HPAI H5N1 virus challenges in SPF chickens. Avian Dis. 56, 928–936 (2012).
Ma, J. et al. Newcastle disease virus-based H5 influenza vaccine protects chickens from lethal challenge with a highly pathogenic H5N2 avian influenza virus. NPJ Vaccines 2, 33 (2017).
Lee, D. H. et al. H9N2 avian influenza virus-like particle vaccine provides protective immunity and a strategy for the differentiation of infected from vaccinated animals. Vaccine 29, 4003–4007 (2011).
Cornelissen, L. A. et al. A single immunization with soluble recombinant trimeric hemagglutinin protects chickens against highly pathogenic avian influenza virus H5N1. PLoS ONE 5, e10645 (2010).
Schultz-Cherry, S. et al. Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects chickens against lethal infection with Hong Kong-origin H5N1 viruses. Virology 278, 55–59 (2000).
Wu, Q. et al. A pseudotype baculovirus-mediated vaccine confers protective immunity against lethal challenge with H5N1 avian influenza virus in mice and chickens. Mol. Immunol. 46, 2210–2217 (2009).
Song, L. et al. Mucosal and systemic immune responses to influenza H7N9 antigen HA1-2 co-delivered intranasally with flagellin or polyethyleneimine in mice and chickens. Front. Immunol. 8, 326 (2017).
Chen, H. Y. et al. Immune responses of chickens inoculated with a recombinant fowlpox vaccine coexpressing HA of H9N2 avain influenza virus and chicken IL-18. Antiviral Res. 91, 50–56 (2011).
Song, L. et al. The optimized fusion protein HA1-2-FliCDeltaD2D3 promotes mixed Th1/Th2 immune responses to influenza H7N9 with low induction of systemic proinflammatory cytokines in mice. Antiviral Res. 161, 10–19 (2019).
Zhu, W. Z., Wen, Y. C., Lin, S. Y., Chen, T. C. & Chen, H. W. Anti-influenza protective efficacy of a H6 virus-like particle in chickens. Vaccines (Basel) 8, 465 (2020).
Wu, P. et al. Single dose of consensus hemagglutinin-based virus-like particles vaccine protects chickens against divergent H5 subtype influenza viruses. Front. Immunol. 8, 1649 (2017).
Gao, W. et al. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J. Virol. 80, 1959–1964 (2006).
Cornelissen, L. A. et al. Protective efficacy of Newcastle disease virus expressing soluble trimeric hemagglutinin against highly pathogenic H5N1 influenza in chickens and mice. PLoS ONE 7, e44447 (2012).
Sistere-Oro, M. et al. Conserved HA-peptides expressed along with flagellin in Trichoplusia ni larvae protects chicken against intranasal H7N1 HPAIV challenge. Vaccine 38, 416–422 (2020).
Ferreira, H. L. et al. Comparison of single 1-day-old chick vaccination using a Newcastle disease virus vector with a prime/boost vaccination scheme against a highly pathogenic avian influenza H5N1 challenge. Avian Pathol. 43, 68–77 (2014).
Steensels, M. et al. Protection afforded by a recombinant turkey herpesvirus-H5 vaccine against the 2014 European highly pathogenic H5N8 avian influenza strain. Avian Dis. 60, 202–209 (2016).
Liu, J. et al. Recombinant duck enteritis virus works as a single-dose vaccine in broilers providing rapid protection against H5N1 influenza infection. Antiviral Res. 97, 329–333 (2013).
Jegerlehner, A., Schmitz, N., Storni, T. & Bachmann, M. F. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J. Immunol. 172, 5598–5605 (2004).
Simhadri, V. R. et al. A human anti-M2 antibody mediates antibody-dependent cell-mediated cytotoxicity (ADCC) and cytokine secretion by resting and cytokine-preactivated natural killer (NK) cells. PLoS ONE 10, e0124677 (2015).
Wang, R. et al. Therapeutic potential of a fully human monoclonal antibody against influenza A virus M2 protein. Antiviral Res. 80, 168–177 (2008).
Ramp, K. et al. Coexpression of avian influenza virus H5 and N1 by recombinant Newcastle disease virus and the impact on immune response in chickens. Avian Dis. 55, 413–421 (2011).
Webster, R. G., Kawaoka, Y., Taylor, J., Weinberg, R. & Paoletti, E. Efficacy of nucleoprotein and haemagglutinin antigens expressed in fowlpox virus as vaccine for influenza in chickens. Vaccine 9, 303–308 (1991).
Yang, M. et al. Production and diagnostic application of monoclonal antibodies against influenza virus H5. J. Virol. Methods 162, 194–202 (2009).
World Organisation for Animal Heath (WOAH). Avian Influenza (Including Infection With High Pathogenicity Avian Influenza Viruses) https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.03.04_AI.pdf (2025).
Spackman, E. et al. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 40, 3256–3260 (2002).
Yang, M. et al. Development and application of monoclonal antibodies against avian influenza virus nucleoprotein. J. Virol. Methods 147, 265–274 (2008).
Acknowledgements
This work has benefited from the facilities and expertize of MIMA2-MET, MIMA2, and INRAE (Microscopy and Imaging Facility for Microbes, Animals and Foods, https://doi.org/10.15454/1.5572348210007727E12). The authors would like to thank the avian, animal care and pathology units’ staff at the National Centre for Foreign Animal Disease, Canadian Food Inspection Agency for their support and contribution to the project. This work was supported by a grant from the Livestock Vaccine Innovation Fund (International Development Research Centre, Bill & Melinda Gates Foundation, Global Affairs Canada) to D.A., C.C., R.L.G., and S.B. (Project ID 108517).
Author information
Authors and Affiliations
Contributions
C.C., D.A., S.B., Y.B., R.L.G., and Cy.C. conceived and designed the study. C.C., D.A., S.B., Y.B., Cy.C., and V.K. produced vaccine candidates. Y.B., M.S., T.N.A., and C.E.-H. performed chicken experiments and histopathology work. C.C., D.A., S.B., Y.B., T.N.A., and Cy.C. performed data analysis. Cy.C., C.C., D.A., S.B., and T.N.A. wrote the original draft of the manuscript. C.C., D.A., R.L.G., and S.B. reviewed and edited the manuscript. All authors contributed and approved the submitted version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that INRAE has filed patent on work related to the generation and application of the vaccine formulation and the associated platforms presented in this article, with C.C., D.A., S.B., Y.B., Cy.C., and R.L.G. included as co-inventors. There is no restriction on the publication of data. The other authors declare that they have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Calzas, C., Alkie, T.N., Suderman, M. et al. M2e nanovaccines supplemented with recombinant hemagglutinin protect chickens against heterologous HPAI H5N1 challenge. npj Vaccines 9, 161 (2024). https://doi.org/10.1038/s41541-024-00944-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-024-00944-7
- Springer Nature Limited