Abstract
The wider use of clozapine is limited by the risk of agranulocytosis and the associated requirement for monitoring of neutrophil counts. We searched local electronic patient records for cases of agranulocytosis occurring during clozapine treatment during the period 2007–2020. We found 23 episodes recorded as agranulocytosis in clozapine patients. Of these, nine met pre-defined criteria and were considered episodes of life-threatening agranulocytosis (LTA). These episodes of clozapine-induced LTA exhibited a distinct pattern of continuous and rapid neutrophil count decline to zero or near zero. Mean time for neutrophils to fall from ANC > 2 to ANC <0.5 × 109/L was 8.4 days (range 2–15 days). Each event was also characterised by a prolonged nadir and delayed recovery (range 4–16 days). Non-LTA episodes were, in contrast, brief and benign. We conclude that an important proportion of cases of agranulocytosis identified in people prescribed clozapine are not life-threatening and may not even be clozapine-related. Monitoring schemes should aim to identify true clozapine-induced LTA as opposed to threshold-defined nominal agranulocytosis. Genetics studies might benefit from examining associations with clozapine-induced LTA rather than with recorded cases of agranulocytosis or neutropenia.
Similar content being viewed by others
Introduction
Clozapine was the first drug found to possess antipsychotic action without causing extrapyramidal effects1. It eventually gained product registration and was marketed in Europe the early 1970s. Clozapine’s association with agranulocytosis was first reported in 1975 when Finnish authorities described eight cases occurring in around 2000 patients prescribed clozapine2 (an incidence of approximately 0.4%). Outside Finland, the risk of clozapine-associated agranulocytosis at that time was estimated to be 0.027%3. A later and more complete analysis of the cases occurring in Finland suggested that there were “17 cases of neutropenia or agranulocytosis amongst about 3000 patients treated”4. In fact, there had been ten cases of agranulocytosis (seven of which were fatal)—an incidence of agranulocytosis of 0.33%. Clozapine was withdrawn worldwide and later reintroduced with strict haematological monitoring.
The initial association detected was with fatal or near fatal agranulocytosis. However, the introduction of mandatory haematological monitoring for clozapine suggested that clozapine was associated with both agranulocytosis (defined, in respect to clozapine use, as neutrophils <0.5 × 109/L) and neutropenia (usually defined as a neutrophil count of <1.5 × 109/L; sometimes <2 × 109/L). In patients registered on formal clozapine monitoring schemes, the incidence of neutropenia has been reported to range from 2.55 to 5.5%6 and the incidence of agranulocytosis from 0%7,8 to as much as 1%6,9. Clozapine remains underutilised because of the fear of both neutropenia and agranulocytosis and because of the burden imposed by regular blood testing10,11.
Clozapine’s association with neutropenia is rarely questioned but clozapine may not in fact cause a benign neutropenia. In the original Finnish analysis4 the incidence of neutropenia that did not progress to agranulocytosis was 7 in 3000, or 0.23%—less than a tenth of later estimates of the incidence of neutropenia. It is possible that the perceived association with neutropenia is a result of surveillance bias, whereby intensive blood monitoring reveals random, clinically silent, and non-pathological episodes of neutropenia occurring coincidentally to the use of clozapine. This is important because a great many people are prevented from receiving clozapine because of erroneously diagnosed clozapine-related blood toxicity12. The concept of clozapine-induced neutropenia might also be a blind-alley for research into genetic predisposition to clozapine-induced blood dyscrasia. Although the same genetic variants seem to be responsible for the increased risk of both neutropenia and agranulocytosis13, a recent study found a strong link between the human leucocyte antigen (HLA) polymorphism rs113332494 and agranulocytosis but not neutropenia14. Moreover, in studies where genetic associations with clozapine-associated neutropenia are suggested15, it is acknowledged that the majority of neutropenia cases do not develop into agranulocytosis.
It could be argued that what really matters in respect to the safe use of clozapine is the detection and treatment of episodes of reduced neutrophil count that are sufficiently severe and prolonged to represent a risk to life. Few studies have investigated the time course of neutrophil count before, during and after confirmed cases of clozapine-induced life-threatening agranulocytosis (LTA). The aim of this study was to explore the pattern of neutrophil count in patients with true clozapine-induced LTA and to characterise this reaction so as to distinguish it from benign and coincidental episodes of neutropenia. Our hypothesis was that true clozapine toxicity might be distinguished from benign events by carefully describing the pattern of neutrophil count changes in life-threatening cases.
Results
Case selection
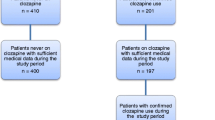
Figure 1 shows a flow diagram of the final case selection. We identified 23 events in which ANC fell to 0.5 × 109/L, 16 of these in which an ANC below 0.5 × 109/L was recorded. Seven of these sixteen cases were excluded; four because their neutrophil count returned to normal on the same day or on the next day without there being two consecutive ANCs below 1.0 × 109, one because the patient had stopped clozapine and switched to quetiapine before neutrophils began to decline, one because they were on concomitant cancer chemotherapy at the time of the event and one because we could not access any ZTAS blood test results via CRIS (and therefore had an incomplete record of ANC results). The study cohort included nine events in eight patients.
Table 1 illustrates the demographic and clinical characteristics of cases.
The temporal profiles of the absolute neutrophil counts for the nine subjects identified are depicted in the graphs appended (Figs. 2–10 (in each graph the y-axis represents neutrophil count (0–15 × 109/L) and the x-axis 38 days before and 38 days after day 0 (the day on which a count below 0.5 × 109/L was first recorded in each case)).
Case details
There were nine events in eight patients who had a sustained neutrophil count <0.5 × 109/L, stopped clozapine and either were hospitalised and/or received GCSF and/or antibiotics (Table 2). Seven of the cases had been prescribed clozapine on a previous occasion. In six of these cases clozapine was previously discontinued owing to neutropenia. None had previously had agranulocytosis (with the exception of the patient contributing two “cases” in this study): the lowest ANC recorded for any patient in previous exposures was 0.8 × 109/L. Two cases were in patients having their first trial of clozapine.
One patient had stopped clozapine previously owing to poor compliance and, upon re-starting treatment, had developed LTA. Following a further break of several months, an attempt to re-challenge with clozapine again resulted in LTA. This patient thus provides two “cases” of LTA.
Six of nine patients required transfer to an acute hospital for close monitoring. Five of these had IV antibiotics for treatment of infection and one had an antiviral plus an antifungal prescribed. Three cases remained on the psychiatric hospital premises, one was administered antibiotics but not GCSF, two had GCSF but not antibiotics. Overall, GCSF was used in seven out of nine events.
All events occurred within the first 18 weeks of starting treatment. In five of the nine events of LTA, the neutrophil count was reduced from above two to less than 0.5 × 109/L within 7 days (the blood testing interval for these patients). The mean time for neutrophils to fall from ANC > 2 to ANC <0.5 × 109/L was 8.4 days (range 2–15 days; median 7 days).
Note: regulations relating to the identification of patients on the CRIS database preclude specific reference to individual details, so as to preserve anonymity.
Discussion
We defined clozapine-associated LTA as that occurring in people stopping clozapine when two consecutive neutrophil counts were recorded as being below 0.5 × 109/L/1.0 × 109/L and where there was evidence of infection, or the prescribing of antibiotics or G-CSF. The nine cases we identified showed a consistent pattern: neurophil counts fell quickly from a normal level and remained substantially below 0.5 × 109/L for several days until natural recovery or until GCSF took effect.
A previous study examined the time course of neutrophil counts in 38 patients stopping clozapine owing to agranulocytosis (defined as ANC <0.5 × 109/L) and found that neutrophil counts fell from above 2.0 × 109/L to below 0.5 × 109/L in an average (mean) of 1 week16. The median time to obtain counts above 0.5 × 109/L on recovery was 4 days and to achieve normal counts, 10 days. All of these observations are remarkably similar to the findings in this study. Further, published individual case studies describe a near identical pattern of neutrophil count change in LTA17,18. Some authors have reported that neutrophil counts increase immediately before falling to levels representing agranulocytosis19 but this phenomenon was evident in only two, perhaps three, of our nine cases.
Clozapine-induced agranulocytosis is a rare event20 and its pathogenesis is not fully understood21. The formation of reactive clozapine metabolites may be directly toxic to neutrophils22. Studies also suggest immunomodulatory effects resulting in transient changes in neutrophil counts after treatment initiation23. Spikes in WCC and neutrophils from the first week of treatment, stabilising by week 10 have been reported20,24. Re-challenge may result in rapid recurrence and more dramatic dyscrasia25, an observation which supports an immune-mediated reaction. Genetic association with clozapine-induced agranulocytosis suggests immune mechanism involving human leucocyte antigen genes13.
Distinct mechanisms and risk factors may underlie clozapine-associated benign neutropenia and clozapine-induced agranulocytosis26. Based on incidence figures, at least two thirds of cases of neutropenia identified in people on clozapine do not “progress” to agranulocytosis. As already mentioned, these cases of neutropenia may be neither clozapine-related nor pathogenic and so may have no mechanism to speak of. In a US general population study27 the prevalence of neutropenia was 4.5% in black participants and 0.78% in white participants. These cases appear to be naturally occurring episodes of low neutrophil counts that may have no clinical significance. The repeated blood testing required for clozapine use undoubtedly increases the chances of finding cases of neutropenia, which may then be inappropriately linked to the use of clozapine. A meta-analysis of twenty studies reporting rates of neutropenia with clozapine and an active comparator, found no elevated risk for clozapine28. In a more recent study in Iceland29 (where clozapine blood monitoring is less frequent than elsewhere), the incidence of neutropenia was no greater with clozapine than with other antipsychotics and, of 34 neutropenia cases identified in people on clozapine, only one progressed to LTA.
Even cases of agranulocytosis in people on clozapine may be either benign and conicidental cases or clozapine-associated LTA. We excluded 14 patients who would normally be diagnosed as having clozapine-induced agranulocytosis (ANC ≤ 0.5 × 109/L). In most of these cases neutrophils rapidly returned to normal with no sequelae. This is not the first time that this phenomenon has been reported—in 1992 Hummer and colleagues30 described transient neutropenia occurring in 22% of patients prescribed clozapine. Two of these patients (2.9% of the total cohort) briefly recorded counts below 1.0 × 109/L. In the Icelandic study mentioned29 several patients recorded ANC results that in other countries would have forced clozapine cessation, but almost all continued without any problems, Interestingly, in the population prevalence study already cited27, neutrophil counts below 1.0 × 109/L were recorded in 0.57% of black patients and 0.11% of white participants. Another population analysis31 suggested the incidence of agranulocytosis (ANC ≤ 0.5 × 109/L) casually detected in people not receiving cancer chemotherapy or immunosuppressant drugs is 10.4/million/year for black people and 4.4/million/year for whites. Thus, there is a natural background of very low neutrophil counts, the incidence of which is fully exposed by the intensive monitoring seen with clozapine.
Our study was not designed to estimate the incidence of LTA and we have no precise figures on clozapine usage over the time period examined. An approximate estimate can be made, however. Our unit has around 1400 patients currently prescribed clozapine and each year around 250 new patients are initiated on clozapine. So, over the 14 years of this study approximately 3500 patients were started on clozapine and there were 23 recorded cases of “agranulocytosis”, giving an incidence of 0.66%—a figure within the range reported by larger and dedicated studies. Note however that the incidence of LTA (nine cases in approximately 3500 starters) was only 0.26%. This illustrates a further important aspect of our findings: that the incidence of LTA is probably substantially less than the accepted risk of agranulocytosis in people taking clozapine.
These observations have considerable importance when we consider rules relating to the use of clozpine. The UK haematological monitoring guidelines for patients on clozapine recommend a weekly ANC and WCC for the first 18 weeks, 2-weekly for weeks 19 to 52 and 4-weekly after 1 year of ongoing treatment with stable blood counts32. Clozapine treatment is discontinued if the WCC drops <3 × 109/L and/or neutrophil count <1.5 × 109/L, which is termed a “red” result and treatment must be stopped. For patients with benign ethnic neutropenia (BEN) this threshold is lower by 0.5, and treatment is discontinued when WCC drops <2.5 × 109/L or ANC <1 × 109/L. Following two “red” results the patient is classed as non-re-challengeable32 and may not receive clozapine again. In the US the threshold for clozapine treatment interruption was reduced to <1 × 109/L in 2015. For patients with BEN this threshold was reduced to ANC <0.5 × 109/L33. The US changes simplify clozapine monitoring and enable more patients to initiate and continue treatment without endangering their health34. A lower threshold of 0.5 × 109/L also is the most cost-effective method of blood monitoring with clozapine35, in the absence of genetic testing. Alignment of the UK guidelines with the revised US Food and Drug Administration monitoring guidelines would have a similar positive impact on improving the use of clozapine without compromising safety12,36. Notably, nonetheless, although sequential falls in ANC may be reported to prescribers, both US and UK regulations classify clozapine-induced blood dyscrasia somewhat crudely according to predefined threshold counts, rather than the identification of a pathological pattern of decline in neutrophils that clearly indicates clozapine as the cause.
As already stated, it is highly likely that standard monitoring thresholds overestimate the proportion of patients who have true clozapine-related LTA. Indeed, it has been shown that the majority of those on the UK non-rechallenge database can be safely re-prescribed clozapine12. A substantial fraction of apparent clozapine-related neutropenia (and even agranulocytosis) is actually a manifestation of benign neutropenia, in which successful rechallenge is possible37.
There are two major consequences to this overdiagnosis. First, many people are unnecessarily precluded from receiving clozapine because of an assumed clozapine-related blood toxicity. Second, genetic association studies are compromised by the inclusion of cases of neutropenia and agranulocytosis that are neither related to clozapine nor of clinical consequence. The highest sensitivity value ever obtained for a genetic test for clozapine-related agranulocytosis is 54%14. However, all studies so far conducted defined agranulocytosis as any measurement of ANC ≤ 0.5 × 109/L and so inevitably included in their study samples a proportion of people with a nominal and inconsequential agranulocytosis. Higher sensitivity values are unlikely to be attained without refining case-selection in line with the findings presented here.
Our results and other cases reported in the literature indicate that clozapine-associated LTA is an all-or-nothing event—a rapidly emerging absence or near absence of circulating neutrophils caused by their almost total chemical or immunological destruction or by the disabling of all neutrophil production by some means (the biological half-life of neutrophils in the blood is 7–9 h38). LTA associated with clozapine has a distinct pattern, which involves a precipitous and continuous fall in neutrophil counts to zero or near zero and their slow recovery after several days, with or without GCSF. The speed at which LTA develops is such that monitoring at intervals longer than 1 or 2 weeks is unlikely to help identify cases. Indeed, evidence is now emerging that longer-than-normal monitoring intervals employed during the COVID-19 pandemic may not have led to adverse consequences39. We further contend that single episodes of reductions in neutrophil counts—even at or those below 0.5 × 109/L officially registering as agranulocytosis—are highly unlikely to be clozapine-related or life-threatening. Likewise, it does not seem likely that clozapine causes benign, non-progressive neutropenia. These episodes can be seen as being an artefact of the intensive blood monitoring programme, which exposes naturally occurring low neutrophils counts which are likely to be seen in any population.
We conclude that clozapine-related blood toxicity should be identified on the basis of the temporal pattern of change of neutrophil counts, not by the attainment of an arbitrary threshold neutrophil count. The value of blood monitoring at intervals of four weeks should be reconsidered because of the low probability of detecting true clozapine-related agranulocytosis (which develops over a period of 2 weeks or less).
Methods
We used the Clinical Record Interactive Search (CRIS) system at the South London and the South London and Maudsley NHS Foundation Trust Biomedical Research Centre (BRC) to extract clinical data. CRIS is a research database that draws anonymised data from the electronic health records of patients at the South London and the Maudsley NHS Foundation Trust (SLAM), a mental health service that serves over 1.3 million people in four London Boroughs40. This research repository allows real-time searches of anonymised free text and structured data of SLAM electronic health records from 2007 onwards. The search results are returned as spreadsheets and exported as CSV files for subsequent analysis. There is data linkage between CRIS and the Zaponex Clozapine Monitoring System (ZTAS) used in SLAM, enabling access to blood test results from this register40,41.
We searched for, and assessed for inclusion, data on all patients who discontinued clozapine owing to a recorded neutrophil count of 0.5 × 109/L or lower (the usual definition of agranulocytosis) over the time period 2007 to the end of 2020.
Selection of LTA cases
We began by conducting an automated search which returned all clozapine patients with a neutrophil event ≤0.5 × 109/L on CRIS. We then excluded all events where patients had an ANC exactly equal to 0.5 × 109/L, and whose count did not fall below this threshold throughout the monitoring period. Further information on signs of infection (e.g., fever/raised CRP), use of GCS-F, transfer to acute hospital, administration of antibiotics during the event and advice sought from a haematologist were extracted by manual search of the anonymised case notes on CRIS. We defined as time zero (t = 0) the day that the neutrophil count was first found to be below 0.5 × 109/L. Under the UK clozapine monitoring rules, patients must discontinue clozapine when their neutrophil count falls below 1.5 × 109/L on two consecutive days.
The LTA inclusion criteria were:
-
A recorded neutrophil count of less than 0.5 × 109/L in patients who stopped clozapine because of this low count.
-
Signs of infection (raised temperature and/or raised CRP) and/or admitted to general hospital for the treatment of infection and/or prescribed antibiotics and/or G-CSF administered for the management of agranulocytosis.
Included patients must have recorded at least two consecutive ANC of less than 1.0 × 109/L (i.e., the first ANC <0.5 × 109/L (as above), and the next ANC <1.0 × 109/L). In this way we excluded those with transient low counts. We also excluded patients on clozapine and concomitant cancer chemotherapy.
Data extraction
Neutrophil counts before and after t = 0 were extracted from laboratory results and from a manual search of the anonymised free text entries on CRIS. Demographic information on gender, ethnicity, diagnosis was also collected from CRIS. The clozapine starting date, the patient’s age at the time of the event (t = 0), concomitant medication, comorbidities, smoking status, frequency of blood count monitoring, as well as whether clozapine treatment was prescribed under the specific protocol Benign Ethnic Neutropenia, were extracted using a structured search. We identified whether the patient had a history of prior clozapine prescription and if clozapine had previously been stopped as a result of clozapine-related neutropenia or agranulocytosis.
Data analysis
The mean, standard deviation (SD) and median values of variables were calculated for descriptive statistics. We used Excel Version 2107 to categorise the data and plot temporal profiles of each subject’s ANC for visualisation.
Data availability
Anonymised data are available from the corresponding author.
References
Crilly, J. The history of clozapine and its emergence in the US market: a review and analysis. Hist. Psychiatry 18, 39–60 (2007).
Idanpaan-Heikkila, J., Alhava, E., Olkinuora, M. & Palva, I. Letter: Clozapine and agranulocytosis. Lancet 2, 611 (1975).
Griffith, R. W. & Saameli, K. Letter: Clozapine and agranulocytosis. Lancet 2, 657 (1975).
Idanpaan-Heikkila, J., Alhava, E., Olkinuora, M. & Palva, I. P. Agranulocytosis during treatment with chlozapine. Eur. J. Clin. Pharmacol. 11, 193–198 (1977).
Veys, P. A., Wilkes, S., Shah, S., Noyelle, R. & Hoffbrand, A. V. Clinical experience of clozapine-induced neutropenia in the UK. Laboratory investigation using liquid culture systems and immunofluorocytometry. Drug Saf. 7, 26–32 (1992).
Toyoda, K. et al. A descriptive study of 10-year clozapine use from the nationwide database in Japan. Psychiatry Res. 297, 113764 (2021).
Pons, A., Undurraga, J., Batalla, A. & Bernardo, M. Clozapine and agranulocitosis in Spain: do we have a safer population? A 5-year hematologic follow-up. Revista de psiquiatria y salud mental 5, 37–42 (2012).
Tunsirimas, N., Pariwatcharakul, P., Choovanichvong, S. & Ratta-Apha, W. Clozapine-induced agranulocytosis and leukopenia: incidence, associated factors, and rate of hematologic adverse-effects monitoring in psychiatric out-patient services in Thailand. Asian J. Psychiatry 41, 13–16 (2019).
Alvir, J. M., Lieberman, J. A., Safferman, A. Z., Schwimmer, J. L. & Schaaf, J. A. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N. Engl. J. Med. 329, 162–167 (1993).
Meltzer, H. Y. Clozapine: balancing safety with superior antipsychotic efficacy. Clin. Schizophr. Relat. Psychoses 6, 134–144 (2012).
Bachmann, C. J. et al. International trends in clozapine use: a study in 17 countries. Acta Psychiatr. Scand. 136, 37–51 (2017).
Oloyede, E. et al. There is life after the UK Clozapine central non-rechallenge database. Schizophr. Bull. https://doi.org/10.1093/schbul/sbab006 (2021).
Legge, S. E. & Walters, J. T. Genetics of clozapine-associated neutropenia: recent advances, challenges and future perspective. Pharmacogenomics 20, 279–290 (2019).
Konte, B. et al. HLA-DQB1 6672G>C (rs113332494) is associated with clozapine-induced neutropenia and agranulocytosis in individuals of European ancestry. Transl. Psychiatry 11, 214 (2021).
Saito, T. et al. Pharmacogenomic study of clozapine-induced agranulocytosis/granulocytopenia in a Japanese population. Biol. Psychiatry 80, 636–642 (2016).
Matsui, K. et al. Clozapine-induced agranulocytosis in Japan: changes in leukocyte/neutrophil counts before and after discontinuation of clozapine. Hum. Psychopharmacol. 35, e2739 (2020).
Patel, N. C., Dorson, P. G. & Bettinger, T. L. Sudden late onset of clozapine-induced agranulocytosis. Ann. Pharmacother. 36, 1012–1015 (2002).
Almaghrebi, A. H. Safety of a clozapine trial following quetiapine-induced leukopenia: a case report. Curr. Drug Saf. 14, 80–83 (2019).
Alvir, J. M., Lieberman, J. A. & Safferman, A. Z. Do white-cell count spikes predict agranulocytosis in clozapine recipients? Psychopharmacol. Bull. 31, 311–314 (1995).
Lee, J. et al. The effect of clozapine on hematological indices: a 1-year follow-up study. J. Clin. Psychopharmacol. 35, 510–516 (2015).
Mijovic, A. & MacCabe, J. H. Clozapine-induced agranulocytosis. Ann. Hematol. 99, 2477–2482 (2020).
Uetrecht, J. P. Metabolism of clozapine by neutrophils. Possible implications for clozapine-induced agranulocytosis. Drug Saf. 7, 51–56 (1992).
Roge, R., Moller, B. K., Andersen, C. R., Correll, C. U. & Nielsen, J. Immunomodulatory effects of clozapine and their clinical implications: what have we learned so far? Schizophr. Res. 140, 204–213 (2012).
Blackman, G. et al. Clozapine response in schizophrenia and hematological changes. J. Clin. Psychopharmacol. 41, 19–24 (2021).
Dunk, L. R., Annan, L. J. & Andrews, C. D. Rechallenge with clozapine following leucopenia or neutropenia during previous therapy. Br. J. Psychiatry 188, 255–263 (2006).
Manu, P., Flanagan, R. & Ronaldson, K. Life-Threatening Effects of Antipsychotic Drugs 118–122 (Elsevier, 2016).
Hsieh, M. M., Everhart, J. E., Byrd-Holt, D. D., Tisdale, J. F. & Rodgers, G. P. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann. Intern. Med. 146, 486–492 (2007).
Myles, N. et al. A meta-analysis of controlled studies comparing the association between clozapine and other antipsychotic medications and the development of neutropenia. Aust. N. Z. J. Psychiatry 53, 403–412 (2019).
Ingimarsson, O., MacCabe, J. H., Haraldsson, M., Jónsdóttir, H. & Sigurdsson, E. Neutropenia and agranulocytosis during treatment of schizophrenia with clozapine versus other antipsychotics: an observational study in Iceland. BMC Psychiatry 16, 441 (2016).
Hummer, M., Kurz, M., Barnas, C. & Fleischhacker, W. W. Transient neutropenia induced by clozapine. Psychopharmacol. Bull. 28, 287–290 (1992).
Strom, B. L., Carson, J. L., Schinnar, R., Snyder, E. S. & Shaw, M. Descriptive epidemiology of agranulocytosis. Arch. Intern. Med. 152, 1475–1480 (1992).
Leyden Delta B. V. https://www.medicines.org.uk/emc/product/10121/smpc (2020).
U.S. Food & Drug Administration. FDA Drug Safety Communication: FDA modifies monitoring for neutropenia associated with schizophrenia medicine clozapine; approves new shared REMS program for all clozapine medicines. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-modifies-monitoring-neutropenia-associated-schizophrenia-medicine (2016).
Sultan, R. S., Olfson, M., Correll, C. U. & Duncan, E. J. Evaluating the effect of the changes in FDA guidelines for clozapine monitoring. J. Clin. Psychiatry 78, e933–e939 (2017).
Ninomiya, K. et al. Cost effectiveness of pharmacogenetic-guided clozapine administration based on risk of HLA variants in Japan and the UK. Transl. Psychiatry 11, 362 (2021).
Whiskey, E. et al. Need to bleed? Clozapine haematological monitoring approaches a time for change. Int. Clin. Psychopharmacol. 34, 264–268 (2019).
Silva, E., Higgins, M., Hammer, B. & Stephenson, P. Clozapine re-challenge and initiation following neutropenia: a review and case series of 14 patients in a high-secure forensic hospital. Ther. Adv. Psychopharmacol. 11, 20451253211015070 (2021).
Hidalgo, A., Chilvers, E. R., Summers, C. & Koenderman, L. The neutrophil life cycle. Trends Immunol. 40, 584–597 (2019).
Hata, M. et al. No adverse events were observed in clozapine-treated patients on extended hematologic monitoring intervals during the coronavirus pandemic in four psychiatric centers in Japan. Neuropsychopharmacol. Rep. 41, 179–184 (2021).
Perera, G. et al. Cohort profile of the South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLaM BRC) Case Register: current status and recent enhancement of an Electronic Mental Health Record-derived data resource. BMJ Open 6, e008721 (2016).
The National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre (BRC). https://maudsleybrc.nihr.ac.uk/facilities/clinical-record-interactive-search-cris/cris-data-linkages/ (2021).
Acknowledgements
We thank Dr Sara Stuart-Smith, Haematologist, King’s College Hospital for advice on the preparation of this manuscript. The author(s) received no financial support for the research, authorship, and/or publication of this article. This research used the Clinical Record Interactive Search (CRIS) platform funded and developed by the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London and a joint infrastructure grant from Guy’s and St Thomas’ Charity and the Maudsley Charity (grant number BRC-2011–10035). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the MRC, the Department of Health and Social Care or H. Lundbeck S/A.
Author information
Authors and Affiliations
Contributions
David Taylor—originated the research, wrote, reviewed, and edited manuscript. Kalliopi Vallianatou screened records, extracted data, analysed data, wrote original draft. Eromona Whiskey and JHM reviewed and edited the manuscript. Olubanke Dzahini supervised the project, verified data, interpreted results, reviewed, and edited the manuscript. All authors were Involved in drafting the manuscript. All authors approved the final version of the manuscript for submission and agree to be accountable for the information provided in the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was performed under a pre-existing approval for CRIS as a data resource for secondary analysis by Oxford Research Ethics Committee C (reference 18/SC/0372). All data were retrieved anonymised, and no patient can be identified from the study and therefore no individual patient consent was required by our ethics approval.
Competing interests
D.T. has received speaker honoraria from Janssen, Servier, Otsuka and Lundbeck. Research funding has been received from Janssen, Lundbeck and BMS. J.H.M. has received research funding from H Lundbeck. The remaining authors do not have any potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taylor, D., Vallianatou, K., Whiskey, E. et al. Distinctive pattern of neutrophil count change in clozapine-associated, life-threatening agranulocytosis. Schizophr 8, 21 (2022). https://doi.org/10.1038/s41537-022-00232-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-022-00232-0
- Springer Nature Limited
This article is cited by
-
Valproate-related neutropenia and lithium-related leukocytosis in patients treated with clozapine: a retrospective cohort study
BMC Psychiatry (2023)
-
Mediation and longitudinal analysis to interpret the association between clozapine pharmacokinetics, pharmacogenomics, and absolute neutrophil count
Schizophrenia (2023)
-
Clozapine
Reactions Weekly (2022)