Abstract
Two-dimensional van der Waals (vdW) magnetic materials have emerged as possible candidates for future ultrathin spintronic devices, and finding a way to tune their physical properties is desirable for wider applications. Owing to the sensitivity and tunability of the physical properties to the variation of interatomic separations, this class of materials is attractive to explore under pressure. Here, we present the observation of direct to indirect band gap crossover and an insulator–metal transition in the vdW antiferromagnetic insulator CrPS4 under pressure through in-situ photoluminescence, optical absorption, and resistivity measurements. Raman spectroscopy experiments revealed no changes in the spectral feature during the band gap crossover whereas the insulator–metal transition is possibly driven by the formation of the high-pressure crystal structure. Theoretical calculations suggest that the band gap crossover is driven by the shrinkage and rearrangement of the CrS6 octahedra under pressure. Such high tunability under pressure demonstrates an interesting interplay between structural, optical and magnetic degrees of freedom in CrPS4, and provides further opportunity for the development of devices based on tunable properties of 2D vdW magnetic materials.
Similar content being viewed by others
Introduction
Ever since the successful exfoliation of stable single-layer graphene1, research on layered two-dimensional van der Waals (2D vdW) materials has attracted significant interests in the past decade. One of the current focuses is the investigation of magnetic 2D vdW materials, which is quite attractive from both a fundamental physics and practical application perspective (e.g., refs 2,3,4). In principle, the discovery of atomically thin materials with intrinsic ferromagnetism and high-magnetic ordering temperature could open up opportunities to realize spintronic devices, such as molecular quantum devices and high-density ultrathin storage devices. Indeed, the recent groundbreaking experimental observations of the intrinsic magnetism in atomically thin FePS3, CrI3, Cr2Ge2Te6, and Fe3GeTe25,6,7,8,9,10,11 have pushed this class of materials to be promising candidates for possible applications in spintronic technology, as they can serve as building blocks for magnetic vdW heterostructures.
In order to explore the wider applications of 2D vdW materials, it is necessary to tune their optical, electronic, and magnetic properties. Several attempts have been made to tune the properties of 2D vdW magnets, such as by intercalation, gating, electrostatic doping, and changing layer thickness to name a few (e.g., refs 9,12,13,14,15,16,17). Pressure or compressive strain might also be an alternative way to tune the properties of the 2D vdW magnets without introducing disorder or impurities to the system. It has been recently shown that pressure can be effectively used to modify the magnetic, electronic, and optical properties of 2D vdW magnets both in bulk and atomically thin samples18,19,20,21,22,23,24,25,26,27,28,29.
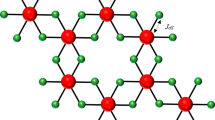
Among the 2D vdW magnetic materials, CrPS4 is currently enjoying a surge of interest30,31,32,33,34,35. CrPS4 was first synthesized in the 1970s and found to possess a monoclinic symmetry (C2/m space group)36. In the structure, puckered layers of S atoms are arranged in hexagonal close packing parallel to the a-axis, whereas the Cr atoms form a square lattice. Each Cr atom is surrounded by six S atoms forming a slightly distorted octahedron, and three CrS6 octahedra are connected by a single P atom (Fig. 1(a)). It is an antiferromagnetic semiconductor with a band gap of ~1.4 eV30,37. Previous magnetic studies suggested a C-type antiferromagnetic structure (antiferromagnetic interaction within a layer and ferromagnetic interaction between the layer) below the Néel temperature (TN) of 36 K38. However, recent neutron diffraction and torque magnetometry investigations revealed an A-type AFM structure with the intralayer ferromagnetic interaction and interlayer antiferromagnetic coupling along the c axis35. X-ray spectroscopy studies demonstrated that the Cr 3d bands are localized within the energy gap in the vicinity of the Fermi level, where the lower and higher energy of the band structure is dominated by the non-bonding S 3p band and the S 3s band, respectively39. Theoretical calculations predicted that CrPS4 is stable in the monolayer form while retaining the intrinsic magnetism and semiconducting properties40. Lee et al.30 reported thickness-dependence studies of the structural, vibrational, and optical properties of CrPS4 where they observed the disappearance of Raman peaks and weakening of the photoluminescence signal in the monolayer limit due to photodegradation of the thin sample30. Symmetry breaking was found to induce the appearance of Fano resonance in the photoluminescence spectra of CrPS4 collected below the antiferromagnetic transition34.
a Crystal structure of CrPS4 with the C2/m space group. b Room temperature photoluminescence and optical absorption spectra of CrPS4 below 1 GPa. Inset shows the deconvoluted PL spectrum fitted with two Voightian peaks. c Molecular orbital energy diagram involved in the optical transitions of CrPS4. The blue and red arrows indicate d−d transitions and the orange arrow represents a possible ligand-to-metal charge-transfer (LMCT) transition. d Configurational diagram of Cr3+ for the observed absorption (blue arrows) and PL (red arrows) spectra.
With its rich optical and electronic properties, exploring the tunability of CrPS4 by the application of pressure is beneficial, not only to improve the clear understanding of the physical properties but also to investigate the wider potential applications of this material. However, the high-pressure behavior of CrPS4 remains unexplored to date. By comparison, high-pressure investigations on the closely related systems such as TMP(S, Se)3 (TM = transition metal) in which the TM ions form a layered honeycomb lattice, have revealed Mott insulator–metal and structural transitions in MnPS318, FePS320,25,27, FePSe325, and recently Mott isostructural transition in V0.9PS326.
In this work, we examine the effects of pressure on the optical, electronic, and vibrational properties of layered CrPS4 through the use of optical absorption, photoluminescence, resistivity, and Raman spectroscopy measurements. We observed anomalous changes in the photoluminescence and optical absorption spectra on compression above ~3 GPa. Further compression leads to an insulator–metal transition, which begins above 15 GPa as evidenced by the optical absorption and resistivity data. Raman spectroscopy measurements indicates that the crystal structure is stable below 10 GPa. However, the Raman spectra significantly changes above 16 GPa, which suggests the formation of a high-pressure phase accompanying the insulator–metal transition in CrPS4. Theoretical calculations show that the anomalies observed in the photoluminescence and optical absorption data above 3 GPa are related to the direct to indirect band gap crossover, which is mainly driven by the rearrangement of the CrS6 octahedra under pressure.
Results and discussion
Optical properties under pressure
The photoluminescence (PL) and optical absorption spectra of CrPS4 at the lowest pressure of our measurements (P < 1 GPa) are presented in Fig. 1(b). Both spectra are similar to those reported previously at ambient pressure, which suggests that the optical and electronic properties of the sample are not affected by the application of pressure <1 GPa. As shown in Fig. 1(b), the sample at 0.9 GPa exhibits a broad PL peak centered at ~1.3 eV, in agreement with the PL spectrum at ambient conditions30,34. The PL spectrum consists of two Voightian components centered at at ~1.3 and ~1.37 eV, respectively (A and B components in the inset of Fig. 1(b)). Previous studies showed that the intensity of the PL peak of CrPS4 increases linearly with increasing laser power without any signs of saturation, ruling out the possible excitonic emission of the PL in CrPS434. In CrPS4 crystal, the Cr3+ ion is located in a distorted octahedral environment formed by six S atoms. In this case, the octahedral crystal field will split the 3d orbital of Cr3+ into t2g and eg orbitals with a splitting energy of Δ ~ 2.0 eV39 (Fig. 1(c)), and the electronic configuration of Cr3+ (d3 configuration, 4F term) splits into the 4A2g ground state and 4T2g and 4T1g excited states (Fig. 1(d)). The PL peak observed in CrPS4 has been previously attributed to the spin-allowed d−d transition of the Cr3+ ion30,34 (red arrows in Fig. 1(c, d)), which are often located around 1.6− 2.2 eV in the Cr-based compounds41,42.
The optical absorption spectrum exhibits three clear features (marked by T1, T2, and T3). The feature at T3 has been assigned to the ligand-to-metal charge-transfer (LMCT) transition from the 3p of the S band to the unoccupied 3d band of the Cr atom30,39 (orange arrow in Fig. 1(c)). In the lower energy region, two weaker (T1 and T2) peaks located at ~1.6 and ~1.8 eV can be observed. These two absorption peaks can be assigned to the spin-allowed d−d transitions (blue arrows in Fig. 1(c, d))30,34, which are forbidden according to the Laporte parity selection rule. Despite being forbidden, these d–d transitions can be weakly allowed due to local symmetry breaking43. A large Stokes shift between the PL peak and the T1 peak in the absorption spectra of ~300 meV is due to the Franck–Condon principle and strong electron-lattice coupling44. Both PL and absorption data, therefore, indicate that CrPS4 possesses a band gap of ~1.3 eV, which is governed by the Cr d−d transition.
In order to investigate the evolution of electronic structures under pressure, we measured in-situ PL and optical absorption data. Figure 2(a) shows the PL spectra of single crystal CrPS4 at room temperature collected up to 11.8 GPa. Under compression to 1.8 GPa, the PL A peak slightly blue-shifts to higher energy whereas the position of the B peak remains constant (Fig. 2(b) and Supplementary Fig. 3). Above 2 GPa the A peak gradually moves to lower energy and the B peak disappears while a new PL peak simultaneously appears at lower energy (marked by C in Fig. 2(a) and Supplementary Fig. 3). The A peak is no longer observable above 8 GPa while the C peak remains up to 10 GPa, above which the entire PL signal of CrPS4 disappears.
The pressure dependence of the PL peak intensity (integrated from 1.2 to 1.5 eV) is shown in Fig. 2(c). The PL peak intensity of CrPS4 continuously decreases with increasing pressure and the PL signal completely disappears above 10 GPa. A clear change in slope is observed at around 2.5 GPa, similar to the pressure where the new PL (C) peak starts to emerge. One possible explanation for the observed change in the PL spectra and intensities of CrPS4 under pressure might be the band gap crossover (direct to indirect band gap). Similar behavior in the PL spectra has been observed previously in several systems such as InP45, InSe46 and monolayer MoS247, and has been attributed to the direct-indirect gap crossover induced by pressure. However, given that the PL signal of CrPS4 arises from the parity forbidden d−d transition, which is sensitive to the CrS6 octahedra, the anomalous behavior of PL data above 3 GPa could be related to a subtle change in the crystal structure, possibly a modification of the CrS6 octahedra under pressure.
In Fig. 3(a), we present the Tauc plot ((αhν)2 versus the photon energy hν) of the low-energy region of the room temperature optical absorption data measured up to 27 GPa. Given that the strong absorption edge at ~2 eV is related to the LMCT transition, we focus our analyses on the evolution of band gap associated with the d−d (T1 and T2) transitions as described in Fig. 1 and Supplementary Fig. 4. The derived band gaps are shown in Fig. 3(b). The band gaps for the T1 and T2 transitions at 0.8 GPa are ~1.5 and ~1.6 eV, respectively. These band gaps are relatively unchanged as the pressure is increased to ~4 GPa. Interestingly, the T1 peak and its associated band edge disappears above 4 GPa, whereas the T2 peak remains (see Supplementary Fig. 5). The band gap for the T2 transition slightly increases above 5 GPa before it starts to decrease above 12 GPa. A clear absorption edge is no longer observable above 19 GPa, which suggests a pressure-induced metallization in CrPS4 above 18 GPa. The overall behavior of the band gap derived from the absorption data (Fig. 3(b)) is similar to that of the PL data. In particular the band gap of CrPS4, governed by the Cr d−d transition, seems to undergo an anomalous change above 4 GPa. The band gap slightly increases on further compression to ~12 GPa, above which it begins to narrow and finally closes above 19 GPa.
Electronic transport properties
The effects of pressure to the electronic properties and possible pressure-induced metallization of CrPS4 is further investigated by electrical transport measurements. In Fig. 4(a) we present the pressure dependence of resistivity of CrPS4 measured at ambient because the resistivity value of CrPS4 below 5 GPa was beyond the sensitivity of the equipment used. The resistivity values between 5 and 10 GPa are in the order of ~105 Ω cm, which indicates an extremely insulating sample. However, on increasing pressure, the resistivity rapidly drops from ~105 Ω cm at 10 GPa to a less than ~10−1 Ω cm above ~15 GPa. Such a huge drop in resistivity indicates that CrPS4 undergoes a pressure-induced insulator–metal transition above ~15 GPa, which is further confirmed by the temperature-dependent data shown in Fig. 4(b). The insulating behavior is recovered upon releasing pressure, which indicates a reversible insulator–metal transition in CrPS4. The corresponding conductivity data (Supplementary Fig. 6) follows a scaling behavior of \((\sigma -{\sigma }_{{\mathrm{c}}}) \sim {(P-{P}_{{\mathrm{c}}})}^{\frac{1}{\gamma }}\) with a critical exponent of γ = 3 and the critical pressure Pc of 13(1) GPa. Similar scaling behavior has been observed in the conductivity of Cr-doped V2O348 and PbCrO349.
The temperature-dependent data below 10 GPa measured from room temperature to 420 K (inset of Fig. 4(b)) reveals that the resistivity decreases as the temperature is increased, thus, confirming the insulating behavior of CrPS4 at low pressures. Similarly, resistivity data at 13.6 GPa measured down to 50 K also shows that the sample possesses semiconducting behavior where the resistivity increases as the temperature is lowered. Further compression leads to the suppression of the semiconducting behavior, and the temperature-dependence data collected above 19 GPa shows a positive slope confirming the fully metallic state of CrPS4 at high pressures, consistent with the optical absorption data. No drop in resistance nor zero resistance at low temperature was observed in the metallic state of CrPS4 thus ruling out the emergence of pressure-induced superconductivity, such as those observed in other van der Waals magnetic insulators such as FePSe325 and CrSiTe329.
High-pressure Raman spectrocopy
High-pressure Raman spectroscopy measurements were then performed to provide insights into the vibrational properties of CrPS4 under pressure. Figure 5(a) shows Raman spectra of CrPS4 measured at various pressures from 1.2 up to 32 GPa. The Raman spectrum taken at the lowest pressure of 1.2 GPa consists of 13 active Raman modes, consistent with the Raman spectrum at ambient conditions30,34. Upon compression to 10 GPa (region I), most of the Raman modes shift towards higher frequencies (hardens) except the M2 mode at around 170 cm−1, which softens with increasing pressure (Fig. 5(b) and Supplementary Fig. 7). Interestingly, the relative intensity between the M6 and M7 modes (I6/I7) anomalously increases above 5 GPa, whereas the M2 and M3 Raman modes seem to undergo a crossover above 4 GPa (Supplementary Fig. 7). Despite these changes, no significant differences were observed in the Raman spectra up to ~14 GPa.
Between 15 and 20 GPa (region II), we observed the disappearance of several Raman modes observed in region I, as well as the emergence of new Raman modes. The intensities of all Raman peaks are weakened above 15 GPa due to the fact that the sample approaches its fully metallic state. Above 20 GPa (region III), where optical absorption and resistivity data show a metallic behavior, the Raman spectra consist of three Raman modes corresponding to the high-pressure phase of CrPS4. Further compression does not lead to the appearance of new Raman modes and no changes in the Raman spectra are observed up to 30.4 GPa. Similar to the electrical transport data, the Raman spectrum returns to its original feature upon decompression to ambient pressure.
Electronic structures of CrPS4 at high pressure
To understand the change in electronic structures observed experimentally, we performed density functional theory (DFT) and DFT + dynamical mean field theory (DMFT) calculations. Owing to the lack of a detailed crystal structure under pressure, we first optimized the structure using the DFT + U method. We note that the additional phonon and Raman calculations we conducted at 0 and 10 GPa to verify the predicted crystal structure under pressure revealed no dynamical instabilities both at 0 and 10 GPa (Supplementary Fig. 8). The calculated Raman spectra are also similar to those measured experimentally (see Supplementary Fig. 8), indicating that our analysis based on the optimized structure from the ab initio calculation (shown below) is reasonable despite the absence of X-ray diffraction data.
Based on the optimized structure from the DFT + U calculations, we performed a paramagnetic (PM) DFT + DMFT calculation because the optical properties under pressure were collected at room temperature (much higher than TN ~ 38 K). The calculated band structure at T ~ 298 K are presented in Fig. 6. Owing to the strong electron correlation effect, half-filled Cr t2g orbitals split into lower Hubbard bands and upper Hubbard band, making a Mott gap (Supplementary Fig. 9). Cr eg bands are located around 2.5 eV while the occupied S p bands dominate the valence band (VB) below −1 eV. CrPS4 at ambient pressure shows a direct-band gap at the Γ point. The band gap is estimated to be around 1.5 eV, which is governed by the Hubbard bands of Cr t2g orbitals. Owing to the nearly flat conduction band (CB) along the Γ − Z direction, an indirect transition between VB at Γ point and CB at Z point cannot be ruled out. The calculated energy gap is consistent with the observation of the PL signal centered at around 1.3 eV.
a Band structures of CrPS4 at 298 K calculated at various pressures. The k-points Γ, X, A, Y, Z represent the (0, 0, 0), (0.5, 0, 0), (0.5, 0.5, 0), (0, 0.5, 0), and (0, 0, 0.5) points in the conventional Brillouin zone. b The CrS6 octahedra environment in CrPS4 and c pressure dependence of the calculated Cr–S bond lengths.
One of the main changes in electronic structure under pressure is the band dispersion in CB along the Γ−Z direction. The Z point energy of CB is downshifted compared with the Γ point due to the decrease in the interlayer distance as pressure increases. At 2 GPa, this shift of the CB slightly changes the nature of the band gap, from a direct to indirect gap between VB at the Γ point and CB at the Z point. Above 2 GPa, another noticeable change is observed where the second highest VB moves upward (see the Γ point). As a result, the VB maximum shifts from Γ to between Γ and Y points at 6 GPa so that the momentum mismatch of the indirect transition increases. This indicates that the anomalous behaviors of the PL and absoption data of CrPS4 above 3 GPa is related to the direct to indirect band gap crossover (Fig. 2).
To understand the origin of the direct-indirect crossover, we investigated the pressure dependence of the bond length of the Cr–S bonds based on the optimized crystal structure obtained from the DFT + U calculations. Owing to the crystal symmetry, there are three types of Cr–S bonds in the CrS6 octahedra as shown in Fig. 6(b). The calculated Cr–S bond lengths at ambient pressure are well matched with the available experimental results, where the Cr–S3 bond is the longest in the octahedra. Upon compression, the Cr–S3 bond length decreases much faster than the Cr–S2 bond length resulting in a bond length crossover where the Cr–S2 bond becomes the longest above 5 GPa (Fig. 6(c)). This bond length crossover agrees well with the VB maximum crossover discussed above. As the bond length difference between Cr-S2 and Cr-S3 decreases, the second highest VB originating from the Cr t2g band moves upward (Supplementary Fig. 9). Above 5 GPa where the Cr–S2 bond becomes longer than the Cr–S3 bond, the VB maximum shifts from Γ to between the Γ and Y point. This implies that the band gap crossover is driven by the shrinkage and rearrangement of the the CrS6 octahedra. We note that the agreement between the Cr–S bond length crossover from our calculations and the M2 and M3 modes crossover observed in the experimental and calculated Raman spectra above 4 GPa (Supplementary Figs. 7 and 9) suggests that the M2 and M3 modes might be related to the vibration of the Cr–S bonds in the CrS6 octahedra.
Having found the occurrence of band gap crossover in CrPS4, we then investigated the possible magnetic transformation under pressure as derived from the DFT + U (U = 2 eV) calculations. At ambient pressure, we found that the A-type AFM structure is the most energetically favorable state, consistent with previous DFT studies40 and recent neutron diffraction experiments35. Our calculations suggest that the A-type AFM structure remains stable and no signs of change in the magnetic state is predicted to occur under pressure up to 20 GPa (see Supplementary Fig. 10). This suggests that the magnetism of CrPS4 is not affected by the band gap crossover. However, further studies using susceptibility measurements under high pressure will clearly be beneficial to investigate the evolution of magnetism of CrPS4 experimentally.
Finally, the nature of the pressure-induced insulator–metal transition in CrPS4 is addressed. As shown above, the Raman spectroscopy experiments suggest a possible structural transition accompanying the insulator–metal transition that occurs above 15 GPa. However, whether the structural transformation is an isostructural transition or rather involves the formation of a high-pressure phase remains unclear. Based on the optimized structure from DFT + U calculations and assuming that the monoclinic C/2m space group is stable at high pressure, our DFT + DMFT calculation at 20 GPa shows that CrPS4 remains an insulator with a band gap of 0.7 eV. We also calculated the electronic structure at 20 GPa using smaller interaction parameters (U = 8 eV, J = 0.8 eV). However, the system is still a Mott insulator with a band gap of 0.4 eV. Our DFT + DMFT calculations indicate that the metallization based on the C/2m structure occurs at much higher pressure (P > 30 GPa) than that observed in the experiment. This implies that the insulator–metal transition in CrPS4 could be accompanied by the formation of a new high-pressure phase as inferred from the Raman spectroscopy data. While the pressure dependence of conductivity follows a mean field exponent (an indication of a Mott transition), it appears that the mechanism behind the insulator–metal transition in CrPS4 cannot be solely attributed to the bandwidth-controlled Mott transition and the formation of high-pressure phase might play an important role in the metallization process. More X-ray diffraction experiments will be carried out in the near future to determine the nature of the structural transition above 15 GPa.
The observation of a direct to indirect band gap crossover and insulator–metal transition in the layered antiferromagnetic insulator CrPS4 under pressure has been demonstrated. While the band gap crossover above 3 GPa is due to the shrinkage and rearrangement of the CrS6 octahedra without a change in the crystal symmetry, it appears that the insulator–metal transition could be accompanied by a structural phase transition above 15 GPa. These highly tunable properties highlight the strong interplay between the structural, optical, electronic and magnetic properties in CrPS4 and show great potential for device applications based on van der Waals materials.
Methods
Sample preparation and characterizations
A single crystal of CrPS4 was prepared by the chemical vapor transport method. The stoichiometry ratio of Cr, P, and S powders were mixed and put into a sealed quartz tube. The sealed tube was then put into a two-zone tube furnace and heated up for 24 h. The hot side was 730 °C while the cold side was about 680 °C, and was maintained for 7 days, then slowly cooled down to room temperature with 100 °C h−1. X-ray diffraction patterns of the single crystal and pulverized samples were collected using the Rigaku Miniflex diffractometer (Cu-Kα radiation) (Supplementary Notes). The magnetic properties were measured by the Quantum Design magnetic property measurement system (MPMS-XL5) (Supplementary Notes).
High-pressure experiments
The resistivity data at high pressure were collected in two runs. In the first run, we measured the high-pressure resistivity data with a symmetric diamond anvil cell (DAC) at ambient temperature using a Keithley 6221 current source, 2182A nanovoltmeter, and a 7001 switch system as the current source, voltmeter, and voltage/current switcher, respectively. In the second run, we measured the resistivity of CrPS4 from 300 to 2 K up to ~31 GPa in a homemade multifunctional measurement system (1.6–300 K; 0–9 T, Cryomagnetics Inc.) using a DAC made of a Be–Cu alloy. The size of the diamond culet used was 300 μm in diameter for all runs. A mixture of epoxy and Cubic boron nitride (cBN) powder was used as a sample chamber and acted as an insulating layer between the electrodes and the stainless steel gasket. The CrPS4 single crystal was cut into a square of approximately 60 μm in width and 10 μm in thickness. Soft hBN powder was used as a pressure medium. We used the van der Pauw four probe method to measure the electrical resistance using platinum (Pt) foil as electrodes. Several ruby balls were loaded into the sample chamber as pressure calibrants50.
The photoluminescence (PL) and Raman measurements were performed in a DAC at room temperature. A small piece of single crystal together with several ruby balls, were placed in a stainless steel gasket with a hole of 120 μm diameter. For the measurement of the Raman and PL emission spectra, a Renishaw in Via Raman system with a laser of 532 nm wavelength was used as an excitation source. High-pressure ultra-visible absorption measurements were performed by a UV–Vis Absorption and Transmission Spectrometer System in a DAC with type-II diamonds up to 25 GPa. A mixture of methanol–ethanol (4:1 volume ratio) or silicone oil was used as a pressure transmitting medium.
Theoretical calculations
To study the correlated electronic structure of CrPS4, we employed the embedded DMFT combined with DFT (DFT + DMFT) as implemented in the WIEN2k + eDMFT package51. The WIEN2k calculations were performed using the PBE-GGA functional52 with 1000 k-points. The correlation effect of Cr 3d orbitals was treated by a DMFT loop on the top of an effective one-electron Hamiltonian generated from the WIEN2k calculation. A hybridization energy window from –10 to 10 eV with respect to the Fermi level was chosen, and the Coulomb interaction of U = 10 eV and Hund’s coupling of J = 1 eV were used for the Cr d orbitals. The local impurity problem was solved by using continuous time quantum Monte Carlo (CTQMC)53. For the structural optimization at different pressure, we used the Vienna ab initio package (VASP)54, where the plane-wave cut off was set to 500 eV. The DFT-D3 functional was used to describe the van der Waals (vdW) interaction55.
Data availability
All relevant data are available from the authors upon reasonable request.
References
Novoselov, K. S. et al. Two-dimensional atomic crystals. Proc. Natl Acad. Sci. USA 102, 10451–10453 (2005).
Park, J.-G. Opportunities and challenges of 2D magnetic van der Waals materials: magnetic graphene? J. Phys. Condens. Matter 28, 301001 (2016).
Samarth, N. Magnetism in flatland. Nature 546, 216–217 (2017).
Burch, K. S., Mandrus, D. & Park, J.-G. Magnetism in two-dimensional van der Waals materials. Nature 563, 47–52 (2018).
Lee, J. U. et al. Ising-type magnetic ordering in atomically thin FePS3. Nano Lett. 16, 7433–7438 (2016).
Gong, C. et al. Discovery of intrinsic ferromagnetism in two-dimensional van der Waals crystals. Nature 546, 265–269 (2017).
Huang, B. et al. Layer-dependent ferromagnetism in a van der Waals crystal down to the monolayer limit. Nature 546, 270–273 (2017).
Fei, Z. et al. Two-dimensional itinerant ferromagnetism in atomically thin Fe3GeTe2. Nat. Mater. 17, 778–782 (2018).
Deng, Y. et al. Gate-tunable room-temperature ferromagnetism in two-dimensional Fe3GeTe2. Nature 563, 94–99 (2018).
Kim, K. et al. Large anomalous Hall current induced by topological nodal lines in a ferromagnetic van der waals semimetal. Nat. Mater. 17, 794–799 (2018).
Seo, J. et al. Nearly room temperature ferromagnetism in a magnetic-metal-rich van der Waals metal. Sci. Adv. 6, eaay8912 (2020).
Léaustic, A. et al. High-Tc magnets in a series of substituted pyridinium FePS3 layered intercalates. Chem. Mater. 8, 1954–1961 (1996).
Evans, J. S. O. et al. Origins of the spontaneous magnetization in MnPS3 intercalates: a magnetic susceptibility and powder neutron diffraction study. Adv. Mater. 7, 735–739 (1995).
Jiang, S. et al. Controlling magnetism in 2D CrI3 by electrostatic doping. Nat. Nanotech. 13, 549–553 (2018).
Wang, Z. et al. Electric-field control of magnetism in a few-layered van der Waals ferromagnetic semiconductor. Nat. Nanotech. 13, 554–559 (2018).
Ciarrocchi, A., Avsar, A., Ovchinnikov, D. & Kis, A. Thickness-modulated metal-to-semiconductor transformation in a transition metal dichalcogenide. Nat. Commun. 9, 919 (2018).
Avsar, A. et al. Defect induced, layer-modulated magnetism in ultrathin metallic PtSe2. Nat. Nanotechnol. 14, 674–678 (2019).
Wang, Y. et al. Pressure-driven cooperative spin-crossover, large-volume collapse, and semiconductor-to-metal transition in Manganese(II) honeycomb lattices. J. Am. Chem. Soc. 138, 15751–15757 (2016).
Lin, Z. et al. Pressure-induced spin reorientation transition in layered ferromagnetic insulator Cr2Ge2Te6. Phys. Rev. Mater. 2, 051004 (2018).
Haines, C. R. S. et al. Pressure-induced electronic and structural phase evolution in the van der Waals compound FePS3. Phys. Rev. Lett. 121, 266801 (2018).
Li, T. et al. Pressure-controlled interlayer magnetism in atomically thin CrI3. Nat. Mater. 18, 1303–1308 (2019).
Song, T. et al. Switching 2D magnetic states via pressure tuning of layer stacking. Nat. Mater. 18, 1298–1302 (2019).
Mondal, S. et al. Effect of hydrostatic pressure on ferromagnetism in two-dimensional CrI3. Phys. Rev. B 99, 180407 (2019).
Wang, X. et al. Pressure-induced modification of the anomalous Hall effect in layered Fe3GeTe2. Phys. Rev. B 100, 014407 (2019).
Wang, Y. et al. Emergent superconductivity in an iron-based honeycomb lattice initiated by pressure-driven spin-crossover. Nat. Commun. 9, 1914 (2018).
Coak, M. J. et al. Isostructural Mott transition in 2D honeycomb antiferromagnet V0.9PS3. NPJ Quant. Mater. 4, 38 (2019).
Coak, M. J. et al. Tuning dimensionality in van-der-Waals antiferromagnetic Mott insulators TMPS3. J. Phys.: Condens. Matter 32, 124003 (2020).
Ahmad, A. S. et al. Pressure-driven switching of magnetism in layered CrCl3. Preprint at https://arxiv.org/abs/1911.03846 (2019).
Cai, W. et al. Pressure-induced superconductivity and structural transition in ferromagnetic Cr2Si2Te6. Preprint at https://arxiv.org/abs/1912.05166 (2019).
Lee, J. et al. Structural and optical properties of single- and few-layer magnetic semiconductor CrPS4. ACS Nano 11, 10935–10944 (2017).
Lee, M. J. et al. Synaptic devices based on two-dimensional layered single-crystal Chromium Thiophosphate (CrPS4). NPG Asia Mater. 10, 23–30 (2018).
Kim, S. et al. Crossover between photochemical and photothermal oxidations of atomically thin magnetic semiconductor CrPS4. Nano Lett. 19, 4043–4051 (2019).
Wu, H. & Chen, H. Probing the properties of lattice vibrations and surface electronic states in magnetic semiconductor CrPS4. RSC Adv. 9, 30655–30658 (2019).
Gu, P. et al. Photoluminescent quantum interference in a van der Waals magnet preserved by symmetry breaking. ACS Nano 14, 1003–1010 (2020).
Peng, Y. et al. Magnetic structure and metamagnetic transitions in the van der Waals antiferromagnet CrPS4. Adv. Mater. https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.202001200 (2020).
Diehl, R. & Carpentier, D. The crystal structure of chromium thiophosphate, CrPS4. Acta Crystallogr. Sect. B 33, 1399–1404 (1977).
Louisy, A., Ouvrard, G., Schleich, D. M. & Brec, R. Physical properties and lithium intercalates of CrPS4. Solid State Commun. 28, 61–66 (1978).
Pei, Q. L. et al. Spin dynamics, electronic, and thermal transport properties of two-dimensional CrPS4 single crystal. J. Appl. Phys. 119, 043902 (2016).
Ohno, Y., Mineo, A. & Matsubara, I. I. Reflection electron-energy-loss spectroscopy, X-ray-absorption spectroscopy, and X-ray photoelectron spectroscopy studies of a new type of layer compound CrPS4. Phys. Rev. B 40, 10262–10272 (1989).
Zhuang, H. L. & Zhou, J. Density functional theory study of bulk and single-layer magnetic semiconductor CrPS4. Phys. Rev. B 94, 195307 (2016).
Wood, D. L., Ferguson, J., Knox, K. & Dillon, J. F. Crystalfield spectra of d3,7 ions. iii. spectrum of Cr3+ in various octahedral crystal fields. J. Chem. Phys. 39, 890–898 (1963).
Nosenzo, L., Samoggia, G. & Pollini, I. Effect of magnetic ordering on the optical properties of transition-metal halides: NiCl2, NiBr2, CrCl3, and CrBr3. Phys. Rev. B 29, 3607–3616 (1984).
Seyler, K. L. et al. Ligand-field helical luminescence in a 2D ferromagnetic insulator. Nat. Phys. 14, 277–281 (2017).
Henderson, B. & Imbusch, G. F. Optical Spectroscopy of Inorganic Solids. (Clarendon Press, Oxford, 1989).
Lee, C.-J. et al. Observation of pressure-induced direct-to-indirect band gap transition in InP nanocrystals. J. Chem. Phys. 113, 2016 (2000).
Manjón, F. J. et al. Band structure of indium selenide investigated by intrinsic photoluminescence under high pressure. Phys. Rev. B 70, 125201 (2004).
Fu, L. et al. K-λ crossover transition in the conduction band of monolayer MoS2 under hydrostatic pressure. Sci. Adv. 3, e1700162 (2017).
Limelette, P. et al. Universality and critical behavior at the Mott transition. Science 302, 89–92 (2003).
Wang, S. et al. Unusual Mott transition in multiferroic PbCrO3. Proc. Natl Acad. Sci. USA 112, 15320–15325 (2015).
Mao, H. K., Xu, J. & Bell, P. M. Calibration of the ruby pressure gauge to 800 kbar under quasihydrostatic conditions. J. Geophys. Res. 91, 4673–4676 (1986).
Haule, K., Yee, C.-H. & Kim, K. Dynamical mean-field theory within the full-potential methods: Electronic structure of CeIrIn5, CeCoIn5, and CeRhIn5. Phys. Rev. B 81, 195107 (2010).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 78, 1396 (1997).
Haule, K. Quantum Monte Carlo impurity solver for cluster dynamical mean-field theory and electronic structure calculations with adjustable cluster base. Phys. Rev. B 75, 155113 (2007).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Acknowledgements
We acknowledge support from the National Natural Science Foundation of China (NSFC) under grant numbers U1530402, 11774015, and 11811530001. This work was supported by the U.S. DOE-BES, Division of Materials Science and Engineering, under Contract No. DE-SC0012704 (BNL materials synthesis). H.L.D. acknowledges the support from the Opening Project of State Key Laboratory of High Performance Ceramics and Superfine Microstructure (Project No. SKL201802SIC.). We thank Saqib Rahman and Nana Li for their help with resistivity measurements, and Yanping Yang for her assistance with EDS measurements. We thank Freyja O’Toole for her assistance with the language editing.
Author information
Authors and Affiliations
Contributions
R.A.S., B.G.Y., and B.C. designed and conceived the project. Q.D. and C.P. synthesized the sample and carried out basic characterizations. R.A.S., J.J.F., and H.L.D. carried out resistivity measurements. Z.Y. performed the UV–Vis optical absorption experiments. R.A.S. and M.Z.Y. performed photoluminescence and Raman spectroscopy experiments. B.G.J., J.H.S., and D.Y.K. performed theoretical calculations. R.A.S., B.G.J, and J.J.F. analyzed the data and wrote the paper with the contributions from other co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Susilo, R.A., Jang, B.G., Feng, J. et al. Band gap crossover and insulator–metal transition in the compressed layered CrPS4. npj Quantum Mater. 5, 58 (2020). https://doi.org/10.1038/s41535-020-00261-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41535-020-00261-x
- Springer Nature Limited
This article is cited by
-
Insulator–metal transition in CrSiTe3 triggered by structural distortion under pressure
npj 2D Materials and Applications (2023)