Abstract
Postural abnormalities are common disabling motor complications affecting patients with Parkinson’s disease (PD). We proposed a summary index for postural abnormalities (IPA) based on Kinect depth camera and explored the clinical value of this indicator. Seventy individuals with PD and thirty age-matched healthy controls (HCs) were enrolled. All participants were tested using a Kinect-based system with IPA automatically obtained by algorithms. Significant correlations were detected between IPA and the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) total score (rs = 0.369, p = 0.002), MDS-UPDRS-III total score (rs = 0.431, p < 0.001), MDS-UPDRS-III 3.13 score (rs = 0.573, p < 0.001), MDS-UPDRS-III-bradykinesia score (rs = 0.311, p = 0.010), the 39-item Parkinson’s Disease Questionnaire (PDQ-39) (rs = 0.272, p = 0.0027) and the Berg Balance Scale (BBS) score (rs = −0.350, p = 0.006). The optimal cut-off value of IPA for distinguishing PD from HCs was 12.96 with a sensitivity of 97.14%, specificity of 100.00%, area under the curve (AUC) of 0.999 (0.997–1.002, p < 0.001), and adjusted AUC of 0.998 (0.993–1.000, p < 0.001). The optimal cut-off value of IPA for distinguishing between PD with and without postural abnormalities was 20.14 with a sensitivity, specificity, AUC and adjusted AUC of 77.78%, 73.53%, 0.817 (0.720–0.914, p < 0.001), and 0.783 (0.631–0.900, p < 0.001), respectively. IPA was significantly correlated to the clinical manifestations of PD patients, and could reflect the global severity of postural abnormalities in PD with important value in distinguishing PD from HCs and distinguishing PD with postural abnormalities from those without.
Similar content being viewed by others
Introduction
Postural abnormalities are disabling motor complications affecting patients with Parkinson’s disease (PD) and become increasingly severe as the disease progresses1,2,3. The most recognized type of postural abnormalities in PD patients is the classic stooped posture, with flexion of the hips and knees, and rounding of the shoulders, which differs them from general population2,4. Moreover, an important subset of patients present with more severe abnormalities of spinal alignment including sagittal abnormalities: camptocormia and anterocollis5,6; frontal abnormalities: Pisa syndrome and scoliosis2,7. Some patients even suffer from a combination of several types of postural abnormalities. Since postural abnormalities in PD subjects usually develop insidiously over months to years before they become obvious8,9, early recognition of them facilitates the diagnosis of the disease, as well as the prompt intervention to avoid worse outcomes.
In clinical practice, there are various methods for evaluating abnormal posture of PD, such as clinical scale, wall goniometer, and photo-based measurement10,11,12. The most commonly used clinical scale for evaluating abnormal posture of PD is the 13th item of the third part of Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS-III 3.13). However, the 5-class ordinal scale gives only a broad classification of postures and is not suitable for a more detailed description because of its insensitivity to small changes which may be clinically relevant10,13. In addition, simple geometric parameters, angles, are generally examined to evaluate postural abnormalities in PD. Conventionally, three common methods, including total camptocormia (TCC) angle, upper camptocormia (UCC) angle, and lower camptocormia (LCC) angle are used to assess the severity of camptocormia6,12,14. Similarly, the drop head angle (DHA) characterizes the severity of anterocollis while the Pisa angle or the lateral trunk bending (LTB) angle serves to assess PD patients with LTB like Pisa syndrome and scoliosis4,15,16,17. Though the wall goniometer method and photo-based measurement method can provide accurate and quantitative measurement of the above angles, they only reflect the severity of postural abnormalities of a certain plane and do not offer a global assessment of postural orientation quality of the patients4,12,15,16,18,19. The global and quantitative assessment of posture is urgently required to monitor the progress of the disease and measure treatment effects.
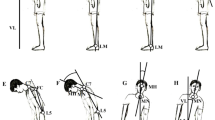
In the last decade, three-dimensional (3D) stereophotogrammetry including Kinect depth camera has been widely used to provide objective information about main joint motions on the three planes of movement20,21,22. To further facilitate the interpretation of these large amount of kinematic data, indices such as Gait Profile Score (GPS)23,24, Trunk Profile Score (TPS)25, and Arm Profile Score (APS)26 have been proposed and show promising clinical values. Inspired by these attempts, we recently developed an intelligent evaluation system to assess postural abnormalities in PD based on Kinect and machine learning27. The automated and accurate assessment of postural abnormalities for each PD patient was realized with only six selected features F1, F2, F3, F4, F5, and F7 (Fig. 1). Their feature importance in the constructed decision tree model was 13.2%, 12.6%, 16.5%, 11.3%, 6.7%, and 40% severally27. Actually, F2, F4, and F5 here shared the same definitions of LTB, TCC, and LCC angles, respectively, which reflected the severity of trunk abnormality12,16. F1 and F3 reflected the severity of head and neck abnormality similar to DHA4,16. F7 was a normalized feature which offered a general assessment in patients with both lower and upper camptocormia27. In this study, we proposed a summary index, the index for postural abnormalities (IPA), which was a combination of all these features (F1, F2, F3, F4, F5, and F7). We explored the correlations between IPA and other clinical manifestations of PD patients and discriminated between participants with different severity of postural abnormalities. In our hypothesis, the IPA is a useful method to assess the clinical severity of postural abnormalities in PD globally with important value in distinguishing PD from healthy general population and distinguishing between PD with and without postural abnormalities.
F1: lateral flexion angle of head, the angle between the connecting line of MH and C7 on the coronal plane and VL (a); F2: lateral flexion angle of trunk, the angle between the connecting line of C7 and L5 on the coronal plane and VL (a); F3: forward flexion angle of head, the angle between the connecting line of MH and MN on the sagittal plane and VL (b); F4: total forward flexion angle of trunk, the angle between the connecting line of L5 and LM and the connecting line of C7 and L5 on the sagittal plane (b); F5: forward flexion angle of trunk at the waist, the angle between the connecting line of L5 and LM and the connecting line of FC and L5 on the sagittal plane (c); F7: F7 was normalized as “D1/D2” with “%” as unit, to eliminate the effect of anthropometrical difference. D1 and D2 referred to the distance between FC and the connecting line of C7 and L5, and distance between C7 and L5 on the sagittal plane, respectively (c). MH the midpoint of head, MN the midpoint of neck, VL the vertical line of the ground, C7 the 7th cervical spinous process, L5 the 5th lumbar spinous process, LM lateral malleolus, FC vertebral fulcrum which indicates the most convex point of the vertebra.
Results
The demographic and clinical characteristics of the participants
Data were obtained from 70 PD patients and 30 HCs with a mean age of 68.0 ± 7.3 years and 66.2 ± 7.8 years (p > 0.05), respectively. Among the PD with postural abnormalities (PwPA) group, 23 patients had sagittal abnormalities, 9 patients had frontal abnormalities, and 4 patients had both planes of abnormalities. Compared with the PD without postural abnormalities (PwtPA) group, the PwPA group had significantly longer disease duration (5.9 ± 3.6 vs 4.0 ± 3.7 years, p = 0.010), significantly higher mean scores on the Hoehn-Yahr scale (2.4 ± 0.7 vs 1.9 ± 0.9, p = 0.005), IPA (24.8 ± 7.3 vs 18.3 ± 3.5, p < 0.001), MDS-UPDRS-Total (70.8 ± 28.4 vs 52.6 ± 31.2, p = 0.005), MDS-UPDRS-III (43.6 ± 18.0 vs 30.9 ± 18.1, p = 0.002), MDS-UPDRS-III B (18.9 ± 7.6 vs 14.6 ± 9.4, p = 0.041), PSQI (9.2 ± 4.8 vs 6.2 ± 4.6, p = 0.013), and lower mean score on BBS (48.6 ± 10.0 vs 51.4 ± 9.7, p = 0.006). However, there was no significant difference in gender, age at admission, onset age, body mass index (BMI), first symptom (tremor or rigidity), MDS-UPDRS-III T, MDS-UPDRS-III G, MMSE, NMSS, CSI, HADS, and PDQ-39 between the two groups (p > 0.05). Compared with HCs, the PD patients had significantly higher mean value of IPA (21.6 ± 6.6 vs 8.0 ± 1.6, p < 0.001) and a higher ratio of male(M)/female(F) (48/22 vs 13/17, p = 0.018). The overall magnitude of F1, F2, F3, F4, F5, and F7 were also presented, which were distributed in a step-like manner from low to high among the HC, PwtPA, and PwPA groups (Table 1). The demographic and clinical characteristics of the participants are shown in Table 1 and Fig. 2.
Comparisons of IPA between PD and HCs (a) and comparisons of MDS-UPDRS-Total, MDS-UPDRS-III, MDS-UPDRS-III B, PSQI and BBS characteristics between groups of PwtPA and PwPA (b–f). Data are mean with error bars representing standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001. PD Parkinson’s Disease, PwtPA PD without postural abnormalities, PwPA PD with postural abnormalities, HCs healthy controls, IPA the index for postural abnormalities, MDS-UPDRS the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale, PSQI the Pittsburgh Sleep Quality Index, BBS the Berg Balance Scale.
The correlation between clinical measurements and IPA
We examined the correlations between the clinical measurements and IPA via Spearman correlation analysis. As shown in Fig. 3, IPA was positively associated with MDS-UPDRS-Total (rs = 0.369, p = 0.002), MDS-UPDRS-III (rs = 0.431, p < 0.001), MDS-UPDRS-III B (rs = 0.311, p = 0.002), MDS-UPDRS-III 3.13 (rs = 0.573, p < 0.001), and PSQI score (rs = 0.272, p = 0.027), but negatively with BBS score (rs = −0.350, p = 0.006). We further explored and identified significant correlations between IPA and other sub-items of MDS-UPDRS-III (rs = 0.241–0.426, p = 0.049–<0.001, Table 2).
Spearman’s correlation analysis revealed significant correlations between MDS-UPDRS-Total, MDS-UPDRS-III, MDS-UPDRS-III B, MDS-UPDRS-III 3,13, PDQ-39 and BBS scores and IPA in all PD patients (a–f). IPA the index for postural abnormalities, MDS-UPDRS the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale, PDQ-39 the 39-item Parkinson’s Disease Questionnaire, BBS the Berg Balance Scale.
To determine the proportion of the variance in clinical measurements explained by IPA, simple linear regression models were constructed with clinical measurements significantly correlated to IPA as dependent variables, and IPA as predictor. Linear regression models revealed that IPA contributed significantly to MDS-UPDRS total score (adjusted R2 = 0.056, p = 0.029), MDS-UPDRS-III total score (adjusted R2 = 0.108, p = 0.003), MDS UPDRS-III 3.3 score (adjusted R2 = 0.046, p = 0.045), 3.7 score (adjusted R2 = 0.099, p = 0.006), 3.8 score (adjusted R2 = 0.055, p = 0.031) and 3.13 score (adjusted R2 = 0.351, p < 0.001), and PDQ-39 score (adjusted R2 = 0.079, p = 0.013), respectively (Table 3).
ROC analysis to identify the optimal cut-off value of IPA
The ROC curve for the IPA level to distinguish PD from HCs and PwPA from PwtPA is presented in Fig. 4. The optimal cut-off value of the IPA for distinguishing PD from HCs was 12.96, with sensitivity, specificity, AUC, and AUC adjusted for gender of 97.14%, 100.00%, 0.999 (0.997–1.002, p < 0.001), and 0.998 (0.993–1.000, p < 0.001), respectively (Fig. 4a, b). The optimal cut-off value of IPA for distinguishing PwPA and PwtPA was 20.14 with sensitivity, specificity, AUC, and AUC adjusted for disease duration and Hoehn-Yahr scale of 77.78%, 73.53%, 0.817 (0.720–0.914, p < 0.001), and 0.783 (0.631–0.900, p < 0.001), respectively (Fig. 4c, d).
The ROC curves for distinguishing PD from HCs adjusted (b) and not adjusted for gender (a). The ROC curves for distinguishing PwPA from PwtPA adjusted (d) and not adjusted for disease duration and H-Y scale (c). PD Parkinson’s Disease, PwtPA PD without postural abnormalities, PwPA PD with postural abnormalities, HCs healthy controls, IPA the index for postural abnormalities, H-Y scale Hoehn-Yahr scale.
Discussion
In this paper, a summary index, the IPA was introduced for quantifying the postural abnormalities of PD patients and comparing them with HCs. This IPA was obtained based on objective kinematic data derived from Kinect depth camera and it had the merit to summarize the overall quality of an individual’s trunk segment alignment during quiet standing. Notably, the IPA showed important value in distinguishing PD from HCs as well as distinguishing PD with postural abnormalities from those without. In a word, IPA is a useful synthetic index for evaluating postural abnormalities in PD.
Specifically, in this study, the IPA performed surprisingly well in distinguishing PD from HCs with an AUC of 0.999 (0.997–1.002, p < 0.001) and adjusted AUC of 0.998 (0.993–1.000, p < 0.001). Meanwhile, it provided moderate to high accuracy in distinguishing PwPA from PwtPA with an AUC of 0.817 (0.720–0.914, p < 0.001) and adjusted AUC of 0.783 (0.631–0.900, p < 0.001). Some researchers have been trying to distinguish PD from healthy individuals or to distinguish PD of different severity stages with the help of kinematic data derived from patients’ gait, posture, and fine movements of the limbs22,28,29,30. Mirelman et al collected multiple gait characteristics obtained with multiple wearable sensors to classify PD motor stages using machine learning methods and they found discriminatory values between motor disease stages with mean sensitivity in the range 72–83%, specificity 69–80%, and AUC 0.76–0.9031. A study used normalized stride length (SL) and gait velocity (GV) for recognizing PD using Microsoft Kinect and achieved a high accuracy rate of 97.2%. Though the sample size was relatively small with 18 PD patients and 18 HCs, it suggested the potential use of Microsoft Kinect image and depth sensors for these applications29. In this study, we focused on assessment of the global quality of postures and proposed the novel summary index of IPA. Our finding indicated that IPA has important value in distinguishing PD from healthy general population and distinguishing between PD with and without postural abnormalities, which may be helpful in early recognition of PD and early intervention of postural abnormalities for patients.
Previous studies have reported the characteristics of postural abnormalities in PD. For instance, Margraf et al. compared TCC, UCC, and Pisa angles of 192 PD patients and 78 HCs with the free NeuroPostureApp© (http://www.neuroimaging.uni-kiel.de/NeuroPostureApp)19. They found that PD patients had a worse posture than HCs in all three angles (p < 0.001). For the TCC angle, 39.1% of the patients had a normal posture (<17.4°), 47.9% a presumed stooped posture (>17.4°, <30.2°), and 6.3% had camptocormia (>30.2°). A large comparative study with general population (GPP) revealed that the dropped head angle (DHA), anterior flexion angle (AFA), and lateral flexion angle (LFA) of the thoracolumbar spine were 21.70 ± 14.40°, 13.13 ± 10.79°, and 5.98 ± 12.67° for PD patients and −3.82 ± 4.04°, 0.86 ± 4.25°, and 1.33 ± 2.16° for age-matched GPPs, respectively4. Our study disclosed significantly higher values of feature F1, F2, F3, F4, F5, and F7 in PD compared to HCs which was consistent with that study. However, all these features alone only reflect the severity of postural abnormalities of a certain plane. To summarize the global quality of an individual’s body segment alignment during quiet standing, another study introduced the Postural Profile Score Index (PPS)20. Twelve joint angles of trunk and of lower limbs, considered representative of the whole-body posture were acquired. The root mean square difference between them and those of the unaffected participants (the Postural Variable Score, PVS) were computed. Then, the PPS was calculated as a combination of the selected PVSs. The authors found significant difference in PPS between PD and HCs (8.59° vs. 6.11°, p < 0.001) but did not reveal any correlation of PPS with respect to UPDRS-III or Hoehn-Yahr scale, nor did they propose the cut-off value to distinguish PD from HCs20. In this study, the IPA was a combination of the selected features (Fig. 1) and the corresponding feature importance. Therefore, it would be an ideal candidate index for well evaluating and reflecting the overall progress of postural abnormalities in PD from the very beginning.
Moreover, significant weak to moderate correlations were detected between IPA and clinical measurements such as many composite scores of MDS-UPDRS, PDQ-39 score and BBS score (shown in Table 2). Results of linear regression models further confirmed that IPA significantly contributed to MDS-UPDRS total score, MDS-UPDRS-III total score, MDS UPDRS-III 3.3, 3.7, 3.8, and 3.13 score, and PDQ-39 score. Among them, MDS UPDRS-III 3.13 score was mostly explained by the IPA (adjusted R2 = 0.351, p < 0.001), indicating IPA is effective in judging postural abnormalities of PD patients. The MDS-UPDRS together with its composite scores is the most commonly used clinical scale for PD and has been recognized as a reliable and valuable tool to assess the severity of PD13,32,33,34,35. The PDQ-39 can provide a summary score of the impact of the illness on functioning and well-being and will be useful in the evaluation of the overall effect of different treatments36,37,38. The BBS has been validated to be a valuable screening tool and ongoing assessment tool for patients with PD39,40. Results from an observational study of 283 PD patients with ≥5° of forward trunk bending (FTB), lateral trunk bending (LTB), or forward neck bending (FNB) revealed that degree of trunk bending was associated only with motor impairment in LTB (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.03–1.22). ROC curves showed that patients with LTB of 10.5° might have moderate/severe motor impairment16. Another two studies demonstrated that camptocormia, antecollis, and Pisa syndrome were associated with severe impairment of neck and back functions, as well as pain in PD patients41,42. In our study, weak to moderate correlations were identified between IPA with common PD clinical scales, which indicates that IPA can reflect the clinical severity of postural abnormalities in PD to some extent.
The present study has some limitations. First, the sample enrolled was only composed by a total of 100 participants and it could not be representative of the general population. Second, we did not further verify the results of the study by carrying out prospective researches. In the future, more studies with a much larger sample size and well-controlled homogeneity of participants will be necessary in order to validate this index as a reliable tool in PD patients’ evaluations.
Despite these noted limitations, there are several highlights of this study. First of all, we proposed cut-off values to distinguish PD from HCs and PD with postural abnormalities from those without via global assessment of postures. Moreover, the IPA was obtained based on objective kinematic features derived from Kinect depth camera and computer algorithm which was accurate and repeatable30,43. In addition, we adopted various statistical analysis methods to explore correlations between clinical manifestations and IPA which guaranteed the clinical value of IPA.
This study proposed the IPA, a summary index aiding in interpreting the complex and highly interdependent kinematic data, to quantitatively grade the global quality of postural abnormalities in PD. It performed as an effective tool in evaluating the clinical severity of postural abnormalities in PD, as well as distinguishing PD from HCs and PD with postural abnormalities from those without.
Methods
Participants
Consecutive patients diagnosed with PD in Tongji Hospital Affiliated to Tongji University from October 2018 to January 2020 were enrolled. The inclusion criteria were: (1) Meeting the 2015 MDS clinical diagnostic criteria for PD44; (2) Being able to stand and walk by oneself for 2 min. The exclusion criteria were: (1) Being suspected or diagnosed with Parkinson’s superimposed syndrome or secondary Parkinson’s syndrome; (2) Patients with deformities or injuries that could affect posture; (3) Patients with marked cognitive impairment (Mini-Mental State Examination ≤24). We also recruited age-matched healthy controls (HCs) of 50 to 80 years old from the patients’ relatives. Power Analysis and Sample Size Software (PASS) version 15 (NCSS, LLC, Kaysville, Utah, United States) were used for sample calculation. A sample of at least 23 from the PD group and 12 from the HC group achieves 90% power to detect a difference of 0.300 between the area under the ROC curve (AUC) under the null hypothesis of 0.500 and an AUC under the alternative hypothesis of 0.800 using a one-sided z-test at a significance level of 0.025. We tried to include as many participants as possible and a total 70 PD patients and 30 HCs were enrolled in the end.
All participants gave a written informed consent prior to testing according to the declaration of Helsinki, and the present study was approved by the Ethics Committee of Shanghai Tongji Hospital (Grant Number, 2018-004).
Device and testing
There are many researchers assessing posture based on Kinect with satisfactory validity and reliability obtained45,46,47. In this study, a Kinect-centered motion analysis device integrating a Kinect v2.0 depth camera (RGB 1920 × 1080 pixels @30fps, depth camera 512 × 424 pixels @30fps, 4-microphone linear phased array, Microsoft) and an independent computer that ran a data capture program was developed by iFLYTEK Suzhou Research Institute27. The participants were asked to stand directly in front of the Kinect camera (at a distance of 2 meters)48 at ease for 5 s and then actively correct their abnormal posture for 5 s. After that, they were asked to turn left for 90°, relax and stand for 5 s, and then actively correct their abnormal posture for another 5 s. Close-fitting clothing were required and long hair should be tied up27,49,50. After the recording, the values of the six selected features F1, F2, F3, F4, F5, and F7 were automatically obtained by computer algorithms. The definition of these features were illustrated in Fig. 1. IPA was calculated with an equation, which was set as follows:
In other words, IPA was defined as the sum of the products of each selected feature and the corresponding feature importance27.
Demographic and clinical information such as gender, age at admission, onset age, first symptom (tremor or rigidity) and disease duration were collected. All the PD patients were assessed with the following scales: Hoehn-Yahr scale, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), the Mini-Mental State Examination (MMSE), the Non-motor Symptoms Scale (NMSS), the Pittsburgh Sleep Quality Index (PSQI), the Constipation Severity Instrument (CSI), the Hospital Anxiety and Depression Scale (HADS), the Berg Balance Scale (BBS), and the 39-item Parkinson’s Disease Questionnaire (PDQ-39). The sub-scores for tremor (items 3.15, 3.16, 3.17, and 3.18), bradykinesia (items 3.4, 3.5, 3.6, 3.7, 3.8, 3.9, and 3.14), and gait (items 3.10, 3.11) were obtained from the MDS-UPDRS-III which were referred to as MDS-UPDRS-III T, MDS-UPDRS-III B, and MDS-UPDRS-III G, respectively. All PD patients performed the assessments 30 min to 2 h after medication intake (based on the participant’s feedback when they usually experience best ON) in ON medication condition51. The PD patients were further divided into group with postural abnormality (PwPA) and group without postural abnormality (PwtPA) based on whether the MDS-UPDRS-III 3.13 item was greater than or equal to 2 points10.
Statistical analysis
Quantitative data were shown as mean ± standard deviation (SD). The normality of distribution of demographic and clinical data was initially tested using the Kolmogorov–Smirnov test. The student’s t test and the Mann–Whitney U test were used for comparison of normally and abnormally distributed data, respectively. The Chi-square test was used to evaluate the differences in categorical variables. Spearman’s correlation analysis was conducted to explore correlations between IPA and other clinical data. A correlation coefficient of 0.00–0.10 indicates negligible correlation, 0.10–0.39 indicates weak correlation, 0.40–0.69 indicates moderate correlation, 0.70–0.89 indicates strong correlation, and 0.90–1.00 indicates very strong correlation52. Linear regression was used to determine the proportion of the variance in clinical measurements explained by the IPA. Receiver operating characteristic (ROC) curves were operated to estimate the optimal IPA cut-off values which were determined by maximizing the Youden’s index on the ROC curve. An area under the curve (AUC) value <0.7 indicates a low diagnostic accuracy, 0.7–0.9 indicates moderate accuracy, and >0.9 indicates high accuracy53. Statistical analyses were performed using GraphPad Prism version 7 (Graph Pad Software Inc, San Diego, CA, USA) and Stata version 16 (StataCorp, College Station, TX, USA), and significance was set at a two-tailed p value < 0.05.
Data availability
The data that support the findings of this study and the algorithms to extract the features mentioned in the article are available from the corresponding author upon reasonable request.
References
Dona, F. et al. Changes in postural control in patients with Parkinson’s disease: a posturographic study. Physiotherapy 102, 272–279 (2016).
Doherty, K. M. et al. Postural deformities in Parkinson’s disease. Lancet Neurol. 10, 538–549 (2011).
Tinazzi, M. et al. Postural abnormalities in Parkinson’s disease: an epidemiological and clinical multicenter study. Mov. Disord. Clin. Pract. 6, 576–585 (2019).
Ando, Y. et al. Postural abnormality in Parkinson’s disease: a large comparative study with general population. Mov. Disord. Clin. Pract. 6, 213–221 (2019).
Revuelta, G. J. Anterocollis and camptocormia in parkinsonism: a current assessment. Curr. Neurol. Neurosci. Rep. 12, 386–391 (2012).
Margraf, N. et al. Clinical definition of camptocormia in Parkinson’s disease. Mov. Disord. Clin. Pract. 4, 349–357 (2017).
Barone, P., Santangelo, G., Amboni, M., Pellecchia, M. T. & Vitale, C. Pisa syndrome in Parkinson’s disease and parkinsonism: clinical features, pathophysiology, and treatment. Lancet Neurol. 15, 1063–1074 (2016).
Valentino, F. et al. Insidious onset of Pisa syndrome after rasagiline therapy in a patient with Parkinson’s disease. Neurol. Sci. 35, 1615–1617 (2014).
Mano, T. Camptocormia induced by a dopaminergic agonist. Clin. Neuropharmacol. 41, 70–72 (2018).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
Tinazzi, M. et al. Validity of the wall goniometer as a screening tool to detect postural abnormalities in Parkinson’s disease. Parkinsonism Relat. Disord. 69, 159–165 (2019).
Margraf, N. et al. Consensus for the measurement of the camptocormia angle in the standing patient. Parkinsonism Relat. Disord. 52, 1–5 (2018).
Martínez-Martín, P. et al. Parkinson’s disease severity levels and MDS-Unified Parkinson’s Disease Rating Scale. Parkinsonism Relat. Disord. 21, 50–54 (2015).
Fasano, A. et al. Diagnostic criteria for camptocormia in Parkinson’s disease: a consensus-based proposal. Parkinsonism Relat. Disord. 53, 53–57 (2018).
Tinazzi, M. et al. Pisa syndrome in Parkinson’s disease: an integrated approach from pathophysiology to management. Mov. Disord. 31, 1785–1795 (2016).
Geroin, C. et al. Does the degree of trunk bending predict patient disability, motor impairment, falls, and back pain in Parkinson’s disease? Front. Neurol. 11, 207 (2020).
Fortin, C. et al. Reliability of a quantitative clinical posture assessment tool among persons with idiopathic scoliosis. Physiotherapy 98, 64–75 (2012).
Orcioli-Silva, D. & Beretta, V. S. Applicability of the Wall Goniometer in Parkinson’s disease. Parkinsonism Relat. Disord. 69, 157–158 (2019).
Schlenstedt, C. et al. Quantitative assessment of posture in healthy controls and patients with Parkinson’s disease. Parkinsonism Relat. Disord. 76, 85–90 (2020).
Arippa, F. et al. A novel summary kinematic index for postural characterization in subjects with Parkinson’s disease. Eur. J. Phys. Rehabil. Med. 56, https://doi.org/10.23736/s1973-9087.19.05803-9 (2020).
Dranca, L. et al. Using Kinect to classify Parkinson’s disease stages related to severity of gait impairment. BMC Bioinforma. 19, 471 (2018).
Buongiorno, D. et al. A low-cost vision system based on the analysis of motor features for recognition and severity rating of Parkinson’s disease. BMC Med. Inform. Decis. Mak. 19, 243 (2019).
Baker, R. et al. The gait profile score and movement analysis profile. Gait Posture 30, 265–269 (2009).
Callais Franco do Nascimento, T. et al. Assessment of the kinematic adaptations in Parkinson’s Disease using the gait profile score: influences of trunk posture, a pilot study. Brain Sci. 11, https://doi.org/10.3390/brainsci11121605 (2021).
Heyrman, L. et al. Three-dimensional head and trunk movement characteristics during gait in children with spastic diplegia. Gait Posture 38, 770–776 (2013).
Jaspers, E. et al. The Arm Profile Score: a new summary index to assess upper limb movement pathology. Gait Posture 34, 227–233 (2011).
Zhang, Z. et al. Automated and accurate assessment for postural abnormalities in patients with Parkinson’s disease based on Kinect and machine learning. J. Neuroeng. Rehabil. 18, 169 (2021).
Eltoukhy, M. et al. Microsoft Kinect can distinguish differences in over-ground gait between older persons with and without Parkinson’s disease. Med. Eng. Phys. 44, 1–7 (2017).
Ťupa, O. et al. Motion tracking and gait feature estimation for recognising Parkinson’s disease using MS Kinect. Biomed. Eng. Online 14, 97 (2015).
Alvarez, I., Latorre, J., Aguilar, M., Pastor, P. & Llorens, R. Validity and sensitivity of instrumented postural and gait assessment using low-cost devices in Parkinson’s disease. J. Neuroeng. Rehabil. 17, 149 (2020).
Mirelman, A. et al. Detecting sensitive mobility features for Parkinson’s disease stages via machine learning. Mov. Disord. 36, 2144–2155 (2021).
Makkos, A. et al. Are the MDS-UPDRS-based composite scores clinically applicable? Mov. Disord. 33, 835–839 (2018).
Skorvanek, M. et al. Relationship between the MDS-UPDRS and quality of life: a large multicenter study of 3206 patients. Parkinsonism Relat. Disord. 52, 83–89 (2018).
Regnault, A. et al. Does the MDS-UPDRS provide the precision to assess progression in early Parkinson’s disease? Learnings from the Parkinson’s progression marker initiative cohort. J. Neurol. 266, 1927–1936 (2019).
Rodriguez-Blazquez, C. et al. The MDS-UPDRS Part II (motor experiences of daily living) resulted useful for assessment of disability in Parkinson’s disease. Parkinsonism Relat. Disord. 19, 889–893 (2013).
Jenkinson, C., Fitzpatrick, R., Peto, V., Greenhall, R. & Hyman, N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing 26, 353–357 (1997).
Neff, C., Wang, M. C. & Martel, H. Using the PDQ-39 in routine care for Parkinson’s disease. Parkinsonism Relat. Disord. 53, 105–107 (2018).
Zhang, J. L. & Chan, P. Reliability and validity of PDQ-39: a quality-of-life measure for patients with PD in China. Qual. Life Res. 21, 1217–1221 (2012).
Qutubuddin, A. A. et al. Validating the Berg Balance Scale for patients with Parkinson’s disease: a key to rehabilitation evaluation. Arch. Phys. Med. Rehabil. 86, 789–792 (2005).
King, L. A., Priest, K. C., Salarian, A., Pierce, D. & Horak, F. B. Comparing the Mini-BESTest with the Berg Balance Scale to evaluate balance disorders in Parkinson’s disease. Parkinsons Dis. 2012, 375419 (2012).
Alwardat, M. et al. The influence of postural deformities on neck function and pain in patients with Parkinson’s disease. NeuroRehabilitation 44, 79–84 (2019).
Alwardat, M. et al. The effect of postural deformities on back function and pain in patients with Parkinson’s disease. NeuroRehabilitation 44, 419–424 (2019).
Schmitz, A., Ye, M., Shapiro, R., Yang, R. & Noehren, B. Accuracy and repeatability of joint angles measured using a single camera markerless motion capture system. J. Biomech. 47, 587–591 (2014).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Clark, R. A. et al. Validity of the Microsoft Kinect for assessment of postural control. Gait Posture 36, 372–377 (2012).
Clark, R. A. et al. Reliability and concurrent validity of the Microsoft Xbox One Kinect for assessment of standing balance and postural control. Gait Posture 42, 210–213 (2015).
Xu, H., Yu, Y., Zhou, Y., Li, Y. & Du, S. Measuring accurate body parameters of dressed humans with large-scale motion using a Kinect sensor. Sensors 13, 11362–11384 (2013).
Ferraris, C. et al. Feasibility of home-based automated assessment of postural instability and lower limb impairments in Parkinson’s disease. Sensors 19, https://doi.org/10.3390/s19051129 (2019).
Okada, Y. J. J. O. N. P. In-home posture evaluation and visual feedback training to improve posture with a Kinect-based system in Parkinson’s disease. J. Nov. Physiother. 4, 232 (2014).
Tan, D. et al. Automated analysis of gait and modified timed up and go using the Microsoft Kinect in people with Parkinson’s disease: associations with physical outcome measures. Med. Biol. Eng. Comput. 57, 369–377 (2019).
Warmerdam, E. et al. Arm swing responsiveness to dopaminergic medication in Parkinson’s disease depends on task complexity. NPJ Parkinsons Dis. 7, 89 (2021).
Schober, P., Boer, C. & Schwarte, L. A. Correlation coefficients: appropriate use and interpretation. Anesth. Analg. 126, 1763–1768 (2018).
Mandrekar, J. N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 5, 1315–1316 (2010).
Acknowledgements
We sincerely thank all participants for their participation in this study. This study was supported by the National Key Research and Development Program (2018YFC1314700), Sub-project of the Yangtze River Delta Regional Innovation Community Project of Shanghai Municipal Science and Technology Commission (20642430100-15002360181/001), Science and Technology Innovation Action Plan of Shanghai Municipal Science and Technology Commission (19441908000), Shanghai outstanding academic leaders’ plan of Shanghai Municipal Science and Technology Committee (20XD1403400), Clinical Technology Innovation Project of Shanghai Shenkang Hospital Development Center (SHDC12020119, SHDC12018X08).
Author information
Authors and Affiliations
Contributions
R.H.: execution of the statistical analysis, drafting the manuscript, review, and critique of manuscript preparation. T.Z. and Z.Z.: organization and execution of the research project, manuscript preparation. Z.W., A.L., X.S., Y.J., Y.G., K.P., L.L., L.P., and H.Z.: execution of the research project. Q.G.: conception and organization of the research project. L.J.: conception, design and organization of the research project, review and critique of manuscript preparation. All authors critically read and approved the final manuscript. R.H., T.Z., and Z.Z. contributed equally to this work as first authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hong, R., Zhang, T., Zhang, Z. et al. A summary index derived from Kinect to evaluate postural abnormalities severity in Parkinson’s Disease patients. npj Parkinsons Dis. 8, 96 (2022). https://doi.org/10.1038/s41531-022-00368-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-022-00368-x
- Springer Nature Limited
This article is cited by
-
Kinect-based objective assessment for early frailty identification in patients with Parkinson’s disease
Aging Clinical and Experimental Research (2023)