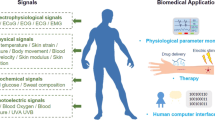
Abstract
Dental, oral, and craniofacial diseases jeopardize health and reduce the quality of life. Accessing disease-related signals in advance is beneficial to prevent the occurrence or progression of those diseases. However, the inconvenience of periodical in-hospital examinations and the difficulty of sustaining daily health monitoring challenge personal compliance and possibly lead to limited prevention or treatment. Medical flexible electronics are electric devices fabricated on soft and extensible substrates to fit the human skin and enable non-invasive continuous monitoring of biophysical/biochemical signals. They provide the possibility of long-term, continuous, comfortable, and wireless healthcare monitoring and are expected to alleviate time and economic consumption by avoiding in-hospital examinations and treatment. Therefore, flexible electronics have emerged for early diagnosis and disease monitoring in stomatology. It is noteworthy that special biophysical/biochemical characteristics and the environment of dental, oral, and craniofacial areas bring distinct challenges that flexible electronics need to address ingeniously to ensure their stability, selectivity, and sensitivity. This review summaries flexible electronics and their specificity when used in dental, oral, and craniofacial applications, including monitoring saliva or cavity-gas related biosignals, sensing the mechanical fluctuation from facial muscle/respiratory activities or orthodontic forces, and executing special functions in the prevention or postoperative recovery of relevant diseases. Furthermore, after analyzing current challenges and proposing potential solutions, the “5I” principles of imperceptibility, intelligence, individualization, integration, and inexpensiveness are presented to help guide the future development of flexible electronics and promote their commercialization for dental, oral, and craniofacial medicine.
Similar content being viewed by others
Introduction
Dental, oral, and craniofacial health is closely related to the quality of both physical and social life. Commonly, dental, oral, and craniofacial diseases include caries, periodontal disease, oral mucosal disease, malocclusion, and benign/malignant tumors in the oral/craniofacial region. Treatment of these diseases is not only time-consuming and causes inconvenience and embarrassment in daily life but also induces a great financial burden1. Given that the progression of most oral and craniofacial diseases is slow, implementing diagnosis and treatment at early pathological stages can effectively prevent exacerbation, significantly improve the prognosis and the quality of life, and also avoid potential time/economy consumption relating to treatment. However, existing diagnostic methods in stomatology mainly rely on artificial examination and imaging technologies and thus have limitations in real-time health monitoring and disease diagnosis regardless of time and place2. After sufficient treatment, monitoring therapeutic effects and recovery also challenges patient compliance due to the inconvenience and excessive costs of re-examination in the hospital. The lack of point-of-care diagnosis hinders the implementation of primary prevention to sustain dental, oral, and craniofacial health.
Medical flexible electronics are electric devices fabricated on soft and extensible substrates that fit the human skin to enable non-invasive continuous monitoring of health and even participate in disease treatment/recovery3. These miniaturized devices have the advantages of portability and wearability to realize continuous and prolonged monitoring of biophysical or biochemical signals without limitations in time and places. These benefits help to avoid interference with daily life, alleviate the economic burden, and thus endow the devices with great potential for application in point-of-care diagnosis. Currently, flexible electronics have been widely used in everyday life as well as medical practises4. In the field of stomatology, flexible electronics have also been gradually utilized for preventing the progression of dental, oral, and craniofacial diseases and judging the corresponding prognosis. After adjusting the design, construction, and material constituents according to the special biophysical/biochemical properties and environment in dental, oral, and craniofacial areas, flexible electronics can detect the levels of various factors relating to oral hygiene, orthodontic forces, and even cancer biomarkers, and thus help to make predictions of oral health status in time.
In this review, we summarize the mechanisms and applications of flexible electronics applied in teeth, oral cavity, and craniofacial regions, and analyze challenges in the current medical practices of these promising devices. Finally, we discuss the potential strategies to improve the device performance and prospect the future trend of flexible electronics in stomatology.
A brief introduction to flexible electronics and their specificity when applied in stomatology
Generally, flexible electronics are designed as wearable sensors5. In daily life, they often present in the form of wireless electronic devices coupled to wristwatches to monitor basic physiological characteristics such as heart rate and skin temperature6. With the development of micromachining, electronics, and sensors, the types of flexible electronics expand greatly. These devices can be mainly categorized into two groups according to their functions of monitoring biophysical or biochemical signals: the former examines electrophysiology, motions (e.g., feedback from prosthetics), temperature changes, intrinsic thermal, electrical, or mechanical properties of the skin, and vascular dynamics, while the latter detects metabolites and electrolytes in body fluids such as saliva, sweat, tears, and interstitial fluid7. Flexible electronics usually consist of a substrate, stretchable interconnects, and sensing elements. The substrate is required to be both flexible and elastically stretchable to ensure that it fits the human skin perfectly. The materials used for substrate fabrication also need to be durable to maintain their sufficient lifespan. Besides adopting intrinsically-specific or chemically-modified materials for fabricating the substrate and interconnection, deterministic geometries, such as serpentine, rotating square tessellations, nanocurve circuits, and wavy structures, are also utilized to enhance the device flexibility and scalability3,8,9. Conductive interconnects are fabricated on the substrate to enable electronic functions such as information transmission or electrochemical detection, mainly using micro/nano-fabrication methods to deposit or print either metals or non-metallic but conductive materials (carbon nanotubes (CNTs), graphene, conductive hydrogels, etc.). Sensors used for flexible electronics are either biosensors or electronic sensors10. Biosensors access biological activities by targeting biomolecules based on immobilized bioactive molecules onto the constructed electrodes. Electronic sensors usually adopt materials with specific electric properties to enable the detection or measurement of the targets. For example, some devices utilize piezo-elastomeric substrates to capture mechanical signals of human tissues, and some other devices use optoelectronic nanohybrids that allow photon absorption, exciton dissociation, and charge-carrier transfer and transport to achieve optical signal acquisition11,12.
Compared to devices applied to other parts of the body, flexible electronics used in dental, oral, and craniofacial areas have to address some special situations (Fig. 1):
Dental, oral, and craniofacial regions have specific features such as frequent motions, special structures, and complex compositions, which bring special challenges for flexible electronics applied here. Currently, applications of flexible electronics in stomatology focus on salivary biomarkers detection, orthodontic signals supervision, facial activities monitoring, and cavity-gas/respiration detection.
(1) Due to the frequent motions of both craniofacial muscles and the tongue during speaking, chewing, and swallowing, flexible electronics for dental, oral, and craniofacial applications are subjected to much more times of deformation and friction (e.g., against teeth and tongue) compared to other kinds of wearable devices. Therefore, they require great extensibility and durability, meanwhile, they should keep good adhesion to the craniofacial skin or the moist surface of teeth, tongue, or oral cavity. It is noteworthy that flexible electronics applied inside the oral cavity should avoid the possibility of accidental detachment, which may lead to respiratory tract block. Moreover, comfort should be considered as well, given the long-time wear and frequent facial motions in daily life.
(2) Materials for fabricating flexible electronics in dental, oral, and craniofacial applications should ensure sufficient biocompatibility. Since these devices adhere to the surface of human tissues for a long time, the substrates, as well as adhesives, need to be chemically friendly to the skin or mucosa and would not cause irritation nor immune response13,14. Especially since that oral cavity directly connects the digestive tract and flexible electronics applied here in long-term contact with various acidic/alkaline substances (from food/drink or microbial metabolism) and enzymes in saliva, materials used for device fabrication should have excellent chemical inertness or been well-encapsulated by other inert and biocompatible materials, or they should be non-toxic and edible (both the materials themselves and their degradation/abrasion products) when intaken into digestive tract15.
(3) The functions of flexible electronics should be stable enough to endure the complex environment with various pH conditions in the moist oral cavity that contains enzyme-rich saliva, food/drink residue, and abundant bacteria. The abundant bioactive molecules and bacteria in the oral cavity greatly challenge the selectivity and sensitivity for target detection and analysis.
According to these peculiarities, various strategies have been developed to ensure the performance of dental, oral, and craniofacial flexible electronics and also the health of the users, mainly through adopting specific materials for device fabrication. Hydrogels that can be attached to skin and have great self-healing ability and load-bearing performance have been excellent choices for fabricating flexible substrates in stomatology scenarios. For instance, organic resin materials such as polyethylene terephthalate (PET), polyimide (PI), and polydimethylsiloxane (PDMS) are widely used due to their excellent durability and flexibility properties. To adapt to the frequent deformation during speaking, chewing, and swallowing, some additives are mixed into the aforementioned hydrogels to increase their stretchability. Some researchers added gallium (Ga) into hydrogel fabricated from sodium alginate (SA) and polyacrylamide (PAm) and obtained good performance when executing repeated mechanical movements since that the dynamic cross-linking between hydrogel ingredients and Ga ions strengthened mechanical properties16,17. MXene is another additive that can improve the hydrogel with satisfactory structural stability and excellent deformability18,19,20. Otherwise, chitosan-rich hydrogel, CNTs, and graphene oxide (GO) nanosheets can also endow flexible electronics with great mechanical properties17,21,22,23. For fabricating electrodes, adopting Cu/PI organic-inorganic composite can help improve deformability when maintaining essential conductivity24,25. It is also necessary to design specific structures (e.g., serpentine) of the electrodes to enable the tensile strength26. In order to enhance the adhesion of flexible electronics to facial skin, silk, poly(N-isopropylacrylamide) (PNIPAM), and other materials can be used to modify hydrogels to increase the covalent and non-covalent interactions21, while some other flexible electronics used to detect physical and biological signals in the oral cavity are mainly attached to mouth guards by using medical-grade epoxy or pastes27,28,29. In addition, for moist surfaces, it has been reported that flexible devices using water-soluble tape or silk can closely attach to the tooth surface after being dissolved by saliva24,30.
For flexible electronics in dental, oral, and craniofacial applications, especially those used inside the oral cavity, the biocompatibility of materials used should be good enough when experiencing various enzymes and pH conditions of saliva. Many natural hydrogels that are edible, such as chitosan and cellulose, are adopted for device fabrication17,19. Another strategy is employing chemically inertial materials (e.g., PDMS, silk film (SF), parylene, and silicone) to embed electric materials19,31,32,33,34. When constructing the electrodes of flexible electronics, metals like Ag, Pt, and Au, and conductive nano-materials such as CNTs and MXene, which have been proven to show little toxicity under limited dosage, are employed to ensure ample biological safety19,21,22,31,35. For sensors that are commonly attached to mouthguards, aligners, or pacifiers, it is crucial to disinfect these items beforehand to ensure their sterility and biosafety29, and their constituents also mainly adopt biocompatible materials such as Prussian-blue (PB) transducer and fluorescent zinc oxide quantum dots (ZnO QDs)27,28,34,35.
In order to ensure the good working performance of dental, oral, and craniofacial flexible electronics, it is important to encapsulate their electrodes and sensors31,35,36 to resist the humid and complex environment in the oral cavity or breathing gas. For example, carbon nanofibers mats are added beneath the surface of mask-like sensors to resist humidity36. In other flexible electronics, inert or hydrophobic PDMS, poly(MPC-co-EHMA-co-MBP) (PMEHB), polyvinyl butyral (PVB), polyethylene naphthalate film, and acrylic pressure sensitive adhesive have been used for package. For instance, to minimize biofouling and interference effects from saliva constituents, poly-orthophenylenediamine (PPD) is utilized for packaging an entire oral sensor27,28, while PMEHB, silicone, Nafion solution, and PVB mainly help to minimize the interference on electrodes31,35,37,38. Additionally, cellulose acetate (CA) membrane can be specifically employed to combat various bacteria present in the mouth35.
Furthermore, it is necessary to enhance the selectivity of flexible electronics applied in complex oral environments. PPD could be embedded in those devices to enhance the performance of enzymatic, ionophore-based, or electrochemical determination of biochemical signals in saliva and oral gases28. For electrochemical analysis, the utilization of multiple capacitive channels can assist in mitigating interference among diverse analytes, thereby improving the accuracy and reliability of the measurements27. Other highly sensitive materials (e.g., MXene, PNIPAM)19,32 can be used as well. For example, the modified polyaniline (PANI) and PVB with increased porous structures coated on electrodes can enhance their ability to enrich ions, thereby boosting the sensitivity of the sensing process31. In addition, improving the electrode patterns or array shapes can also improve the sensitivity21,26.
Flexible electronics applied in oral, dental, and craniofacial scenarios
Flexible electronics have the advantages of good sensitivity, short response time, and non-invasiveness39, therefore they can be applied in the dental, oral, and craniofacial areas to realize the real-time monitoring of health state and play an important role in the prevention of disease occurrence/progression, timely feedback of therapeutic effects and finally benefit long-term oral health maintenance.
Detecting biochemical signals
Biochemical signals in the oral cavity coming from saliva or cavity gas can be used as indicators for oral health status (Table 1). Common saliva-based biomarkers include uric acid (UA), glucose, lactic acid (LA), and various electrolytes, while oral cavity gas-based biomarkers are mainly ammonia (NH3), volatile sulfide compounds (VSCs), and so on.
Saliva-based biomarkers
Metabolites, biomolecules, and a variety of ionic compounds in saliva can reflect the health status of human body40,41. Measuring levels and changes of various substances in saliva by flexible electronics can guide disease diagnosis and treatment, and is a good alternative to invasive tests such as blood tests.
Uric acid is a biomarker for a variety of diseases such as gout and hyperuricemia. Kim et al. fabricated a mouthguard biosensor to monitor UA in saliva using a well-established method by screen-printing the sensor on a flexible polyethylene terephthalate substrate (Fig. 2a)27. A working electrode, PB transducer, was made by crosslinking the uricase enzyme and electropolymerizing o-phenylenediamine. A Bluetooth low energy chipset was integrated to achieve wireless connectivity with smartwatches, cell phones, or computers. The device employed the uricase to implement high selectivity, sensitivity, and stability to measure salivary UA and avoid interference from ascorbic acid. It could respond stably to 300 μM UA, showing sufficient sensitivity when the abnormal UA value usually exceeded greater than 400 μM. Diabetes is a common cardiovascular disease whose diagnosis mostly depends on blood sugar detection (with fasting blood sugar (FBS) higher than 7 mmol L−1)42. Researches reveal that salivary glucose levels of healthy people (ranging from 0.5 to 4.8 mg dL−1) are statistically lower than diabetic patients (ranging from 1.26 to 11 mg dL−1)43,44, and the correlation of saliva glucose and blood glucose in individuals can be quantitatively determined as previously reported: FBS = 76.548 + (0.423 × saliva glucose) for healthy people, FBS = 106.35 + (0.116 × saliva glucose) for pre-diabetic patients, and FBS = 51.064 + (2.964 × saliva glucose) for diabetic patients45. Accordingly, Arakawa et al. invented a cavity sensor to measure glucose in saliva, thus avoiding conventional invasive blood glucose testing and facilitating continuous dynamic monitoring of diabetes (Fig. 2b)38. The measurement of salivary glucose was mainly implemented by detecting the concentration of hydrogen peroxide produced by the enzymatic reaction of glucose oxidase (GOD). GOD was embedded within poly(2-methacryloyloxyethyl phosphorylcholine-co-2-ethylhexyl methacrylate) (poly(MPC-co-EHMA) or PMEH) and fixed on a customized monolithic mouthguard bracket with a wireless transmitter. This device ensured the specific monitoring of salivary glucose concentrations ranging from 1 to 1000 μmol L−1, covering the concentration variation of 20–200 μmol L−1 required for medical monitoring. Afterward, they attached a CA coating to this sensor to prevent contamination from saliva (Fig. 2c)35. Kim et al. developed a mouthguard sensor based on a printable PB transducer and a PPD/lactate oxidase (LOx) reagent layer (Fig. 2d)28. This miniaturized and integrated device could be worn without professional guidance, enabling continuous noninvasive monitoring of lactate levels in a point-of-care manner for widespread scenarios. PB is highly selective for the products of LOx to detect lactate with a sensitivity of 0.1–1.0 mM. Newborns usually have difficulty in cooperating with invasive blood tests, and routine blood tests may lead to infections and even pediatric blood clots, sepsis, etc.46. Lim et al. developed a smart pacifier that continuously monitors salivary electrolytes in real-time. Several miniature ionic sensors made of fine metal wires were mounted in a microfluidic channel and inserted into a commercial pacifier. The microfluidic device was able to continuously draw saliva from the baby’s mouth to monitor sodium (5.7–9.1 mM) and potassium (4.2–5.2 mM) levels in newborns (Fig. 2e)29. This device avoided the pain and risks associated with skin pricking during traditional blood tests, enabled continuous monitoring of the salivary electrolyte status of infants, and increased the cooperation of newborns with essential diagnosis.
Biomacromolecules in saliva can indicate the physiological/pathological status of the oral cavity as well. Mannoor et al. designed graphene nanosensors coupled with silk membranes and self-assembled antimicrobial peptides onto graphene monolayers to achieve biorecognition30. This device could monitor Helicobacter pylori in the oral cavity and thus prevent gastric ulcers. Li et al. designed a flexible conducting polymer electrode that could monitor salivary steroid hormones with low concentrations to reflect the female menstrual cycle47. The sensitivity of this device could reach 11 ± 4 fM and eliminate a series of interferences in saliva such as urea, LA, glucose, and ascorbic acid.
Besides, salivary Ca2+ concentration and pH values, as a reflection of the degree of enamel demineralization, can also indicate the situation and risk of individual caries48,49. Ling et al. designed a Ca2+ and pH sensor electrode fabricated using Ca2+ ionophore II (ETH 129) and polyaniline, supplemented with a temperature sensor to reflect the effect of temperature fluctuation on electrochemistry (Fig. 2f)31. This sensor enabled the monitoring of the distribution of Ca2+ and pH values on the tooth surface. The Ca2+ sensor exhibited a linear response from 0.25 to 4 mM with a sensitivity of 30.3 mV decade−1, and the pH sensor exhibited a linear response to pH values from 4 to 8 with a sensitivity of 60.6 mV decade−1 based on electrochemical measurement. The device contained PVB to enhance stability and electrodeposited gold nanoparticles (AuNPs) on the electrodes to improve the sensing performance, whose lifetime could last more than 20 days. In addition, the monitoring of pH variation in the oral microenvironment using a dental patch was realized50. Some flexible electronics could detect ion variation caused by dental caries during orthodontic treatment51,52. Liu et al. designed an integrated multiplex sensing clear aligner system that could attach to transparent aligners37. The device could reflect the varied Ca2+, pH, and PO43− in the oral environment in a real-time manner with electrochemical sensitivity of 29.52 mV decade−1 of concentration for the Ca2+ sensor, −10.52 nA mM−1 for the PO43− sensor, and 54.38 mV pH−1 for the pH sensor, so as to prompt tooth demineralization.
a A mouthguard biosensor monitoring salivary uric acid27. b A cavity sensor measuring salivary glucose38. c A cavity sensor for glucose detection with a cellulose acetate coating35. d A lactate detection mouthguard sensor based on the LOx products-selective ability of Prussian blue28. e A smart pacifier continuously monitoring salivary electrolytes29. f A sensor monitoring the distribution of Ca2+ and pH values on the tooth surface31. Panels a–e reproduced with permission. Panel f reproduced under Creative Commons (CC BY) license.
In order to detect more than a single metabolite, Liu et al. proposed a dual-channel electrochemical biosensor for the simultaneous detection of lactose (with a sensitivity of 21.8 μA mM−1 cm−2) and glucose (with a sensitivity of 18.7 μA mM−1 cm−2) in saliva53. Since lactate is an intermediate product of glucose metabolism, simultaneous detection of dynamic trends of both lactate and glucose levels is important for preventing diabetic complications. This dual-channel biosensor was based on a flexible screen-printed PET-based electrode with two working electrodes so that the sensitivity and linear range of the two substances could be distinguished, thus avoiding interference between the two channels.
Cavity gas-based biomarkers
Detection of gases from the oral cavity, when dental caries or periodontitis occurs, is helpful for early diagnosis of these diseases. Pathogenic anaerobes potentially inducing periodontal disease and dental caries produce NH3 and VSCs when consuming food residues54,55. Jin et al. utilized a degradable bacterial cellulose/Ti3C2Tx MXene (BC/MXene) bioaerogel sandwiched between the patterned Au/PET substrates to design a multifunctional flexible sensing platform, which could detect the localized release of NH3 (Fig. 3a)19. The 3D porous structure of BC/MXene bioaerogels ensured good gas sensitivity, and this material was easy to degrade to achieve environmental protection. Experiments proved that the device could use gas signals to reveal imperceptible dental lesions. Li et al. designed a transparent, wearable fluorescent mouthguard consisting of the zinc oxide–poly(dimethylsiloxane) (ZnO-PDMS) nanocomposite to detect the local release of VSCs (Fig. 3b)34. Through observing fluorescence brightness variation, the device not only located VSCs to screen hidden dental lesion sites but also reflected the degree of the lesion. Although this research ruled out the influence of VSCs in gas and liquid states, factors such as different food residues would impact the results and the detection accuracy still needed to be improved for oral flexible electronics.
a A multifunctional flexible sensing platform detecting the localized release of NH319. b A wearable fluorescent mouthguard detecting the local release of volatile sulfur compounds34. c A CNT/SF hybrid materials combined into a facial mask to monitor respiration22. d Flexible electronics attached to teeth to achieve biorecognition30. Panels a–d reproduced with permission.
Detecting biophysical signals
Dental, oral, and craniofacial biophysical signals mainly cover mechanics, temperature, humidity, and fluid dynamics (Table 2). Appropriate stress on oral tissues is vital to maintain the health of teeth and periodontal tissues, and the mechanical movements of craniofacial muscles are related to functions such as pronunciation, facial expression, and swallowing. In addition, the dynamic temperature, humidity, and gas flow can reflect the state of breathing as well.
Muscle activities
Beyond applications for detecting the above biochemical signals, flexible electronics also have a wide range of applications for monitoring physical signals from dental, oral, and craniofacial muscle functions56. Cao et al. prepared a composite hydrogel with covalently crosslinked polyacrylamide as flexible “elastin”, Ca2+ crosslinked SA as rigid “collagen” and Ga as active conductive soft “filler”, whose properties were very similar to the human skin (Fig. 4a)16. The SA/PAm/Ga hydrogel strain sensors made from this material could monitor the subtle changes in facial muscles and generate and transmit signals through the resistance variety corresponding to different pronunciations. This device could help people with speech disorders to recover their speech function. Lee et al. designed a skin-like electrode that could reduce motion artifacts compared to rigid electrodes and assist patients suffering from dysphagia in swallowing training (Fig. 4b)24. This electrode had a significantly lower false-positive detection rate for sub-chin muscle monitoring than rigid electrodes and could distinguish swallowing from other movements. Song et al. fabricated a structurally stable and highly sensitive hollow structure of MXene-polydimethylsiloxane composites (MPCs) (Fig. 4c)18. MPCs were piezoresistive pressure sensors allowing fine measurement of the vibration of facial muscles by detecting the resistance change of compressive strain, which might help facial paralysis patients to practice more effectively in their rehabilitation exercises. Du et al. utilized functionalized adenine molecules and chitosan-grafted polyaniline copolymer (chi-g-PANI) to form conductive hydrogels to sensitively measure the activity of facial muscles for a long period. Polyaniline endowed the material with electrical conductivity, and chitosan compensated for the brittleness of PANI (Fig. 4d)17. The quaternate adenine here not only acted as an adhesive modulator by breaking the hydrogen bond between PANI and chitosan but also promoted the dispersion of the rigid polymer network to avoid the deterioration of mechanical and electrochemical properties.
a Hydrogel that could be applied for the function recovery of oral and craniofacial muscles16. b A skin-like electrode assisting swallowing training24. c MXene-PDMS composites (MPCs) based piezoresistive pressure sensors for facial expression rehabilitation18. d Conductive hydrogel that could be applied as flexible electronics for measuring facial muscles17. Panels a–d reproduced with permission.
Orthodontic forces
Traditional orthodontic treatment requires patients to make regular follow-up visits and adjust their orthodontic plan several times, which is likely to cause a decrease in patient compliance, leading to poor treatment results or even failure57. Wang et al. integrated a piezoelectric electret field generator into a personalized mechanical orthodontic aligner, which could stimulate the proliferation and differentiation of osteoclasts and osteoblasts through electrostimulation (Fig. 5a)26. This device could promote orthodontic tooth movement through a piezoelectric-excited alternating electric field for bone remodeling. Furthermore, the six-dimensional force sensors could help orthodontists refine their protocols, given that the device could keep directional tracking and precise monitoring while applying complex multiaxial stimuli. Hu et al. mimicked mortise and tenon structures to fabricate flexible sensors that could be interlocked between the upper and lower structures by concave and convex interlocking and integrated them with an orthodontic aligner (Fig. 5b)58. This device could effectively detect the magnitude and direction of orthodontic forces on the teeth to minimize potential side effects and treatment periods. Lee et al. developed a flexible hemispherical sensor that could be affixed between the tooth surface and a clear aligner (Fig. 5c)33. The thermoplastic hemispherical sensor fixed its semicircular bottom on the tooth surface, and its top side touched the clear aligner. When the aligner was stressed, the contact area between the top of the hemispherical sensor and the aligner surface changed, which could be measured by a portable microscope to quantify orthodontic forces. In order to measure the large loads of biting force and ensure the sensitivity, Lin et al. utilized a combination of multilayer ceramic capacitors (MLCCs) that had good piezoelectric properties and flexible small force sensors made of polyimide to form an MLCC force sensor (Fig. 5d)59. This device could be tightly attached to the teeth and was able to measure the force distribution on the occlusion surface or the force response during actual food chewing, which would be beneficial in aiding dental treatment.
a Piezoelectric electret field generator (PEFG) promoting orthodontic tooth movement26. b Flexible sensors for orthodontic forces detection58. c A hemispherical sensor for orthodontic forces detection33. d A multilayer ceramic capacitor (MLCC) force sensor for detecting force distribution on the occlusal surface59. Panels a, b, d reproduced with permission. Panel c reproduced under Creative Commons (CC BY) license.
Respiratory dynamics
Flexible electronics are also able to measure the flow rate of gas, which endows them with the ability to determine the respiratory status and prevent the adverse effects of mouth breathing on intraoral dentition and craniofacial development. In order to prevent mouth breathing from adversely affecting mandibular development, cranial shape, or dental occlusion60, Pang et al. designed a flexible pressure and temperature dual-mode smart mouthpiece that could continuously monitor and recognize eight different respiratory conditions, including coughing, breath-holding, mouth-normal breathing, and slow breathing, to prevent the occurrence and progression of mouth-breathing syndrome36. Using a humidity sensor to replace the monitor of airflow rate, the inaccuracy detection of respiration caused by the displacement of flexible electronics could be avoided61. Thiyagarajan et al. used multi-walled carbon nanotubes (MWCNT)/PDMS composite materials on a cellulose paper surface to detect respiration21. Porous cellulose paper was very sensitive to the moisture of the environment and changed the ionic conductivity according to the change of relative humidity during breathing. Flexible nanowires applied in masks could also realize high anti-bacterium for medical treatment. Ma et al. reconstructed the mesoscopic structures of the bombyx mori SF materials with carbon nanotubes to give them stable mesoscopic structures (Fig. 3c)22. The CNT/SF hybrid materials showed great superiorities in biocompatibility, electrical conductivity, pressure sensitivity, and humidity sensitivity, which were then combined into facial masks to monitor respiration. This device could quickly respond to the change of humidity and had long-term stability in a humid environment, laying a foundation for oral and craniofacial telemedicine. Mannoor et al. printed the resonant coil onto water-soluble silk film substrates to fabricate graphene nanosensors (Fig. 3d)30. The silk membrane dissolved rapidly when contacting with water to ensure strong binding of the graphene-Au electrode structure onto oral tissues. The sensor could monitor respiration through signal changes as well because the conductivity of graphene could change in real-time with breathing. Sim et al. investigated an Ag nanowires and GO nanosheet mixture to make a flexible and transparent conductive electrode and used this electrode to make a facial mask layer as a self-heating and antibacterial coating23. The special mask could fight against Staphylococcus aureus and Klebsiella pneumonia, with a high bacteriostatic rate of up to 99.7%.
Other applications
Besides the above applications, flexible electronics also apply to beauty, diet, and other aspects (Table 3). Li et al. designed a hyaluronic acid-deliverable stretchable electronic facial mask that could increase skin moisture content by 20% with the help of sonophoresis25. The single-side soft pressing technique made the bending curvature of the mask reach 0.04 mm−1, corresponding to a radius of 2.5 cm, so it could perfectly fit human skin with the same bending curvature. In addition, experiments showed that the maximum temperature of the device could be maintained at 42 °C, making individuals feel comfortable. In the diet aspect, flexible electronics are helpful in evaluating the relationship between dietary intake and nutrition with low burden and, therefore, are helpful for monitoring the nutritional recovery of patients after surgery. Tseng et al. designed radiofrequency-trilayer sensors to monitor oral food consumption, and the sensitivity (in vitro) could reach around 0.6 MHz in 1 g L−1 of glucose32. They used broadside-coupled, split ring resonators to sandwich sensing interlayer and encapsulated this device with hygroscopic silk film. The sandwich structure provided a short-term reservoir to average the concentration of solution over time so as to cope with the transient nature of the solution in the mouth. Zhao et al. reported a self-powered wearable taste bud-mimicking e-skin based on enzyme-modified ZnO nanowire arrays on a patterned flexible substrate62. The e-skin modified with ascorbic acid oxidase and alcohol oxidase could reflect the ascorbic acid or alcohol content in food through the piezoelectric-enzyme reaction coupling process. The e-skin was able to taste beverages or fruits, for instance, to distinguish fruits such as cherries and oranges. This device showed potential applications in treating taste-related diseases. In addition, there is research on the application of flexible temperature sensors in the postoperative monitoring of dental implants so as to respond to the subtle changes of temperature rise caused by inflammation63,64. Kim et al. designed an implantable micro-temperature sensor attached to the abutment wing of the dental implant65. The temperature measurement range could reach 20–100 °C, and it had high functional stability and biocompatibility. Although the physical stability was affected by the processing conditions, the device could still adapt to the moist conditions in the oral cavity.
Challenges and prospects
Flexible electronics need to fulfill three principles (3 S): stability, selectivity, and sensitivity66, which, however, encounter various challenges when applied in the field of stomatology. Temperature and stress are two major factors influencing the stability of flexible sensors. Complex environments or changes in external physical conditions, such as the oral cavity, which often undergo drastic temperature changes during various functional activities, can exacerbate the instability of the device material. In the continuously moist or fluidic environment of the oral cavity, biological receptors, such as enzymes in the sensors, are prone to lose their biometric functions67. Additionally, accumulated biological contaminants can also challenge the stability of sensors, leading to issues like incomplete attachment and artifacts between sensors and the target surface. Selectivity refers to the ability of a sensor to distinguish the analyte of interest from possible interferences68. Due to the complexity of the oral and craniofacial biological environment and frequent movement/deformation of the oral cavity, flexible electronics often work under conditions with a variety of chemical compounds (chemical/biological signals) or under persistent mechanical stresses (physical signals), making it difficult to realize monitoring of a specific target signal. High sensitivity is crucial for accurate monitoring of oral and craniofacial status, especially considering that the concentration of many biological indicators in oral biofluid is much lower than in blood69. Moreover, transmitting wireless signals from within the mouth across the cheeks to the exterior is highly challenging70. All these above challenges can reduce the sensitivity of flexible electronics. Flexible electronic devices also suffer from single function and delayed response time, and functional integration of the devices may be a potential solution53. Furthermore, the promotion of advanced technologies requires that flexible electronics are practical, and their future applications should cover the prevention, treatment, and monitoring of treatment effects and prognosis of oral and craniofacial diseases.
At present, in order to meet the 3 S challenges of flexible electronics for dental, oral, and craniofacial medicine, the experience of flexible electronics applied in other fields can be utilized. To overcome the challenges of stability, introducing compensating elements such as temperature sensors or using temperature-insensitive materials can be considered to avoid the effect of temperature. And the challenge of biofouling can be solved by using a protective film to prevent biological contamination. Also, encapsulating the key components, such as enzymes, into the electrodes using 3D printing helps to improve the device package and to prevent unwanted detaching70. Artificial receptors that collect target signals can be employed to improve stability as well. Compared to natural receptors like enzymes, artificial receptors are also simpler to process and thus can reduce costs71. The protective film or encapsulation can also help flexible electronics used in dental, oral, and craniofacial environments to resist moisture and corrosion. Besides, the challenges of selectivity can be solved by the efforts that have been made by several researchers: (1) For measuring chemical/biological signals, highly selective enzyme-based methods or techniques using artificial receptors are developed71,72. Moreover, it is possible to use selective sensor arrays to identify different analytes and achieve accurate analysis of the target70. (2) For measuring physical signals, designing sensor geometry to stretch in a specific direction when subjected to a force helps to achieve response and analysis of the mechanics of a single direction73, thus realizing the measurement of physical signals with a certain orientation. One possible solution to improve sensitivity is to find other highly-concentrated biomarkers that correlate with corresponding diseases5, meanwhile, improving the sensitivity of the sensor itself is also a critical issue. Using signal amplifier transistors to improve signal transduction or labeling with fluorescent proteins are reliable ways to improve sensitivity. For example, based on chemical stripping lithography, field effect transistors (FETs) with nanoscale strips could be produced, and they showed greater sensitivities than conventional methods due to the fact that these FETs had a high specific surface area to access many more targets74. To resolve signal transmission obstacles caused by cheeks, the intensity of the emitted signal or the sensitivity of the receiver for detecting radio signals can be enhanced. The improvement of the power supply capacity and antenna design, as well as the use of high-frequency signals, are also expected to benefit the signal-receiving performance75,76.
However, beyond the 3 S principles, flexible electronics have yet to be improved in terms of biosafety, price, comfort, intelligence, and so on. In our opinion, the prospect of the applications of flexible electronics in oral and craniofacial clinical practises should follow the 5I principles: imperceptibility, intelligence, individualization, integration, and inexpensiveness (Fig. 6). (1) Imperceptibility can promote the acceptance of flexible electronics in stomatology by providing high comfort and esthetics to device wearers while minimizing interference with their daily lives. To achieve comfort, imperceptibility requires flexible electronics to be soft and small enough to fit the complex biological structure and sensitive organs in oral, dental, and craniofacial regions. Otherwise, the device may irritate the oral mucosa and increase saliva secretion, potentially leading to flexible electronics shedding. For esthetics, they prefer to be invisible or disguised by applying transparent substrates and electrodes (such as metals, metal oxides, and metal carbides). (2) Intelligent, flexible electronics possess the capability to not only transmit data to associated electronic devices but also process the data, diagnose and analyze diseases in a real-time manner, and provide suggestions and emergency measures without assistance from doctors. The real-time data analysis and decision-making support not only conserves medical resources but also contributes significantly to enhancing the accuracy of diagnosis. Besides, this intelligent device should be able to detect various biomarkers concurrently or simultaneously monitor physical signals, chemical signals, and biological signals to realize multi-functional integration and even achieve comprehensive dynamic simulation and modeling of the oral cavity and craniofacial region. (3) Flexible electronics are expected to be more individualized. Given the significant variations in oral health status and dental morphology among individuals arising from genetic and habitual differences, it is crucial to design individualized flexible electronics that cater to the specific needs of each individual, encompassing esthetics, size, and comfort, among other factors. (4) Realize the integration of advanced techniques of diagnosis and treatment into flexible electronics. For instance, flexible electronics could combine drug delivery systems such as microneedle patches to automatically trigger drug release when monitoring pathological situations. (5) Additionally, identifying low-cost and high-performance materials is paramount for promoting the widespread applications of flexible electronics in dental clinics. Inexpensive, flexible electronics can alleviate the financial burden on patients, preventing them from incurring high inspection costs in hospitals.
The development of flexible electronics also needs a combination of multidiscipline technologies. For example, the lack of long-term energy supply in flexible electronics is an important reason for the lack of durability. Research has demonstrated that flexible electronics can collect the energy generated during the specific movement of the attachment site, thus providing continuous energy for wearable devices77. In addition to maintaining the self-power supply of the device, it is also possible to code the program to detect at intervals and then remain in “sleep mode” at other times, thus improving endurance. Currently, the diverse bacterial population in the oral cavity makes it possible to quickly form an acquired film that absorbs a large number of bacteria after cleaning teeth, posing a threat to oral hygiene, especially for flexible electronics attached to this area. To address this issue, it is crucial to reduce bacterial adhesion on flexible electronics or apply an antibacterial coating to the surface of device78,79,80. In addition, incorporating a self-heating electrode into the flexible electronic mask can eliminate bacteria through heating23. Moreover, according to market research, mechanical mismatches in Young’s moduli between the human body and flexible electronics can lead to poor sensing accuracy81. If flexible electronics dislocate from soft body surfaces during eating or swallowing, the artifact arises and results in inaccurate data records. Consequently, the use of conductive hydrogel with excellent adhesion as a substrate has gradually become prevalent82,83,84. Furthermore, the employment of ionic additives that enhance stretchability and electrical conductivity can lower the materials Young’s modulus85. Oral flexible electronics require attachment to mouthguards or orthodontic accessories, thereby necessitating a higher degree of miniaturization. Employing semi-solid/solid electrolytes under the technique of 3D printing and thin-film sputtering makes possibilities to construct microscale flexible electronics86. It could also develop smaller and ultrathin sensors based on flexible semiconducting materials (e.g., crystalline silicon carbide nanomembranes)87. Researchers have designed microneedle sensors for sample extraction, where the embedded electrodes or responsive materials at the microneedle tips can detect biomarkers. Integrating these microneedle sensors into a flexible patch is expected to further reduce the device size50. The size of the wireless system can be diminished by shifting from near-field or radio frequency identification-like approaches, which necessitate bulky proximal reader devices, to far-field radios capable of communicating with compact receivers that can potentially be situated at considerable distances27. When implanting flexible electronics in a moist oral environment, it can realize precise surface fit with the help of water-responsive super contractile polymer (WRAP) films88. When relative humidity exceeds 80%, WRAP films can contract instantly, achieving Young’s modulus of less than 100 kPa after contraction. Hydrogels can also be integrated with moisture-driven energy generators to provide a long-term energy supply for flexible electronics in humid environments89,90. The substrate for dental, oral, and craniofacial flexible electronics must possess sufficient flexibility and stretchability to withstand and recognize complex muscle movements. It is also a strategy to apply self-healing materials in flexible electronics for both electronic devices and substrates91,92. For example, adding materials such as SF and tannic acid to the hydrogel to enhance the chemical force, such as hydrogen bonds inside the hydrogel93, or using hydrogel that can heal itself under the stimulation of water94, can be used to improve the self-healing ability of flexible electronics.
Moreover, it is noteworthy that most of the medical flexible electronics in stomatology are currently still in the proof-of-concept stage, therefore extensive clinical studies should be conducted to advance the applications of flexible electronic devices in preclinical and clinical situations29. It is insufficient that most of the clinical trials of flexible electronics in oral and craniofacial contain no more than ten participants. At present, the results of these clinical experiments show that the measurement of flexible electronics is reliable, including the monitoring of various components and pH of saliva or oral cavity gases, which can meet the requirements of clinical pathological detection and accurately judging facial expressions or muscle movements according to the frequency of different electrical signals. However, the results of a small number of clinical investigations are inaccurate and non-universal, so it is necessary to carry out large-scale clinical experiments to verify the practicability and accuracy of flexible electronics.
Conclusion
Flexible electronics have been emerging in the clinical practices of stomatology, which greatly aids in preventing the development of dental, oral, and craniofacial diseases and in assessing disease prognosis. With their assistance, it is anticipated that individuals will be able to reformulate the management of their oral healthcare status and reduce the time/economic costs associated with treating oral diseases. Currently, flexible electronics can detect the level of a variety of substances in saliva and cavity gas and determine the status of systemic diseases as well as monitor oral bacterial conditions and pH changes to prevent caries and safeguard periodontal health. By measuring mechanical signals, flexible electronics can also be utilized to assist in the development and adjustment of orthodontic strategies, the reconstruction of taste and smell sensations, and the analysis of respiratory dynamics. Moreover, flexible electronics contribute to the monitoring of postoperative recovery levels, facilitating training in swallowing and occlusal functions.
As a result of the achievements in various technologies, flexible electronics combine multiple disciplines, such as materials science, physics, algorithm and software development, and clinical medicine, to facilitate innovative and patient-centered diagnostic, monitoring, and therapeutic approaches. They can achieve non-invasive and portable testing around the clock without interfering the life quality. These advantages assist oral patients as well as stomatologists in establishing regimes pertaining to primary prevention and treatment, assistance in rehabilitation, and improvement of prognostic tracking.
The application potentiality of flexible electronics in dental, oral, and craniofacial is magnificent. 5I principles are expected to steer the field development and facilitate the realization of this ambitious plan. In the future, flexible electronics should have the ability to adapt to the intricate environment in dental, oral, and craniofacial regions, such as dynamic temperature and pressure, humid environments, and the interference of various food residues. We believe that by fostering the utilization of flexible electronics, the prevention of oral diseases can be significantly advanced, leading to a more equitable distribution of medical resources.
Data availability
The graphical data and other findings in this paper are available from the corresponding author upon reasonable request.
Code availability
All custom codes regarded as central to the conclusions are available from the corresponding authors upon reasonable request.
References
Peres, M. A. et al. Oral diseases: a global public health challenge. Lancet 394, 249–260 (2019).
Patil, S. et al. Identification of oral immune disorders—a review and a diagnostic algorithm. Dis. Mon. 69, 101350 (2023).
Gao, Y., Yu, L., Yeo, J. C. & Lim, C. T. Flexible hybrid sensors for health monitoring: materials and mechanisms to render wearability. Adv. Mater. 32, e1902133 (2020).
Min, J., Sempionatto, J. R., Teymourian, H., Wang, J. & Gao, W. Wearable electrochemical biosensors in North America. Biosens. Bioelectron. 172, 112750 (2021).
Yang, Y. & Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 48, 1465–1491 (2019).
Wong, C. K. et al. Artificial intelligence mobile health platform for early detection of COVID-19 in quarantine subjects using a wearable biosensor: protocol for a randomised controlled trial. BMJ Open 10, e038555 (2020).
Ray, T. R. et al. Bio-integrated wearable systems: a comprehensive review. Chem. Rev. 119, 5461–5533 (2019).
Deng, Y. et al. Rotating square tessellations enabled stretchable and adaptive curved display. npj Flex. Electron. 8, 4 (2024).
Su, M. et al. Nanoparticle based curve arrays for multirecognition flexible electronics. Adv. Mater. 28, 1369–1374 (2016).
Wang, L., Chen, D., Jiang, K. & Shen, G. New insights and perspectives into biological materials for flexible electronics. Chem. Soc. Rev. 46, 6764–6815 (2017).
Sadighbayan, D., Hasanzadeh, M. & Ghafar-Zadeh, E. Biosensing based on field-effect transistors (FET): recent progress and challenges. Trends Anal. Chem. 133, 116067 (2020).
Verma, G. et al. Multiplexed gas sensor: fabrication strategies, recent progress, and challenges. ACS Sens. 8, 3320–3337 (2023).
Albrektsson, T. et al. Bone loss around oral and orthopedic implants: an immunologically based condition. Clin. Implant Dent. Relat. Res. 21, 786–795 (2019).
Miron, R. J. & Bosshardt, D. D. OsteoMacs: key players around bone biomaterials. Biomaterials 82, 1–19 (2016).
Fakhri, E. et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 162, 956–974 (2020).
Cao, Q. et al. Highly elastic, sensitive, stretchable, and skin-inspired conductive sodium alginate/polyacrylamide/gallium composite hydrogel with toughness as a flexible strain sensor. Biomacromolecules 23, 2603–2613 (2022).
Du, P., Wang, J., Hsu, Y. I. & Uyama, H. Bio-inspired homogeneous conductive hydrogel with flexibility and adhesiveness for information transmission and sign language recognition. ACS Appl. Mater. Interfaces 15, 23711–23724 (2023).
Song, D. et al. Hollow-structured MXene-PDMS composites as flexible, wearable and highly bendable sensors with wide working range. J. Colloid Interface Sci. 555, 751–758 (2019).
Jin, X. et al. Assessment of occlusal force and local gas release using degradable bacterial cellulose/Ti3C2Tx MXene bioaerogel for oral healthcare. ACS Nano 15, 18385–18393 (2021).
Li, T. et al. A skin-conformal and breathable humidity sensor for emotional mode recognition and non-contact human-machine interface. npj Flex. Electron. 8, 3 (2024).
Thiyagarajan, K., Rajini, G. K. & Maji, D. Flexible, highly sensitive paper-based screen printed MWCNT/PDMS composite breath sensor for human respiration monitoring. IEEE Sens. J. 21, 13985–13995 (2021).
Ma, L. et al. From molecular reconstruction of mesoscopic functional conductive silk fibrous materials to remote respiration monitoring. Small 16, e2000203 (2020).
Sim, H.-M. & Kim, H.-K. Highly flexible Ag nanowire network covered by a graphene oxide nanosheet for high-performance flexible electronics and anti-bacterial applications. Sci. Technol. Adv. Mater. 22, 794–807 (2021).
Lee, Y. et al. Soft electronics enabled ergonomic human-computer interaction for swallowing training. Sci. Rep. 7, 46697 (2017).
Li, S. et al. Stretchable electronic facial masks for sonophoresis. ACS Nano 16, 5961–5974 (2022).
Wang, Q. et al. Effective orthodontic tooth movement via an occlusion-activated electromechanical synergistic dental aligner. ACS Nano https://doi.org/10.1021/acsnano.3c03385 (2023).
Kim, J. et al. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 74, 1061–1068 (2015).
Kim, J. et al. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 139, 1632–1636 (2014).
Lim, H. R. et al. Smart bioelectronic pacifier for real-time continuous monitoring of salivary electrolytes. Biosens. Bioelectron. 210, 114329 (2022).
Mannoor, M. S. et al. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 3, 763 (2012).
Ling, W. et al. Continuously quantifying oral chemicals based on flexible hybrid electronics for clinical diagnosis and pathogenetic study. Research 2022, 9810129 (2022).
Tseng, P., Napier, B., Garbarini, L., Kaplan, D. L. & Omenetto, F. G. Functional, RF-trilayer sensors for tooth-mounted, wireless monitoring of the oral cavity and food consumption. Adv. Mater. 30, e1703257 (2018).
Lee, S., Lee, C., Bosio, J. A. & Melo, M. A. S. Smart flexible 3D sensor for monitoring orthodontics forces: prototype design and proof of principle experiment. Bioengineering (Basel) https://doi.org/10.3390/bioengineering9100570 (2022).
Li, X. et al. A transparent, wearable fluorescent mouthguard for high-sensitive visualization and accurate localization of hidden dental lesion sites. Adv. Mater. 32, e2000060 (2020).
Arakawa, T. et al. A wearable cellulose acetate-coated mouthguard biosensor for in vivo salivary glucose measurement. Anal. Chem. 92, 12201–12207 (2020).
Pang, Z., Zhao, Y., Luo, N., Chen, D. & Chen, M. Flexible pressure and temperature dual-mode sensor based on buckling carbon nanofibers for respiration pattern recognition. Sci. Rep. 12, 17434 (2022).
Liu, Z. et al. Integrated multiplex sensing clear aligner for in situ monitoring of dental enamel demineralization. ACS Biomater. Sci. Eng. 9, 3680–3689 (2023).
Arakawa, T. et al. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: a novel cavitas sensor. Biosens. Bioelectron. 84, 106–111 (2016).
Wang, C. et al. Advanced carbon for flexible and wearable electronics. Adv. Mater. 31, e1801072 (2019).
Zhou, Y. & Liu, Z. Saliva biomarkers in oral disease. Clin. Chim. Acta 548, 117503 (2023).
Liang, X. Y., Du, X. M. & Zhou, X. D. Latest research findings on health management based on saliva testing. Sichuan Da Xue Xue Bao Yi Xue Ban 53, 1110–1117 (2022).
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 32 S62–S67 (2009).
Pérez-Ros, P., Navarro-Flores, E., Julián-Rochina, I., Martínez-Arnau, F. M. & Cauli, O. Changes in salivary amylase and glucose in diabetes: a scoping review. Diagnostics (Basel) 11, 453 (2021).
Pullano, S. A. et al. Glucose biosensors in clinical practice: principles, limits and perspectives of currently used devices. Theranostics 12, 493–511 (2022).
Fares, S., Said, M. S. M., Ibrahim, W., Amin, T. T. & Saad, N. E. S. Accuracy of salivary glucose assessment in diagnosis of diabetes and prediabestes. Diabetes Metab. Syndr. 13, 1543–1547 (2019).
Baserga, M. C., Puri, A. & Sola, A. The use of topical nitroglycerin ointment to treat peripheral tissue ischemia secondary to arterial line complications in neonates. J. Perinatol. 22, 416–419 (2002).
Li, Z., Chen, F., Zhu, N., Zhang, L. & Xie, Z. Tip-enhanced sub-femtomolar steroid immunosensing via micropyramidal flexible conducting polymer electrodes for at-home monitoring of salivary sex hormones. ACS Nano 17, 21935–21946 (2023).
Gao, X., Jiang, S., Koh, D. & Hsu, C. Y. Salivary biomarkers for dental caries. Periodontol 2000 70, 128–141 (2016).
Bijle, M. N. A., Yiu, C. K. Y. & Ekambaram, M. Calcium-based caries preventive agents: a meta-evaluation of systematic reviews and meta-analysis. J. Evid. Based Dent. Pract. 18, 203–217.e204 (2018).
Shi, Z. et al. Wearable battery-free theranostic dental patch for wireless intraoral sensing and drug delivery. npj Flex. Electron. 6, 49 (2022).
Wierichs, R. J. et al. Masking-efficacy and caries arrestment after resin infiltration or fluoridation of initial caries lesions in adolescents during orthodontic treatment—a randomised controlled trial. J. Dent. 138, 104713 (2023).
Pinto, A. S., Alves, L. S., Maltz, M., Susin, C. & Zenkner, J. E. A. Does the duration of fixed orthodontic treatment affect caries activity among adolescents and young adults? Caries Res 52, 463–467 (2018).
Liu, M., Yang, M., Wang, M., Wang, H. & Cheng, J. A flexible dual-analyte electrochemical biosensor for salivary glucose and lactate detection. Biosens. (Basel) 12, 120 (2022).
Rosier, B. T. et al. The Importance of Nitrate Reduction for Oral. Health J. Dent. Res. 101, 887–897 (2022).
Huang, X. et al. Diversity in antagonistic interactions between commensal oral streptococci and streptococcus mutans. Caries Res 52, 88–101 (2018).
Rafeedi, T. et al. Wearable, epidermal devices for assessment of swallowing function. npj Flex. Electron. 7, 52 (2023).
Shaadouh, R. I., Hajeer, M. Y., Mahmoud, G. & Murad, R. M. T. Systematic review: is high-energy laser therapy (HELT) with flapless corticotomy effective in accelerating orthodontic tooth movement? Cureus 14, e22337 (2022).
Hu, J. et al. Flexible six-dimensional force sensor inspired by the tenon-and-mortise structure of ancient Chinese architecture for orthodontics. Nano Energy 96, 107073 (2022).
Lin, K. R., Chang, C. H., Liu, T. H., Lin, S. W. & Lin, C. H. Experimental and numerical estimations into the force distribution on an occlusal surface utilizing a flexible force sensor array. J. Biomech. 44, 1879–1884 (2011).
Surtel, A., Klepacz, R. & Wysokińska-Miszczuk, J. The influence of breathing mode on the oral cavity. Pol. Merkur Lekarski 39, 405–407 (2015).
Folke, M., Cernerud, L., Ekström, M. & Hök, B. Critical review of non-invasive respiratory monitoring in medical care. Med. Biol. Eng. Comput. 41, 377–383 (2003).
Zhao, T. et al. Self-powered gustation electronic skin for mimicking taste buds based on piezoelectric-enzymatic reaction coupling process. Nanotechnology 29, 075501 (2018).
Gassen, J., Nowak, T. J., Henderson, A. D. & Muehlenbein, M. P. Dynamics of temperature change during experimental respiratory virus challenge: relationships with symptoms, stress hormones, and inflammation. Brain Behav. Immun. 99, 157–165 (2022).
Shen, Y. et al. Reliable and remote monitoring of absolute temperature during liver inflammation via luminescence-lifetime-based nanothermometry. Adv. Mater. 34, e2107764 (2022).
Kim, J. J., Stafford, G. R., Beauchamp, C. & Kim, S. A. Development of a dental implantable temperature sensor for real-time diagnosis of infectious disease. Sensors 20, 3953 (2020).
Deng, X. et al. Dramatic responsivity enhancement through concentrated H(2) SO(4) treatment on PEDOT:PSS/TiO(2) heterojunction fibrous photodetectors. Small 17, e2101674 (2021).
Bandodkar, A. J., Jeerapan, I. & Wang, J. Wearable chemical sensors: present challenges and future prospects. ACS Sens. 1, 464–482 (2016).
Ballantine, D. S. et al. In Acoustic Wave Sensors (eds. D. S. Ballantine et al.) 222-330 (Academic Press, 1997).
Kim, J., Campbell, A. S., de Ávila, B. E.-F. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
Luo, Y. et al. Technology roadmap for flexible sensors. ACS Nano 17, 5211–5295 (2023).
Tu, J., Torrente-Rodríguez, R. M., Wang, M. & Gao, W. The era of digital health: a review of portable and wearable affinity biosensors. Adv. Funct. Mater. 30, 1906713 (2020).
Tommasone, S. et al. The challenges of glycan recognition with natural and artificial receptors. Chem. Soc. Rev. 48, 5488–5505 (2019).
Araromi, O. A. et al. Ultra-sensitive and resilient compliant strain gauges for soft machines. Nature 587, 219–224 (2020).
Zhao, C. et al. Narrower nanoribbon biosensors fabricated by chemical lift-off lithography show higher sensitivity. ACS Nano 15, 904–915 (2021).
Liu, Y. et al. Stretchable sweat-activated battery in skin-integrated electronics for continuous wireless sweat monitoring. Adv. Sci. 9, e2104635 (2022).
Glavin, N. R. et al. Flexible gallium nitride for high-performance, strainable radio-frequency devices. Adv. Mater. 29, e1701838 (2017).
Mariello, M., Qualtieri, A., Mele, G. & De Vittorio, M. Metal-free multilayer hybrid PENG based on soft electrospun/-sprayed membranes with cardanol additive for harvesting energy from surgical face masks. ACS Appl. Mater. Interfaces 13, 20606–20621 (2021).
Narciso, F. et al. 3D-printed biosurfactant-chitosan antibacterial coating for the prevention of silicone-based associated infections. Colloids Surf. B Biointerfaces 230, 113486 (2023).
Ye, Z. et al. Hybrid nanocoatings of self-assembled organic-inorganic amphiphiles for prevention of implant infections. Acta Biomater. 140, 338–349 (2022).
Xie, Y. et al. Gold nanoclusters-coated orthodontic devices can inhibit the formation of streptococcus mutans biofilm. ACS Biomater. Sci. Eng. 6, 1239–1246 (2020).
Ling, Y. et al. Disruptive, soft, wearable sensors. Adv. Mater. 32, e1904664 (2020).
Shu, L., Wang, Z., Zhang, X. F. & Yao, J. Highly conductive and anti-freezing cellulose hydrogel for flexible sensors. Int. J. Biol. Macromol. 230, 123425 (2023).
Wang, C. et al. Flexible patch with printable and antibacterial conductive hydrogel electrodes for accelerated wound healing. Biomaterials 285, 121479 (2022).
Zheng, M. et al. Skin-inspired gelatin-based flexible bio-electronic hydrogel for wound healing promotion and motion sensing. Biomaterials 276, 121026 (2021).
Wang, Y. et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 3, e1602076 (2017).
Fan, X. et al. Opportunities of flexible and portable electrochemical devices for energy storage: expanding the spotlight onto semi-solid/solid electrolytes. Chem. Rev. 122, 17155–17239 (2022).
He, R. et al. Flexible miniaturized sensor technologies for long-term physiological monitoring. npj Flex. Electron. 6, 20 (2022).
Yi, J. et al. Water-responsive supercontractile polymer films for bioelectronic interfaces. Nature 624, 295–302 (2023).
Zhao, K. et al. Humidity-tolerant moisture-driven energy generator with MXene aerogel-organohydrogel bilayer. ACS Nano 17, 5472–5485 (2023).
Zhang, Y. et al. An asymmetric hygroscopic structure for moisture-driven hygro-ionic electricity generation and storage. Adv. Mater. 34, e2201228 (2022).
Li, Z. et al. Self-healing hydrogel bioelectronics. Adv. Mater. e2306350 (2023).
Zhou, Y. et al. Self-healing polymers for electronics and energy devices. Chem. Rev. 123, 558–612 (2023).
Zheng, H., Lin, N., He, Y. & Zuo, B. Self-healing, self-adhesive silk fibroin conductive hydrogel as a flexible strain sensor. ACS Appl. Mater. Interfaces 13, 40013–40031 (2021).
Wang, Y. et al. Ultrafast self-healing, reusable, and conductive polysaccharide-based hydrogels for sensitive ionic sensors. ACS Sustain. Chem. Eng. 8, 18506–18518 (2020).
Acknowledgements
This study was supported by China Postdoctoral Science Foundation (2018M630883 and 2019T120688), Hubei Province Chinese Medicine Research Project (ZY2023Q015), Natural Science Foundation of China (61904057 and 22193051) and Hubei Province (2023AFB665 and 2018CFB124), Fundamental Research Funds for the Central Universities (Wuhan University, Clinical Medicine + X, 2042024YXB017), Medical Young Talents Program of Hubei Province and Wuhan Young Medical Talents Training Project.
Author information
Authors and Affiliations
Contributions
K.N.W.: Investigation, Methodology, Conceptualization, Writing—Original Draft, Visualization, Writing—Review & Editing. Z.Z.L.: Investigation, Methodology, Conceptualization, Writing—Original Draft, Writing—Review & Editing. Z.N.N.: Investigation, Methodology, Conceptualization, Writing—Original Draft, Writing – Review & Editing. C.L.M.: Methodology, Conceptualization, Writing—Original Draft, Writing—Review & Editing. L.B.: Supervision, Writing—Review & Editing, Project Administration. K.Z.: Investigation, Methodology, Conceptualization, Writing—Original Draft, Writing—Review & Editing. H.F.Y., B.C. & L.L.B: Conceptualization, Supervision, Project Administration, Funding Acquisition, Writing—Review & Editing. All authors have reviewed and approved the final version of this paper for publication. Each author agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the authors read and revised the manuscript. K.N.W. and Z.Z.L. contributed equally to this work. Correspondence to Fang-Yi Huo (kq001106@whu.edu.cn), Bo Cai (bcai@jhun.edu.cn) or Lin-Lin Bu (lin-lin.bu@whu.edu.cn).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, KN., Li, ZZ., Cai, ZM. et al. The applications of flexible electronics in dental, oral, and craniofacial medicine. npj Flex Electron 8, 33 (2024). https://doi.org/10.1038/s41528-024-00318-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41528-024-00318-y
- Springer Nature Limited