Abstract
Organic electrochemical transistors (OECTs) are being researched for various applications, ranging from sensors to logic gates and neuromorphic hardware. To meet the requirements of these diverse applications, the device fabrication process must be compatible with flexible and scalable digital techniques. Here, we report a direct-write additive process to fabricate fully 3D-printed OECTs, using 3D printable conducting, semiconducting, insulating, and electrolyte inks. These 3D-printed OECTs, which operate in the depletion mode, can be fabricated on flexible substrates, resulting in high mechanical and environmental stability. The 3D-printed OECTs have good dopamine biosensing capabilities (limit of detection down to 6 µM without metal gate electrodes) and show long-term (~1 h) synapse response, indicating their potential for various applications such as sensors and neuromorphic hardware. This manufacturing strategy is suitable for applications that require rapid design changes and digitally enabled direct-write techniques.
Similar content being viewed by others
Introduction
Organic electrochemical transistors (OECTs) are an enabling technology for many (bio-)electronic applications. OECTs owe their success to several compelling features such as simple device structure, tunable electronic properties to match different applications, biocompatibility, and simple manufacturing1,2,3,4. OECTs consist of a patterned organic (semi-)conductor thin film connecting the source and drain electrodes. An electrolyte is in contact with the semiconductor channel and the gate electrode. Applying a voltage to the gate (VG) induces ion injection from the electrolyte into the semiconductor channel, causing the latter to change its doping state and conductivity. This modifies the current flowing between the source and drain electrodes (drain current, ID). Contrary to standard thin-film transistors (TFTs), the doping mechanism is volumetric (i.e., occurring throughout the bulk of the channel material), leading to large ID variations even for small changes in VG. Therefore, OECTs have shown remarkably high transconductance (gm = ∂ID/∂VG), despite being typically slower than TFTs, as ions have to penetrate the entire channel layer5. Because of this, OECTs have been successfully applied in bioelectronics, exploiting their high transconductance for biosensing6,7,8,9,10 and printable electronics for low-power devices11,12. In addition, recent studies have underlined the possibility of using OECTs to emulate the functioning of biological synapses13,14,15 and neurons16,17, offering an opportunity to develop neuromorphic hardware for beyond-von-Neumann computing applications.
While many recent works have demonstrated the possibility of fabricating OECTs using a variety of additive manufacturing techniques, such as screen printing18,19, inkjet printing20,21,22, aerosol-jet printing23,24 and gel extrusion6,25,26, photolithography remains by far the most common approach for fabricating OECTs and circuits thereof, with high yields, resolution, and scalability27,28,29,30. There is, however, a growing need for rapid design changes and digitally enabled direct-write techniques to enable high throughput screening of new materials and device concepts. To this end, 3D-printing offers several advantages compared to commonly used screen printing, gravure printing, or inkjet printing, such as superior flexibility in terms of material selection, ink preparation, and pattern design/change4. 3D printing allows for the deposition of a broad spectrum of materials (from insulators to semiconductors and conductors) with a large range of viscosity31, similar to screen printing techniques. However, compared to screen printing, 3D printing allows for much higher flexibility in pattern modification since no new screens need to be produced for any changes to the pattern. In addition, compared to inkjet printing, 3D printing enables the patterning of highly viscous plastic materials with a good resolution, allowing the integration of OECTs with, for example, traditional microfluidics, thus opening new possibilities in the field of biosensing and bioelectronics in general22,32,33. Previous attempts to 3D print OECTs6,25,26,34,35,36 have also used other manufacturing techniques because of difficulties in developing suitable metallic and semiconducting inks. In addition, attempts have yet to be made to create alternatives to liquid electrolytes, which is fundamental for integrating OECT into applications that might not be limited to simple demonstrators.
Here, we propose a simple direct-write additive manufacturing approach to fabricating OECTs, based on a wet extrusion-based 3D printing system (BIOX by Cellink). We developed 3D printable functional inks for each OECT’s components (source, drain, gate electrodes, semiconductor channel, insulator, gate electrolyte, and substrate) and demonstrated fully 3D-printed OECTs. PEDOT:PSS was chosen as the OECT channel material, a benchmark mixed ion-electron conducting polymer for OECTs37,38. The fully 3D-printed OECTs show remarkable electrical and mechanical properties and can be used for biosensing and neuromorphic computing applications. As wet extrusion-based 3D printers can be used to pattern cells39,40, tissues41,42, and organs43,44, we anticipate that the possibility to fully 3D printing OECTs will pave the way for easier interfacing of electronics with biology.
Results
OECTs fabrication process overview
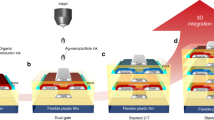
Figure 1 illustrates the OECT fabrication process and final device layout. The source, drain, and gate electrodes were 3D-printed on a microscope glass slide coated with a thin (<4 µm) Parylene layer using a blend of graphene oxide (GO) and carbon nanotubes (CNTs) (see Methods section for details about the ink formulation). The printed GO/CNT electrodes were treated with 50 wt% potassium iodide (KI) in 1 M hydrochloric acid (HCl) overnight to yield a reduced GO (rGO)45, resulting in rGO/CNT electrodes with electrical conductivity as large as 600 S cm–1 (Supplementary Fig. 1). The oxidation level of GO and rGO was measured by X-ray photoelectron spectroscopy (XPS) (see Supplementary Fig. 2a for the C1s spectra). For GO, deconvolution of the C1s spectra shows three peaks corresponding to sp2 (C=C, 284.8 eV), epoxy/hydroxyl (C–O, 286.2 eV), and carbonyl (C=O, 287.8 eV) carbon atoms46. In contrast, the C1s spectra of the rGO samples only show the presence of C=C and C–C (285.4 eV). The C/O atom ratio calculated from XPS survey spectra also increases from 1.55 for GO to 7.38 for rGO, indicative of an effective GO reduction47. Ultraviolet photoelectron spectroscopy (UPS) was used to evaluate the work function (WF) changes of GO, rGO, and rGO/CNT (Supplementary Fig. 2b). The WF of GO was measured to be about 5.05 eV and decreased to 4.34 eV after reduction to rGO. As the WF of pristine CNTs is about 4.8 eV48, a final WF of 4.54 eV for rGO/CNT indicates a homogeneous dispersion of rGO and CNTs. To further decrease the gate electrode’s electrical resistance and increase its ion injection capability and switching speed, we 3D printed a high conductivity PEDOT:PSS formulation (4 wt%, ≈200 S cm−1, Supplementary Fig. 1) on top of the rGO/CNT source, drain, and gate electrodes. The high conductivity PEDOT:PSS formulation was prepared following a procedure reported previously49.
Having defined the source, drain, and gate electrodes, we 3D printed the OECT channel using another PEDOT:PSS formulation with a lower concentration (2.5 wt%). To ensure fast OECT response time and low operational voltage, we added an excess of D-Sorbitol (20 wt%) to the PEDOT:PSS formulation. D-Sorbitol is a well-known ion reservoir capable of maintaining mobile ions inside the semiconducting polymers and a suitable plasticizer for PEDOT:PSS, promoting its adhesion to Parylene50,51. Additionally, we used divinyl-sulfone (DVS) to boost the PEDOT:PSS channel water stability without sacrificing the electrical conductivity52,53. Images of the OECT channel, showing the different layers’ morphology, are available in Supplementary Fig. 3. A PDMS insulating layer with an opening area of about 0.5 × 3 mm2 was then printed on top of the PEDOT:PSS channel (width W = 240 μm and length L = 77 μm), and the gate electrode (2 × 6 mm2) to define a well for the electrolyte. For the latter, we printed a poly(sodium 4-styrenesulfonate) (PSSNa) based hydrogel (50 wt%) containing 0.2 M aluminum chloride (AlCl3) to increase its ionic conductivity through the addition of more ionic species (Supplementary Fig. 4). The fully printed OECTs, possessing a footprint of 7 × 7 mm2, are lightweight and show good mechanical flexibility (see photograph in Fig. 1b).
3D-printed OECT characterization
The electrical characteristics of the fully 3D-printed PEDOT:PSS-based OECTs are reported in Fig. 2a (W × L = 77 µm × 240 µm). The transfer curves, recorded at drain voltage VD = −1 V, are typical for a depletion mode PEDOT:PSS-based OECT, with an ON/OFF current ratio exceeding 2800 at gate voltage VG < 1 V and a geometry-normalized transconductance (gm) as high as 34 S cm−1. The latter is comparable to typical values of inkjet, screen, or aerosol-jet printed PEDOT:PSS-based OECTs18,24. The transfer curves also show low hysteresis (<20 mV), indicative of easily reversible dedoping/doping processes. The output characteristics are also consistent with typical PEDOT:PSS-based OECTs (Supplementary Fig. 5a). The transient response of our 3D-printed PEDOT:PSS-based OECTs, measured using gate voltage pulses of VG = 0.8 V, is reported in Supplementary Fig. 5b. The switching off (τOFF) and switching on (τON) times were extracted by fitting the ID temporal response with a single exponential decrease function (Supplementary Fig. 5c), yielding a τOFF ≈ 27 ms and a τON ≈ 100 ms. These results are remarkable, considering they refer to fully 3D-printed OECTs and are comparable to screen-printed devices (see Supplementary Table 1 and Supplementary Fig. 6)18,19,54.
a Transfer curve and normalized transconductance of the 3D-printed OECT. b OECT transfer curves and (c) transconductances under different mechanical stress. d OECT response time under different mechanical stress. e OECT electrical stability under VG cycling (0–1 V) for over 3 h. f Comparison of the normalized transfer curves before and after cycling stability test and their recovery after resting at 6 °C and high humidity.
To investigate if the channel or the electrolyte material caused the asymmetry in ON-to-OFF and OFF-to-ON switching speeds, we performed the exact measurements using 0.1 M NaCl liquid electrolyte. The results are reported in Supplementary Fig. 7 and show τOFF = 7.5 ms and τON = 7.8 ms when aqueous NaCl is used as the electrolyte. The similar τOFF and τON values suggest that ion trapping inside the PEDOT:PSS channel occurs when PSSNa+AlCl3-based hydrogel is used as the gel electrolyte, likely due to the trapping of large ionic species inside the semiconducting channel material. Note that a similar asymmetry in the switching speeds has already been observed in OECTs and linked to steric hindrance of the counterions50,55,56 or dimensions of the electrolyte ions57,58.
As the devices are fabricated on top of a thin (<4 µm) Parylene layer, they can be easily peeled off from the glass slide. These free-standing devices can be wrapped around a glass pipette (radius r = 0.7 mm) and even folded in half, perpendicularly to the channel (radius r < 0.1 mm), without any appreciable changes in the electrical performance, as also visible from the transfer and transconductance curves reported in Fig. 2b, c. We observed a < 2× decrease in the switching speed of the bent device compared to the unfolded devices. However, since the ON-state current is the same for all cases, we speculate that the changes in device time response are due to partial delamination of the gel electrolyte from the PEDOT:PSS channel. Despite the slightly slower switching speed, our devices show remarkable mechanical stability even under high stresses.
Finally, we tested the electrical performance stability of our 3D-printed PEDOT:PSS OECTs under 3 h continuous operation in ambient at pulsed VG = 1 V (Fig. 2e) and observed a 10× increase of the OFF current (from 0.1 µA to about 1 µA after 3 h) as well as a slight 20% increase in the ON current (from 1 mA to 1.2 mA after 3 h). To better understand the origin of the slight rise in ID, we measured the transfer curves before and after the cycling test (Fig. 2f, unnormalized curves in Supplementary Fig. 8). When the transfer curves are normalized to the ON current, a less effective gate modulation can be observed, with the OECT not completely turning OFF at VG = 0.8 V (ON/OFF ratio decreases from 2400 before stress to 140 after the cycling stress). However, the electrical characteristics are partially recovered after storing the devices in the fridge for two days (ON/OFF ratio >1000) and are completely recovered after one week of storage inside the refrigerator. Therefore, we speculate that some of the water inside the gel electrolyte evaporated due to heating during continuous operation, leading to a decrease in ion mobility inside the electrolyte. The gel could absorb moisture from the environment by storing the device in the fridge, which creates a favorable environment for ion motion.
Beyond traditional printing techniques
In the previous section, we discussed the potential of 3D printing to fabricate all the elements of an OECT. This section highlights the advantages of this fabrication process compared to more traditional printing techniques. Other ways of patterning devices would allow for higher throughput (for example, screen printing or gravure printing) or higher resolution (for example, inkjet/aerosol-jet printing and standard photolithography/spin coating). However, high throughput deposition/patterning techniques are severely limited to flat, flexible, or stretchable substrates with minimal possibility of dealing with unconventional (irregular, curved, or soft) surfaces59. On the other hand, techniques such as inkjet or spin-coating are more limited in terms of processable materials, only allowing the patterning of low-viscosity functional inks. While a few reports have shown the potential of printing devices on very thin substrates, which are then laminated or wrapped around more complex objects, after processing60,61,62, direct deposition/patterning on complex structures using these techniques is typically troublesome. On the contrary, exploiting the high viscosity of the inks, it is possible to 3D print structures on unconventional objects with irregular surfaces. Thus, to demonstrate the full potential of 3D printing, we targeted two different objectives: (1) the printing of a complete device, including a flexible and self-standing substrate, and (2) the patterning on irregular surfaces.
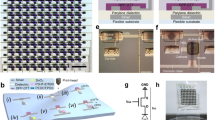
Regarding the first point, while using Parylene allows us to fabricate flexible free-standing devices, it clashes with the idea of devices manufactured using low-cost printing techniques. Indeed, the deposition of Parylene requires vacuum systems and a high temperature of >600 °C, with most of the material wasted inside the deposition chamber. Thus, we used the capability of our 3D printing system to handle highly viscous formulations to print the OECT substrate directly. To use only low-cost materials/processes, we developed an anionic nanofibrillated cellulose (a-NFC)/polyvinyl alcohol (PVA) (1:1 ratio) hydrogel that can be quickly processed at low temperatures (<100 °C). To obtain a water-insoluble substrate, we added glutaraldehyde to our formulation, which is known to crosslink PVA after acid treatment63,64. In addition, the cross-linking of the substrate is mediated by the presence of an acidic environment, which allows us to use the same solution used to reduce the GO electrodes to process both substrate and electrical connections simultaneously. First, we 3D printed the a-NFC/PVA substrate and dried it in an oven at 80 °C for 1 h. The resulting 60-µm-thick film was then used as the substrate upon which we 3D printed the GO/CNT electrodes. Then, we immersed the a-NFC/PVA film and GO/CNT electrodes overnight in the reducing solution, as previously described in the OECT fabrication process overview. After removing the iodine contaminants in the rGO/CNT electrodes by immersing the substrate and electrical connections in ethanol, the devices were dried inside an oven at 80 °C for 2 h. The subsequent channel and electrolyte layers were deposited following the OECT fabrication process described in the previous section.
Figure 3a–d shows the electrical characteristics of the OECTs 3D printed on a-NFC/PVA substrates. The ON current matches the ID values recorded for the OECTs fabricated on Parylene, indicative of the high reproducibility of the printing process. The most significant differences, however, concern the OFF-state current and switching characteristics. We observed a significantly reduced ON/OFF ratio of 700 against 2400 of the OECTs printed on Parylene and a slower temporal response with τOFF ≈ 70 ms and τON ≈ 170 ms (Fig. 3c, d). We attributed the slightly lower performance to using anionic NFC as the substrate material, which is known to be a good ionic conductor65,66. We then speculate that part of the ions is injected into the underlying cellulose substrate from the gel electrolyte. This seems to be confirmed by the observation that applying a negative gate bias (−0.7 V) increases the ON/OFF ratio and time response (Fig. 3c, d). This bottleneck could be solved in the future using cationic cellulose-based materials, thus preventing the injection of cations into the substrate.
a Photographs of the cellulose ink (left), printed film (top right), and OECT (bottom right). b Transfer curve and normalized transconductance of the OECTs printed on cellulose substrate. c Evolution of the time response (τOFF and τON) after biasing the gate at −0.7 V for different times (up to 1 h in total). d Evolution of transconductance and ON/OFF ratio after biasing the gate at −0.7 V for different times (up to 1 h).
To verify this hypothesis, we prepared another set of devices using a blend of cationic NFC (c-NFC) and PVA, following the same procedure described above for the OECTs printed on a-NFC/PVA. The transfer characteristics and time response of the OECTs printed on c-NFC/PVA are shown in Fig. 4a, b. These OECTs had a higher ON/OFF ratio (over 1200, 4× the initial values of the OECTs on a-NFC) and faster response times (τOFF = 14.6 ± 0.5 ms and τON = 19.9 ± 0.4 ms) compared to the OECTs printed on a-NFC/PVA, as shown in Supplementary Fig. 9.
As for the possibility of printing devices onto unconventional substrates, we 3D printed the OECTs on soft, curved, and irregular surfaces such as red bell peppers and orchid flowers. Given the fragile nature of these substrates, we modified the printing process by replacing the rGO/CNT ink with the high conductivity PEDOT:PSS formulation used on the gate of the OECTs described above. This is because reducing the rGO/CNT layers requires acid treatment and a heating step that would otherwise compromise the integrity of the plant-based substrate. Figure 4c, d shows the electrical characteristics of the OECTs 3D printed on orchid petals and bell pepper. Because of the rough and complex substrate surface, we used a larger channel area and thickness for this device. For this reason, the ID,ON is higher for both devices than those printed on Parylene and NFC, with an ON/OFF ratio of around 300 (orchid) and 160 (bell pepper). We ascribed the lower ON/OFF ratio primarily to two factors: (1) a thicker channel layer and (2) the soft/ion permeable nature of the plant-based surface, similar to NFC. In addition, we estimated device response times in the range of 0.5–1 s (Supplementary Fig. 10), which are expected considering the thicker channel layers and the use of electrochemically active PEDOT:PSS source/drain electrodes67,68. Despite the somewhat lower performance of these unoptimized devices compared to OECTs 3D printed on standard Parylene substrates, pattering working devices on complex surfaces––challenging with traditional printing techniques and photolithography––have significant implications for the field of biosensing and edible electronics where modulation speed is not always a stringent requirement. Future optimization should focus on developing milder curing/post-processing conditions to reduce the rGO/CNT electrodes.
Applications of 3D-printed OECTs
Having characterized the electrical properties of the 3D-printed OECTs, we tested these devices for two representative applications: biosensing and neuromorphic.
BIOSENSING: dopamine detection
First, we evaluated the capability of our 3D-printed OECTs to detect biomolecules of interest in neuromedicine. Specifically, we tested the detection of dopamine (DA), a neurotransmitter regulating the correct functioning of several organs and whose loss in some areas of the brain is linked to, for example, Parkinson’s disease. Another motivation for choosing DA detection is that DA is a benchmark molecule for testing OECT biosensing due to its easy detection mechanism69,70,71,72. Neurotransmitters show different oxidation potentials, which helps aid their detection and distinguish them73. The reduction of dopamine at the gate electrode modulates the effective VG by an offset voltage proportional to the analyte concentration, which in turn modulates ID7. Due to the amplification capabilities of the OECTs, it is more favorable to measure small changes in ID rather than VG. We modified our OECT structure for DA detection by removing the PEDOT:PSS layer at the gate electrode. The reason is to shift the transconductance peak at higher values of VG, to have dopamine reduction at the gate, which generally happens at VG > 0.4 V74,75,76. We drop-casted the phosphate buffer solution containing different analyte concentrations in the PDMS well, defined using our 3D printing systems.
The 3D-printed OECT response to changes in DA concentration and relative calibration curve are reported in Fig. 5. To minimize device differences and measurement noise, we used the normalized response (NR) against the fit curve (Fig. 5a, Eq. 1):
Where IFit is the fitted ID of the blank buffer and IDA is the drain current in the presence of dopamine. To obtain the dose curve, the dopamine concentration was changed from 1 µM to 10 mM every 100 s, and the value before switching to a higher concentration was taken for the dose curve.
Both samples followed the same trend when the PBS buffer solution was changed multiple times. With increasing the DA concentration, a rise in the normalized response (decrease in ID) is observed due to a faradaic current at the gate electrode. The devices showed a fast response to changes in DA concentration and exhibited a near-step-like behavior (Supplementary Fig. 11). While the devices showed a linear behavior at low DA concentrations (up to 100 µM), they started saturating at higher DA concentrations (above 1 mM). For these concentrations, a maximum NR of about 0.4 is achieved, leaving ample room for improvement. For example, a possible strategy is reducing the gate dimension, which causes a more significant potential drop at the gate/electrolyte interface, thus increasing sensitivity when sensing peroxide77. The transfer curves before and after dopamine sensing exhibit similar characteristics (Fig. 5b). The device showed a slight ID shift in transfer sweeps (at VG = 0 V) before and after DA sensing. This could be attributed to device bias stress during measurement or irreversible interaction of DA with the gate electrode (for example, through the formation of polydopamine74,75,76). The latter could change the transfer curve by altering the capacitance at the gate/electrolyte interface.
To obtain the limit of detection (LOD)––the lowest DA concentration our devices can detect––we used a linear regression model78. As LOD is equal to 3.3 × Sa/b, with Sa the response’s standard deviation (ID in our measurement) and b the curve’s slope in the linear regime of the dose curve, we extrapolated a value of 6 µM. This value is remarkable considering the absence of metal gates like Pt, typically used to detect lower dopamine concentrations and is well within physiological values8,35,69,70. To further improve the LOD, future investigations could focus on functionalizing the gate electrode (for example, with Pt nanoparticles) and improving the gate/channel geometry, thus increasing the signal-to-noise ratio and sensitivity.
NEUROMORPHIC: PEDOT:PSS channel long-term depression
Synapse connects two neurons and enables information exchange through chemical and electrical signals. Learning in the human brain is caused by permanent/temporary strengthening and weakening of the synaptic connections. The strength of these connections is referred to as the synaptic weight and is modulated by processes called potentiation and depression. Long-term changes in the synaptic weight (minutes to hours or longer), called long-term potentiation/depression (LTP/LTD), are the basis for experience-dependent alteration of the neural network. In contrast, short-term changes in synaptic weights (ms to s), known as short-term potentiation/depression (STP/STD), assist in decoding temporal information79. OECTs have already been employed to emulate synaptic functionality14,80,81,82 and even to simulate neural behavior16,17. Yet, no reports of fully 3D-printed devices with state retention longer than 1 h and employing gel-based electrolytes have been reported. In particular, using non-liquid electrolytes could push the integration of neuromorphic devices with other electronic systems, bringing them closer to real-life applications.
Analogous to the biological synapses, the voltage input to the gate of the 3D-printed OECT-based artificial synapses represents the presynaptic input. At the same time, the current flowing across the channel is the postsynaptic output referred to as the excitatory postsynaptic current (EPSC). The artificial synapse operates in short-term and long-term plasticity modes like the biological counterpart. Ionic buildup in the PEDOT:PSS channel during gate-induced doping/dedoping causes a short-term increase/decrease in channel conductance over several seconds (Fig. 6a). Short-term synaptic facilitation (ms to s) in biological synapses enabled by the accumulation of excess Ca2+ ions and neurotransmitters on facing recurrent action potentials from the neuron is analogous to this process. The EPSC can be modulated by changing the gate bias from −0.4 V to −0.8 V, resulting in an enhanced excitatory effect.
a Excitatory postsynaptic current measured at the drain (−0.05 V) on application of various gate pulse voltages of 90 ms duration. b Paired-pulse facilitation indices of the synapse operating in the short-term doping/dedoping mode. PPF index is given by (A2/A1) × 100 (see inset). The increase in EPSC on application of consecutive gate pulses separated by Δt. c Long-term depression and potentiation in the synapse exhibiting 25 distinct levels. Depression is induced by 1.1 V pulses of 2.5 s duration and potentiation by −0.6 V pulses of 4 s duration. The time delay between two consecutive write pulses is 20 s. The conductance state is read using a drain voltage pulse of −0.05 V, 10 s after writing. d Retention of two states, A and B, over 1 h.
Paired-pulse facilitation (PPF) is a type of short-term plasticity where the EPSC increases progressively with repeated action potentials. PPF is involved in decoding temporal information in biological systems. PPF index is the ratio of the amplitudes of the second and the initial EPSC on the application of consecutive presynaptic inputs. PPF indices of the artificial synapse exhibited an exponential decay with increasing pulse intervals resembling the signaling characteristics of biological synapses (Fig. 6b) and can be fitted with the exponential decay equation (Eq. 2):
where x represents pulse interval duration, c = 100.38 represents resting magnitude, A1 = 12.31 and A2 = 9.92 are the facilitation constants, and t1 = 50 ms and t2 = 134.5 ms are the characteristic time constants. These characteristic time constants closely resemble the values occurring in biological synapses (40 ms and 300 ms)82.
Increasing the gate pulse width and amplitude resulted in long-term changes in the conductivity of the OECT channel. This is similar to the long-term plasticity in biological synapses, where the insertion of new receptors leads to permanent increases in synaptic strength. LTP is induced by applying −0.6 V pulses of 4 s duration and LTD using 1.1 V pulses for 2.5 s (Fig. 6c). Twenty-five distinct conductance states are achieved in the synapse with retention of around 1 h (Fig. 6d). This is comparable to state-of-the-art OECT-based synapses80,81 and does not require a floating gate setup to enable retention. The reason for the prolonged retention in the system is currently elusive and under investigation. However, we hypothesize that it may be due to ion trapping in the 3D-printed channel80.
Discussion
In conclusion, we showed that extrusion-based wet 3D printing––a digital approach to additive manufacturing––can be used to fabricate OECTs rapidly. The fully 3D-printed OECTs showed remarkable electrical characteristics, good biosensing capabilities (without needing standard metal gates for detection), and neuromimicking paired-pulse depression behavior. Due to the mechanical flexibility of our 3D-printed OECTs, we demonstrate the possibility of integrating biosensing and neuromorphic applications into personalized, low-cost devices fabricated by additive manufacturing.
Wet 3D additive manufacturing, commonly used for microfluidics and printing biological structures40,83,84,85,86, is a relatively new approach in the field of organic electronics. However, it offers several benefits over traditional manufacturing techniques like screen printing and gravure printing, including the ability to pattern devices on complex structures with flexibility in design and minimal material waste (typical of drop-on-demand techniques). This is particularly important considering the high cost of materials, the limited flexibility, and the high material waste associated with traditional methods. Photolithography, while widely used in the semiconductor industry, is not cost-effective for low-cost processing and generates significant material waste through the spin coating process. In contrast, the research presented here demonstrates the potential to optimize materials and use 3D printing to create flexible and self-standing OECTs with good electrical performance, the ability to detect biological signals, and neuromorphic behavior. This work shows a clear path towards creating more complex structures and devices for bioelectronics applications1,87 and future developments could involve transitioning to a vertical device geometry to achieve better miniaturization and thus, higher performance88.
Methods
GO synthesis
All chemicals, including graphite flakes (~325 mesh), sulfuric acid (H2SO4, 98%), potassium permanganate (KMnO4), hydrochloric acid (HCl), and hydrogen peroxide (H2O2), were obtained from Sigma-Aldrich and used as received. GO samples were prepared as reported in Ref. 46. In brief, 3 g graphite flakes were dispersed in 90 mL 98% H2SO4 in a 250 mL flask and kept stirring at room temperature for 30 min. Then 9 g KMnO4 was slowly added to the flask under vigorous stirring. The oxidation process was performed at room temperature for 3 h. After that, the reaction was determined by pouring the reaction system into 500 mL deionized water with 10 mL H2O2 to reduce Mn (VII) species. The suspension was stirred for 10 min and allowed to stand overnight. Then the supernatant was decanted and washed with 10 vol% HCl solution three times by centrifuge (10,000 rpm for 5 min) to remove sulfate ion. Three times washing with deionized water was followed by centrifuge (10,000 rpm for 50 min) to remove any acid residue.
GO/CNT ink preparation
The GO/CNT ink was prepared by dispersing 150 mg GO and 75 mg CNT (~50 μm purchased from Jiangsu XFNANO Materials Tech Co., Ltd) into 10 ml of 50 vol% dimethyl sulfoxide (DMSO)/H2O and ultrasonicated for 6 h.
Conductive PEDOT:PSS ink preparation
100 ml of PEDOT:PSS commercial formulation (PH1000, Heraeus) was frozen in liquid nitrogen and then inserted in our freeze-dryer system (BenchTop Pro, SP-Scientific), where it was left for 72 h to eliminate all the solvent inside, following the procedure described in ref. 49. Then the dry PEDOT:PSS was redispersed in 4 wt% in a DMSO/H2O (vol% 5/95). To increase the plasticity of the formulation, 1 wt% of D-Sorbitol and 4 w% of Triton X were added. The components were mixed first using a homogenizer (T 10 basic ULTRA-TURRAX®, IKA) for 5 min and then magnetically stirred overnight at 120 °C on a hotplate. Afterward, (3-glycidyloxypropyl) trimethoxysilane (GOPS, 0.1 wt%) was added, and the solution was then placed in a vacuum chamber for 30 min to remove all the air bubbles.
OECT channel PEDOT:PSS ink preparation
100 ml of PEDOT:PSS commercial formulation (PH1000, Heraeus) was frozen in liquid nitrogen and then inserted in our freeze-dryer system (BenchTop Pro, SP-Scientific), where it was left for 72 h to eliminate all the solvent inside, following the procedure described by ref. 49. Then the dry PEDOT:PSS was redispersed in 2.5 wt% in a DMSO/H2O (vol% 5/95). To increase the plasticity of the formulation, 2 wt% of Triton X was added subsequently. In addition, 20 wt% of D-Sorbitol was added to increase plasticity and promote ion diffusion inside the PEDOT:PSS film. The components were mixed first using a homogenizer (T 10 basic ULTRA-TURRAX®, IKA) for 5 min and then magnetically stirred overnight at 120 °C on a hotplate. Afterward, glycerol (1 wt%) and divinyl-sulfone (DVS, 3 vol%) were added to the solution to have PSS– cross-linking at room temperature without losing electrical conductivity52,53. Finally, the solution was placed in a vacuum chamber for 30 min to remove all the air bubbles.
Cellulose ink preparation
The cellulose ink was fabricated as reported in Ref. 89. In brief, 51.35 g carboxymethylated nanofibrillated cellulose (1 wt% solid content, ordered from Research Institutes of Sweden(RISE)), 11 g of 2 mg mL−1 polyvinyl alcohol (PVA, Mowiol® 18–88, Sigma-Aldrich) solution and 44 mg of 25 wt% glutaraldehyde solution (Sigma-Aldrich) were homogenized together using a laboratory mixer (T 10 basic ULTRA-TURRAX®, IKA) for 10 min and then followed by degassing in a vacuum desiccator overnight to remove excessive bubbles in the viscous mixture. The same procedure was followed for both a-NFC and c-NFC.
PSSNa gel electrolyte ink preparation
2.5 g of PSSNa (Sigma, Mw 2,000,000) and 0.5 g of D-Sorbitol were dispersed in 5 ml of 0.2 M of aluminum chloride (AlCl3) and 10 ml of glycerol. The components were magnetically stirred overnight at 150 °C on a hotplate and then at room temperature for an entire day to remove all the air bubbles inside the gel.
PDMS ink preparation
Two batches of different PDMS formulations, 10 g each of Sylgard 184 (Sy 184) and SE 1700 (Dow Corning, Auburn, MI), were separately prepared by mixing with their respective curing agent in a 10:1 ratio. Each blend was then mixed independently to ensure the proper dispersion of the curing agent (ARE-250 CE, Thinkymixer). Then the Sy 184 and SE 1700 were combined with a 6:4 ratio and centrifuged to ensure a homogeneous dispersion. Afterward, isopropyl alcohol (IPA) was added in a 1:2 ratio as thinning agent; the final formulation was then centrifuged once again to obtain a homogeneous 3D ink.
OECT 3D-printing
The devices were fabricated using a commercial extrusion-based 3D-Printing system (BIOX by Cellink). The patterns employed to fabricate the OECTs have been drawn using Blender Software and saved as.stl files. These files were then sliced using the inbuilt BIOX software. The printing parameters for each material are reported here:
-
GO/CNT. Nozzle: 30G. Pressure: 70 Pa. Speed: 10 mm/s. Resolution between 120 and 150 µm
-
PEDOT:PSS for the channel. Nozzle: 34G. Pressure: 80 Pa. Speed: 5 mm/s. Resolution between 100 and 120 µm
-
PEDOT:PSS for the gate. Nozzle: 30G. Pressure: 200 Pa. Speed: 15 mm/s. Resolution 150 µm
-
PDMS. Nozzle: 30G. Pressure: 200 Pa. Speed: 15 mm/s. Resolution 150 µm
-
PSSNa electrolyte. Nozzle: 32G. Pressure: 500 Pa. Speed: 10 mm/s. Resolution 120 µm
-
Cellulose. Nozzle: 27 G. Pressure: 70 Pa. Speed: 25 mm/s. Resolution 200 µm
These parameters result from an optimization process to ensure a suitable filament extrusion and pattern infill with the best resolution. Higher pressure values caused material over-deposition (thus, lower resolution), while lower pressure led to a non-completely filled pattern due to nonoptimal filament extrusion. Regarding the printing speed, higher values led to a nonoptimal filament deposition yielding an incomplete pattern, while lower values contributed to material spreading and thus reduced resolution.
OECT 3D printing of unconventional surfaces
All-PEDOT:PSS-based OECTs were directly printed on orchid and bell pepper surfaces. The S, D, and G electrodes were fabricated using the conducting PEDOT:PSS formulation used for the gate electrode. The channel areas were 150 µm × 800 µm (orchid) and 300 µm × 800 µm. The PEDOT:PSS channel formulation was then printed inside the channel area. The OECTs were inserted in a vacuum desiccator for 2 h to dry and then removed and left in air for 2 h to remove any solvent from the printed formulations entirely. Before measuring the OECT on the orchid, the petals were placed for 15 min on a hotplate at 100 °C to remove any liquid inside the plant that would cause large parasitic currents. Finally, the PSSNa electrolyte was 3D-printed on the devices to obtain the complete OECT structure.
OECT and electrical conductivity measurements
Electrical conductivity measurements of the semiconducting/conducting materials and OECT testing were performed using a 4200-SCS Semiconductor Characterization System. The obtained data were then analyzed and fitted using Python software.
Ionic conductivity measurements
The ionic conductivity was measured by electrochemical impedance spectra (Bio-logic Instrument) as reported previously90. The NaCl or PSSNa gel was modified with an area of 0.071 cm2 and 0.35 cm-thick PDMS well and then sandwiched between two 100-nm-thick Ti-coated glasses. Impedance measurements were carried out between 100 kHz to 0.1 Hz with an AC amplitude of 10 mV. The real impedance at the highest frequency was taken as the bulk resistance.
Dopamine detection measurements
Electrical measurements for device response were performed with a Keithley 2612 and a Labview program in a 1X PBS, pH 7.4, buffer solution. Before sensing experiments, the device operation was confirmed and stabilized with transfer sweeps. The response of the sensors to the analyte was measured in constant bias mode with VD = −0.1 V and VG = 0.5 V and reading ID. Transfer characteristics were obtained by applying VD = −0.1 V and sweeping VG from 0 to 0.55 V with a scan rate of 25 mV s–1 until stabilization was obtained. Only the last complete cycle was examined to compare the sample before and after dopamine sensing. The buffer solution was changed multiple times to get a stable signal and a better basis for fitting the device drift before dopamine sensing. These changes in the blank buffer solution can be fitted with an exponential decay fit.
Photoelectron spectroscopy measurements
XPS and UPS were performed in a UHV surface analysis system equipped with a Scienta-200 hemispherical analyzer. The excitation source for UPS was a standard He-discharge lamp with hν = 21.22 eV (He I), and for XPS, monochromatized Al Kα radiation with 1486.6 eV energy was used.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
The coding generated for the measurement and the device fabrication during the current study are available from the corresponding author upon reasonable request.
References
Rivnay, J. et al. Organic electrochemical transistors. Nat. Rev. Mater. 3, 17086 (2018).
Moser, M. et al. Materials in Organic Electrochemical Transistors for Bioelectronic Applications: Past, Present, and Future. Adv. Funct. Mater. 29, 1807033 (2019).
Sun, H., Gerasimov, J., Berggren, M. & Fabiano, S. n-Type organic electrochemical transistors: materials and challenges. J. Mater. Chem. C. 6, 11778–11784 (2018).
Chen, S. et al. Recent Technological Advances in Fabrication and Application of Organic Electrochemical Transistors. Adv. Mater. Technol. 5, 2000523 (2020).
Friedlein, J. T., McLeod, R. R. & Rivnay, J. Device physics of organic electrochemical transistors. Org. Electron. 63, 398–414 (2018).
Majak, D., Fan, J. & Gupta, M. Fully 3D printed OECT based logic gate for detection of cation type and concentration. Sens. Actuators B Chem. 286, 111–118 (2021).
Bernards, D. A. et al. Enzymatic sensing with organic electrochemical transistors. J. Mater. Chem. 18, 116–120 (2008).
Tang, H., Lin, P., Chan, H. L. W. & Yan, F. Highly sensitive dopamine biosensors based on organic electrochemical transistors. Biosens. Bioelectron. 26, 4559–4563 (2011).
Marks, A., Griggs, S., Gasparini, N. & Moser, M. Organic Electrochemical Transistors: An Emerging Technology for Biosensing. Adv. Mater. Interfaces 9, 2102039 (2022).
Chen, J. et al. Highly stretchable organic electrochemical transistors with strain-resistant performance. Nat. Mater. 21, 564–571 (2022).
Wu, H.-Y. et al. Influence of Molecular Weight on the Organic Electrochemical Transistor Performance of Ladder-Type Conjugated Polymers. Adv. Mater. 34, 2106235 (2022).
Yang, C.-Y. et al. Low-Power/High-Gain Flexible Complementary Circuits Based on Printed Organic Electrochemical Transistors. Adv. Electron. Mater. 8, 2100907 (2022).
Gkoupidenis, P., Schaefer, N., Garlan, B. & Malliaras, G. G. Neuromorphic Functions in PEDOT:PSS Organic Electrochemical Transistors. Adv. Mater. 27, 7176–7180 (2015).
Zhou, B. et al. Organic electrochemical transistors toward synaptic electronics. J. Phys. D: Appl. Phys. 55, 304006 (2022).
Sun, J., Fu, Y. & Wan, Q. Organic synaptic devices for neuromorphic systems. J. Phys. D: Appl. Phys. 51, 314004 (2018).
Perez, J. C. & Shaheen, S. E. Neuromorphic-based Boolean and reversible logic circuits from organic electrochemical transistors. MRS Bull. 45, 649–654 (2020).
Harikesh, P. C. et al. Organic electrochemical neurons and synapses with ion mediated spiking. Nat. Commun. 13, 901 (2022).
Andersson Ersman, P. et al. All-printed large-scale integrated circuits based on organic electrochemical transistors. Nat. Commun. 10, 5053 (2019).
Zabihipour, M. et al. High yield manufacturing of fully screen-printed organic electrochemical transistors. npj Flex. Electron. 4, 15 (2020).
Demuru, S. et al. All-Inkjet-Printed Graphene-Gated Organic Electrochemical Transistors on Polymeric Foil as Highly Sensitive Enzymatic Biosensors. ACS Appl. Nano Mater. 5, 1664–1673 (2022).
Basiricò, L. et al. Electrical characteristics of ink-jet printed, all-polymer electrochemical transistors. Org. Electron. 13, 244–248 (2012).
Schmatz, B., Lang, A. W. & Reynolds, J. R. Fully Printed Organic Electrochemical Transistors from Green Solvents. Adv. Funct. Mater. 29, 1905266–1905967 (2019).
Makhinia, A., Hübscher, K., Beni, V. & Andersson Ersman, P. High Performance Organic Electrochemical Transistors and Logic Circuits Manufactured via a Combination of Screen and Aerosol Jet Printing Techniques. Adv. Mater. Technol. 7, 2200153 (2022).
Tarabella, G. et al. Aerosol jet printing of PEDOT:PSS for large area flexible electronics. Flex. Print. Electron. 5, 014005 (2020).
Mangoma, T. N., Yamamoto, S., Malliaras, G. G. & Daly, R. Hybrid 3D/Inkjet-Printed Organic Neuromorphic Transistors. Adv. Mater. Technol. 7, 2000798 (2022).
Granelli, R. et al. High-Performance Bioelectronic Circuits Integrated on Biodegradable and Compostable Substrates with Fully Printed Mask-Less Organic Electrochemical Transistors. Small 18, 2108077 (2022).
Rashid Reem, B. et al. Ambipolar inverters based on cofacial vertical organic electrochemical transistor pairs for biosignal amplification. Sci. Adv. 7, eabh1055 (2021).
Rashid, R. B. et al. A Semiconducting Two-Dimensional Polymer as an Organic Electrochemical Transistor Active Layer. Adv. Mater. 34, e2110703 (2022).
Khodagholy, D. et al. High transconductance organic electrochemical transistors. Nat. Commun. 4, 2133 (2013).
Huang, L. et al. Porous Semiconducting Polymers Enable High-Performance Electrochemical Transistors. Adv. Mater. 33, 2007041 (2021).
Malda, J. et al. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 25, 5011–5028 (2013).
Abdalla, A. & Patel, B. A. 3D-printed electrochemical sensors: A new horizon for measurement of biomolecules. Curr. Opin. Electrochem. 20, 78–81 (2020).
Liyarita, B. R., Ambrosi, A. & Pumera, M. 3D-printed Electrodes for Sensing of Biologically Active Molecules. Electroanalysis 30, 1319–1326 (2018).
Fan, J., Montemagno, C. & Gupta, M. 3D printed high transconductance organic electrochemical transistors on flexible substrates. Org. Electron. 73, 122–129 (2019).
Bertana, V. et al. Rapid prototyping of 3D Organic Electrochemical Transistors by composite photocurable resin. Sci. Rep. 10, 13335 (2020).
Tarabella, G. et al. Multifunctional Operation of an Organic Device with Three-Dimensional Architecture. Materials 12, 1357 (2019).
Inal, S., Malliaras, G. G. & Rivnay, J. Benchmarking organic mixed conductors for transistors. Nat. Commun. 8, 1767 (2017).
Zozoulenko, I. et al. Electronic, Optical, Morphological, Transport, and Electrochemical Properties of PEDOT: A Theoretical Perspective. Macromolecules 54, 5915–5934 (2021).
Goranov, V. et al. 3D Patterning of cells in Magnetic Scaffolds for Tissue Engineering. Sci. Rep. 10, 2289 (2020).
Rastin, H. et al. 3D printing of cell-laden electroconductive bioinks for tissue engineering applications. J. Mater. Chem. C. 8, 5862–5876 (2020).
Kilian, D. et al. 3D Bioprinting of osteochondral tissue substitutes – in vitro-chondrogenesis in multi-layered mineralized constructs. Sci. Rep. 10, 8277 (2020).
Leucht, A. et al. Advanced gelatin-based vascularization bioinks for extrusion-based bioprinting of vascularized bone equivalents. Sci. Rep. 10, 5330 (2020).
Dey, M. & Ozbolat, I. T. 3D bioprinting of cells, tissues and organs. Sci. Rep. 10, 14023 (2020).
Tappa, K. & Jammalamadaka, U. Novel Biomaterials Used in Medical 3D Printing Techniques. J. Funct. Biomater. 9, 17 (2018).
Das, A. K. et al. Iodide-mediated room temperature reduction of graphene oxide: a rapid chemical route for the synthesis of a bifunctional electrocatalyst. J. Mater. Chem. A 2, 1332–1340 (2014).
Chen, H. et al. Efficient room-temperature production of high-quality graphene by introducing removable oxygen functional groups to the precursor. Chem. Sci. 10, 1244–1253 (2019).
Tang, B. et al. Are vacuum-filtrated reduced graphene oxide membranes symmetric? Nanoscale 8, 1108–1116 (2016).
Zhang, S. et al. Synergistic Effect of Multi-Walled Carbon Nanotubes and Ladder-Type Conjugated Polymers on the Performance of N-Type Organic Electrochemical Transistors. Adv. Funct. Mater. 32, 2106447 (2022).
Yuk, H. et al. 3D printing of conducting polymers. Nat. Commun. 11, 1604 (2020).
Spyropoulos George, D., Gelinas Jennifer, N. & Khodagholy, D. Internal ion-gated organic electrochemical transistor: A building block for integrated bioelectronics. Sci. Adv. 5, eaau7378 (2019).
He, H. & Ouyang, J. Enhancements in the Mechanical Stretchability and Thermoelectric Properties of PEDOT:PSS for Flexible Electronics Applications. Acc. Mater. Res. 1, 146–157 (2020).
del Agua, I. et al. DVS-Crosslinked PEDOT:PSS Free-Standing and Textile Electrodes toward Wearable Health Monitoring. Adv. Mater. Technol. 3, 1700322 (2018).
Mantione, D. et al. Low-Temperature Cross-Linking of PEDOT:PSS Films Using Divinylsulfone. ACS Appl. Mater. Interfaces 9, 18254–18262 (2017).
Andersson Ersman, P. et al. Screen printed digital circuits based on vertical organic electrochemical transistors. Flex. Print. Electron 2, 045008 (2017).
Zeglio, E. et al. Highly Stable Conjugated Polyelectrolytes for Water-Based Hybrid Mode Electrochemical Transistors. Adv. Mater. 29, 1605787 (2017).
Tu, D. & Fabiano, S. Mixed ion-electron transport in organic electrochemical transistors. Appl. Phys. Lett. 117, 080501 (2020).
Yamamoto, S., Polyravas, A. G., Han, S. & Malliaras, G. G. Correlation between Transient Response and Neuromorphic Behavior in Organic Electrochemical Transistors. Adv. Electron. Mater. 8, 2101186 (2022).
Yamamoto, S. & Malliaras, G. G. Controlling the Neuromorphic Behavior of Organic Electrochemical Transistors by Blending Mixed and Ion Conductors. ACS Appl. Electron. Mater. 2, 2224–2228 (2020).
Wu, X. et al. Universal Spray-Deposition Process for Scalable, High-Performance, and Stable Organic Electrochemical Transistors. ACS Appl. Mater. Interfaces 12, 20757–20764 (2020).
Stucchi, E., Scaccabarozzi, A. D., Viola, F. A. & Caironi, M. Ultraflexible all-organic complementary transistors and inverters based on printed polymers. J. Mater. Chem. C. 8, 15331–15338 (2020).
Viola, F. A. et al. A sub-150-nanometre-thick and ultraconformable solution-processed all-organic transistor. Nat. Commun. 12, 5842 (2021).
Stucchi, E. et al. Biodegradable all-polymer field-effect transistors printed on Mater-Bi. J. Inf. Disp. 22, 247–256 (2021).
Morandim-Giannetti, Ad. A. et al. Characterization of PVA/glutaraldehyde hydrogels obtained using Central Composite Rotatable Design (CCRD). J. Biomed. Mater. Res. B Appl. Biomater. 106, 1558–1566 (2018).
Yang, W. et al. Effect of Cellulose Nanocrystals and Lignin Nanoparticles on Mechanical, Antioxidant and Water Vapour Barrier Properties of Glutaraldehyde Crosslinked PVA Films. Polymers 12, 1364 (2020).
Jiao, F. et al. Ionic thermoelectric paper. J. Mater. Chem. A 5, 16883–16888 (2017).
Jiao, F. et al. Nanofibrillated Cellulose-Based Electrolyte and Electrode for Paper-Based Supercapacitors. Adv. Sustain. Syst. 2, 1700121 (2018).
Johansson, T., Persson, N.-K. & Inganäs, O. Moving Redox Fronts in Conjugated Polymers Studies from Lateral Electrochemistry in Polythiophenes. J. Electrochem. Soc. 151, E119 (2004).
Andersson Ersman, P. et al. Fast-switching all-printed organic electrochemical transistors. Org. Electron. 14, 1276–1280 (2013).
Liao, C. et al. Organic electrochemical transistors with graphene-modified gate electrodes for highly sensitive and selective dopamine sensors. J. Mater. Chem. C. 2, 191–200 (2014).
Gualandi, I. et al. Selective detection of dopamine with an all PEDOT:PSS Organic Electrochemical Transistor. Sci. Rep. 6, 35419 (2016).
Tybrandt, K., Kollipara, S. B. & Berggren, M. Organic electrochemical transistors for signal amplification in fast scan cyclic voltammetry. Sens. Actuators B Chem. 195, 651–656 (2014).
Ji, W. et al. Carbonized silk fabric-based flexible organic electrochemical transistors for highly sensitive and selective dopamine detection. Sens. Actuators B Chem. 304, 127414 (2020).
Heien, M. L. A. V. et al. Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst 128, 1413–1419 (2003).
Almeida, L. C. et al. Electrosynthesis of polydopamine films - tailored matrices for laccase-based biosensors. Appl. Surf. Sci. 480, 979–989 (2019).
Almeida, L. C. et al. Electrosynthesis of polydopamine-ethanolamine films for the development of immunosensing interfaces. Sci. Rep. 11, 2237 (2021).
Marchesi D’Alvise, T. et al. Ultrathin Polydopamine Films with Phospholipid Nanodiscs Containing a Glycophorin A Domain. Adv. Funct. Mater. 30, 2000378 (2020).
Cicoira, F. et al. Influence of Device Geometry on Sensor Characteristics of Planar Organic Electrochemical Transistors. Adv. Mater. 22, 1012–1016 (2010).
Alankar, S. & Vipin, B. G. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young-. Sci. 2, 21–25 (2011).
Purves, D., Augustine, G. J., Fitzpatrick, D., Hall, W. C., LaMantia, A.-S., McNamara, J. O., & Williams, S. M. (Eds.). Neuroscience 3rd ed. (2004), (Sinauer Associates, Publishers).
Ji, X. et al. Mimicking associative learning using an ion-trapping non-volatile synaptic organic electrochemical transistor. Nat. Commun. 12, 2480 (2021).
van de Burgt, Y. et al. A non-volatile organic electrochemical device as a low-voltage artificial synapse for neuromorphic computing. Nat. Mater. 16, 414–418 (2017).
Zucker, R. S. & Regehr, W. G. Short-Term Synaptic Plasticity. Annu. Rev. Physiol. 64, 355–405 (2002).
Ozbolat, V. et al. 3D Printing of PDMS Improves Its Mechanical and Cell Adhesion Properties. ACS Biomater. Sci. Eng. 4, 682–693 (2018).
Ozbolat, V., Dey M Fau-Ayan, B., Ayan B Fau-Ozbolat, I. T. & Ozbolat, I. T. Extrusion-based printing of sacrificial Carbopol ink for fabrication of microfluidic devices. Biofabrication 11, 034101 (2019).
Jammalamadaka, U. & Tappa, K. Recent Advances in Biomaterials for 3D Printing and Tissue Engineering. J. Funct. Biomater. 9, 22 (2018).
Gopinathan, J. & Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 22, 11 (2018).
Khodagholy, D. et al. In vivo recordings of brain activity using organic transistors. Nat. Commun. 4, 1575 (2013).
Huang, W. et al. Vertical organic electrochemical transistors for complementary circuits. Nature 613, 496–502 (2023).
Zhang, F. et al. Highly Strong and Solvent-Resistant Cellulose Nanocrystal Photonic Films for Optical Coatings. ACS Appl. Mater. Interfaces 13, 17118–17128 (2021).
Zhang, S. et al. An adaptive and stable bio-electrolyte for rechargeable Zn-ion batteries. J. Mater. Chem. A 6, 12237–12243 (2018).
Acknowledgements
S.Z. thanks Jianfei Li for writing the program to extract the cycle stability data reported in Fig. 2e. M.M. thanks Chi-Yuan Yang for taking pictures of the OECTs on the orchid and bell pepper. This work was financially supported by the Knut and Alice Wallenberg Foundation, the Swedish Research Council (2020–03243), ÅForsk (18–313 and 19–310), the European Commission through the MSCA-IF-2020 project BEACON (GA-101024191), the FET-OPEN project MITICS (GA-964677), and the Swedish Government Strategic Research Area in Materials Science on Functional Materials at Linköping University (Faculty Grant SFO-Mat-LiU 2009–00971).
Funding
Open access funding provided by Linköping University.
Author information
Authors and Affiliations
Contributions
M.M. and S.Z. contributed equally. M.M. designed, fabricated, and characterized the OECTs, and optimized the PEDOT:PSS and electrolyte formulations. S.Z. synthesized the GO, the cellulose/PVA formulation, and helped characterize the inks. M.M. and S.Z. contributed to the layout and writing of the paper. H.P. characterized the neuromorphic properties of the OECTs, while B.B. and C.D. performed the dopamine detection experiments, supervised by D.S.. D.T. helped with the device layout and optimization. X.L. and M.F. performed the XPS/UPS characterization of the rGO. S.F. conceived and supervised the project and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Massetti, M., Zhang, S., Harikesh, P.C. et al. Fully 3D-printed organic electrochemical transistors. npj Flex Electron 7, 11 (2023). https://doi.org/10.1038/s41528-023-00245-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41528-023-00245-4
- Springer Nature Limited
This article is cited by
-
Designing organic mixed conductors for electrochemical transistor applications
Nature Reviews Materials (2024)
-
Intrinsically healing conducting polymer/hydrogel nanocomposite films and their novel volumetric channel for high-performance, flexible, and healable organic phototransistors
Science China Materials (2024)
-
Metal 3D nanoprinting with coupled fields
Nature Communications (2023)
-
High-performance organic electrochemical transistors gated with 3D-printed graphene oxide electrodes
Nano Research (2023)