Abstract
The Characterizing Arabidopsis Root Attractions (CARA) spaceflight experiment provides comparative transcriptome analyses of plants grown in both light and dark conditions within the same spaceflight. CARA compared three genotypes of Arabidopsis grown in ambient light and in the dark on board the International Space Station (ISS); Col-0, Ws, and phyD, a phytochrome D mutant in the Col-0 background. In all genotypes, leaves responded to spaceflight with a higher number of differentially expressed genes (DEGs) than root tips, and each genotype displayed distinct light / dark transcriptomic patterns that were unique to the spaceflight environment. The Col-0 leaves exhibited a substantial dichotomy, with ten-times as many spaceflight DEGs exhibited in light-grown plants versus dark-grown plants. Although the total number of DEGs in phyD leaves is not very different from Col-0, phyD altered the manner in which light-grown leaves respond to spaceflight, and many genes associated with the physiological adaptation of Col-0 to spaceflight were not represented. This result is in contrast to root tips, where a previous CARA study showed that phyD substantially reduced the number of DEGs. There were few DEGs, but a series of space-altered gene categories, common to genotypes and lighting conditions. This commonality indicates that key spaceflight genes are associated with signal transduction for light, defense, and oxidative stress responses. However, these key signaling pathways enriched from DEGs showed opposite regulatory direction in response to spaceflight under light and dark conditions, suggesting a complex interaction between light as a signal, and light-signaling genes in acclimation to spaceflight.
Similar content being viewed by others
Introduction
Plants have utilized light and gravity as environmental cues throughout their evolutionary history to navigate and shape their growth and development1,2. Microgravity spaceflight environments are being explored with considerable effort to inform understanding of growth signals as well as the initiative to create suitable habitats and approaches for including plants in long-term space missions3,4. On orbit, where gravity does not act as a primary tropic force applied on plants, light is used as an orienting tropism for plant growth5,6. Plants remodel their growth strategy in different light conditions during spaceflight5,7. In the Advanced Plant Experiment 01 (APEX-01) on board the International Space Station (ISS), Arabidopsis (Arabidopsis thaliana) seedlings utilized the established light gradient within the growth hardware to direct their tropic growth5,8. Experiments have also revealed the dramatic impact of light wavelengths on the morphology of Arabidopsis seedlings grown in microgravity and fractional gravity6,9,10,11. Due to space facility limitations, as well as payload capacity in spaceflight, most of plant transcriptomic analyses have been performed using either constant light or constant dark conditions8,12,13,14,15. While extensive analyses have been conducted on plants cultivated in various spaceflight-associated environments, including some experiments without light, direct comparisons of transcriptomic responses to spaceflight under light and dark conditions in the same flight are still limited. As one of the very few examples, Villacampa et al. 16 grew Arabidopsis seedlings onboard ISS in a light/dark cycle for 4 days, prior to treatment with constant red light or dark for 2 days16. Compared with dark treatment, photo-stimulation of red light enhanced the adaptive response to 0.33 g by upregulating multiple stress-responsive pathways. In contrast, the CARA experiment comprised prolonged plant growth in constant light or in constant dark environments after germination to reveal the role of light in spaceflight-associated transcriptome regulation17.
The Characterizing Arabidopsis Root Attractions (CARA) experiment set up a side-by-side comparison between the plants grown in the light and plants kept dark in the same location on the ISS. Half of the plants were exposed to ambient cabin light to provide a primarily non-directional source of light for growth and development. The other half were kept wrapped in blackout cloth. This comparison thereby facilitated the direct dissection of the role of light in plant molecular responses in space17. Three genotypes of Arabidopsis were used: Col-0 and Ws wildtypes, and a mutant in the Col-0 background for the phytochrome D (PHYD) gene, which encodes a member of phytochrome family, the plant red/far-red photoreceptors18. Previous studies of the roots from CARA plants identified the genes and signaling pathways engaged by the root tip cells on orbit17. The influences of genotype on the plant physiological adaptation to spaceflight were measured by transcriptomic patterns. Presented here is using the same set of plants as reported by Paul et al. 17 to perform a comprehensive analysis of the impact of light on the genome-wide transcriptional response in Arabidopsis leaves in the spaceflight environment, showing genotype- and ecotype-related signaling pathways and gene categories that are changed in response to spaceflight in the light and in the dark. The comparison of tissue-related transcriptomic responses to space between leaves and root tips is discussed. The present work has revealed a sharp contrast between leaf transcriptomic responses to orbital flight in the light and the responses that occur in the dark.
Results
Leaves showed stronger transcriptional responses to spaceflight than root tips
As described in Paul et al. 17, the CARA experiment deployed Flight (F) or Ground Control (G) samples composed of Arabidopsis plants with three genotypes, Col-0 (C), phyD (P) and Ws (W), grown in the light (L) or in the dark (D) conditions17. Figure 1 summarizes the differential analyses of the transcriptome in leaf tissues. The comparative analysis generated differentially expressed genes (DEGs) with significant changes that were more than two-fold in the transcription level (log2(fold-change) >1 or < -1, and FDR adjusted P value (Padj) < 0.05) between samples of Flight and Ground Control (Fig. 1a). More DEGs were detected in the light than in the dark for each genotype in leaves, while there was an opposite trend of DEG number in the comparison between the light and the dark in root tips (Fig. 1b). Overall, leaf and root tip tissues showed little overlap of DEGs in both the light and the dark. Substantially more DEGs were detected in leaves than in root tips, suggesting that leaves are more sensitive in transcriptional response to spaceflight than root tips, especially in the light (Fig. 1b).
Samples include Flight (F) or Ground Control (G) for Arabidopsis with three genotypes, Col-0 (C), phyD (P) and Ws (W), grown in the light (L) or in the dark (D) conditions. The threshold is log2(fold-change) >1 or < −1, Padj <0.05. a Numbers of DEGs detected in pair-wise comparisons between samples of Flight (F) and Ground Control (G). b Venn diagrams show overlap between DEGs detected in leaves and root tips for each comparison.
Light condition is a critical factor affecting transcriptional regulation in plant response to spaceflight
In leaves, 8477 DEGs were detected between Flight and Ground Control in at least one comparison for Col-0, phyD or Ws ecotype in the light or in the dark (Fig. 2a and Supplementary Table 1). Distinct patterns of spaceflight responses were observed between plants grown in the light (left three columns of heatmap in Fig. 2a) and the dark (right three columns of heatmap in Fig. 2a). The significant regulation of DEGs in the same direction in both the light and the dark were defined as “similar” space-altered patterns, otherwise they were designated as “distinct” patterns. There were 99.7%, 99% and 97.3% DEGs in Col-0, phyD and Ws showed distinct space-altered patterns in the light and in the dark, respectively (Fig. 2b). Although there were 58, 89 and 678 DEGs presented in both the light and the dark in three genotypes, only 11, 43 and 153 DEGs showed similar space-altered patterns, respectively (Fig. 2b, c). In other words, 81%, 52% and 77% DEGs in three genotypes shown in both the light and the dark were regulated in opposite direction by spaceflight under the two conditions. Overall, the overlap of spaceflight responsive DEGs under light and dark environments was also smaller than that between different genotypes (Fig. 2c, d). Enrichment analyses of gene ontology and classification were performed on DEGs in each condition to reveal signaling pathways and gene families that were significantly altered in response to spaceflight (Fig. 3a, b). The orientation of comparisons was shown in Fig. 3c. Distinct pathways were altered by spaceflight in the light (left three columns of each heatmap in Fig. 3a) and in the dark (right three columns of each heatmap in Fig. 3a). Responses to abiotic stresses including salt, heat and abnormal oxygen levels as well as aging pathways were enriched for up-regulated genes of all three genotypes only in the light. More cell wall organization and biogenesis related pathways were repressed in the light than in the dark. In Ws, there were unique dark-responses typically associated with hypoxic and immune responses that were up-regulated, and chemical homeostasis and ion transport related pathways that were down-regulated. In contrast, no down-regulated pathway was enriched for Col-0 in the dark. There were also some similar signaling pathways deployed for response to spaceflight in both the light and the dark, but with different regulatory patterns. For instance, some photosynthesis related pathways were repressed in the light but induced in the dark for both Col-0 and Ws. Defense responses to chitin and insect were also repressed in the light but induced in the dark for Ws, while secondary metabolic process was induced in the light but repressed in the dark for Ws and phyD. Accordingly, the enriched gene families related to each signaling pathway were identified (Fig. 3b). Genes with significant responses to spaceflight in both the light and the dark could also be regulated in different patterns. As an example, light-harvesting chlorophyll a/b-binding (LHC) genes functioning in photosynthesis and stress responses19 were repressed in the light and induced in the dark in all three genotypes. The repression of genes encoding chlorophyll a/b binding proteins was among the first transcriptome responses associated with the spaceflight20.
The threshold is log2(fold-change) >1 or < -1, Padj <0.05. a Expression pattern of 8477 DEGs that were significantly changed between Flight (F) and Ground Control (G) in at least one comparison for Col-0 (C), phyD (P) or Ws (W) in the light or in the dark. Hierarchical clustering of the heatmap is done using one minus cosine similarity. Genes with no significant changes or no expression data detected in RNA-seq are indicated in gray. b DEGs with distinct or similar space-altered patterns in response to spaceflight for leaves grown in the light and in the dark. There are 4014 out of 4025 (99.7%), 4179 out of 4222 (99%) and 5470 out of 5623 (97.3%) DEGs showing distinct space-altered expression patterns in the light and in the dark for Col-0, phyD and Ws, respectively. Venn diagrams show the overlap of DEGs between leaf responses to spaceflight in the light and in the dark for each genotype (c), and the overlap of DEGs among leaf responses to spaceflight in three genotypes in the light or in the dark (d).
(a) and gene family (b) using CARA leaf DEGs between Flight and Ground Control in different comparisons. The threshold for DEGs is log2(fold-change) >1 or < -1, Padj <0.05, and for enrichment is Padj <0.05. Heatmaps are showing the -log10(Padj) for each significantly enriched item. Significantly enriched pathways or gene families are clustered according to the enrichment pattern. Clusters are indicated using colored bars on the left of the heatmaps. c The order of the comparisons used to generate the DEGs for enrichment analysis is shown. Each column in the heatmaps indicates one comparison. Heatmaps in (a) and (b) use the same order as shown in (c).
The expression patterns of DEGs from gene families enriched in spaceflight were compared between the light and the dark in both leaves and root tips. Gene family enrichment analyses were performed on 8477 DEGs from leaves and 716 DEGs from root tips17 DEGs between Flight and Ground Control (Fig. 4a). Glutathione-S-Transferase (GST) and WRKY families involved in stress response and metabolism signaling21,22 were enriched in both tissues. Differential expression patterns in response to spaceflight were plotted for leaf DEGs from GST, WRKY gene families, and the most highly enriched families of LHC and APETALA2/ethylene-responsive element binding protein (AP2/EREBP), as well as root tip DEGs from GST and WRKY families (Fig. 4b). Consistent with the overall transcriptomic responses to spaceflight in leaves, the majority of DEGs from these enriched gene families showed distinct space-altered patterns in the light (left three columns of each heatmap in Fig. 4b) and the dark (right three columns of each heatmap in Fig. 4b). Especially for LHC, none of the 25 genes showed significant regulation in the same direction in any of three genotypes (Fig. 4c). More than 90% DEGs from GST, AP2/EREBP and WRKY in leaves as well as GST in root tips showed distinct space-altered patterns under light and dark conditions in all three genotypes. The only exception is WRKY genes in root tips, exhibiting 17% and 50% DEGs with distinct space-altered patterns in the light and in the dark for Col-0 and phyD, respectively, implying the light-independent regulation of WRKY genes in the ecotype of Col-0.
a Gene family enrichment analysis for 8477 leaf DEGs and 716 root tip DEGs between Flight and Ground Control in at least one comparison of the CARA experiments. The threshold for DEGs is log2(fold-change) >1 or < –1, Padj <0.05, and for enrichment is Padj <0.05. Heatmap is showing the -log10(Padj) for each significantly enriched gene family. b The differential expression pattern of genes from LHC, GST, AP2/EREBP and WRKY families in leaves, and GST and WRKY families in root tips. The heatmap is plotted in the same way as Fig. 2c. c Percentage of DEGs from listed gene families with distinct space-altered patterns in response to spaceflight under light and dark conditions in leaves and root tips, respectively.
PHYD-dependent spaceflight-altered genes
PHYD functions in red/far-red light sensing in Col-0, but Ws is naturally deficient in the gene encoding PHYD18. In root tips, mutation of PHYD in Col-0 disrupted the regulation of multiple gene categories during spaceflight, many associated with cell wall metabolism and defense17. In leaves, pathways related to cell wall metabolism and defense were also influenced in the spaceflight response in Col-0 but not in the phyD mutants (Fig. 3a), suggesting that both root tips and leaves share a set of key pathways regulated by PHYD in response to spaceflight. There were a set of DEGs with significant changes between Flight and Ground Control only in Col-0, but not in phyD, as well as significant changes between phyD and Col-0 only in spaceflight, but not on earth (Fig. 5a). These genes were defined as PHYD-dependent spaceflight-altered genes, which showed similar expression levels between Col-0 and phyD on the ground, but had significant responses only in wild type plants in space (Supplementary Table 2). In the light, there were 71 PHYD-dependent space-altered genes, with 63 up-regulated and 8 down-regulated only in Col-0 (Fig. 5b). GSTU12 from GST family21 and a lipoxygenase gene LOX223 were examples showing the up- and down-regulation in space response of Col-0 but not phyD, respectively (Fig. 5c). In the dark, 28 PHYD-dependent space-altered genes were identified (Fig. 5d), with 20 up-regulated and 8 down-regulated only in Col-0 (Fig. 5e). LHCA1 from LHC family24 and an auxin-responsive gene AT3G6184025 were examples of PHYD-dependent space-altered genes in the dark (Fig. 5f).
a 71 PHYD-dependent spaceflight-altered genes identified using overlap analysis for DEGs in the light. These genes are significantly changed in transcription by spaceflight in Col-0, but not in phyD, and they only show significant difference of transcription between Col-0 and phyD in space, not on earth. b Differential expression pattern of 71 genes from (a) between Flight and Ground Control in Col-0 and phyD. c Examples of PHYD-dependent spaceflight-altered genes in the light, which were significantly induced (GSTU12) or repressed (LOX2) by spaceflight in Col-0, but not in phyD. d 28 PHYD-dependent spaceflight-altered genes are identified using overlap analysis for DEGs in the dark. e Differential expression pattern of 28 genes from (d) between Flight and Ground Control in Col-0 and phyD. f Examples of PHYD-dependent spaceflight-altered genes in the dark, which were significantly induced (LHCA1) or repressed (AT3G61840) by spaceflight in Col-0, but not in phyD. In histograms, gene expression level is indicated by average count per million mapped reads (CPM) in RNA-seq. Lowercase letters indicate significant differences between samples (Padj <0.05 in DESeq2). The heatmap is plotted in the same way as Fig. 2c.
Ecotype-related spaceflight-altered genes in Col-0 or Ws
Next, ecotype-related spaceflight-altered genes were identified through overlap analysis of DEGs in response to spaceflight from Col-0 and Ws. These identified genes showed similar expression levels between Col-0 and Ws on the ground, and had significant differential responses in only one of these two ecotypes in space (Supplementary Table 2). In the light, 486 spaceflight-altered genes presented in Col-0 were not significantly changed in the response of Ws to spaceflight (Fig. 6a). These genes differentially expressed in Col-0 but not in Ws in space, which were defined as Ws-deficient spaceflight-altered genes. They were enriched in four gene families functioning in plant developmental processes and stress responses, including ATP-binding cassette (ABC) transporter26, GST21, NAC27 and WRKY22 (Fig. 6b). GSTU24 and GSTU18 were two examples of this kind (Fig. 6c). Meanwhile, there were 292 genes altered in Ws but not in Col-0 in response to spaceflight, and they had similar expression levels between Col-0 and Ws on earth (Fig. 6a). They were defined as Col-0-deficient spaceflight-altered genes. No gene family was enriched for these Col-0-deficient spaceflight-altered genes, but abiotic stress responsive genes were observed, such as DES128,29 that was only induced in Ws, and CBF130 that was only repressed in Ws (Fig. 6d). In the dark, there were 51 and 408 spaceflight-altered genes without space response in Ws and Col-0, respectively (Fig. 7a). The 408 Col-0-deficient spaceflight-altered genes were enriched in AP2/EREBP and SWEET families functioning in plant development and abiotic stress responses31,32 (Fig. 7b). JAZ8 and RAP2.1 were two examples of this kind (Fig. 7d). No gene family was enriched for 51 Ws-deficient spaceflight-altered genes, but LHC and GST genes were observed (Fig. 7c).
a 486 Ws-deficient and 292 Col-0-deficient spaceflight-altered genes were identified in overlap analysis in the samples of the light. These genes are significantly changed in transcription by spaceflight either in Col-0 or in Ws, not in both, and they only show significant difference of transcription between Col-0 and Ws in space, not on earth. Differential expression patterns between Flight and Ground Control in Col-0 and Ws are shown for Ws-deficient and Col-0-deficient genes. The heatmap is plotted in the same way as Fig. 2c. b Gene family enrichment analysis using 486 Ws-deficient spaceflight-altered genes. Heatmap is plotted in the same way of Fig. 4a. c Examples of Ws-deficient spaceflight-altered genes, which were significantly induced (GSTU24) or repressed (GSTU18) by spaceflight in Col-0, but not in Ws. d Examples of Col-0-deficient spaceflight-altered genes, which were significantly induced (DES1) or repressed (CBF1) by spaceflight in Ws, but not in Col-0. Gene expression level is presented in the same way as Fig. 5c.
a 51 Ws-deficient and 408 Col-0-deficient spaceflight-altered genes were identified in overlap analysis in the samples of the dark. Differential expression patterns between Flight and Ground Control in Col-0 and Ws are shown for Ws-deficient and Col-0-deficient genes. The heatmap is plotted in the same way as Fig. 2c. b Gene family enrichment analysis using 408 Col-0-deficient spaceflight-altered genes. The heatmap is plotted in the same way as Fig. 4a. c Examples of Ws-deficient spaceflight-altered genes, which were significantly induced (LHCA2) or repressed (GSTU15) by spaceflight in Col-0, but not in Ws. d Examples of Col-0-deficient spaceflight-altered genes, which were significantly induced (JAZ8) or repressed (RAP2.1) by spaceflight in Ws, but not in Col-0. Gene expression level is presented in the same way as Fig. 5c.
PHYD-dependent and ecotype-related spaceflight-altered genes were regulated in a light-dependent manner in response to spaceflight
Between Arabidopsis leaves grown in the light and in the dark, zero PHYD-dependent space-altered genes were overlapped, indicating that PHYD regulated distinct transcriptional downstream sigaling of adaptive responses to space under light and dark environments (Fig. 8a). Similarly, there was also a small overlap for ecotype-related spaceflight-altered genes in leaves under light and dark conditions (Fig. 8b). In contrast, there were ~43% (31 out of 71 in the light and 12 out of 28 in the dark) PHYD-dependent spaceflight-altered genes were also Ws-deficient in response to spaceflight in both light and dark conditions (Fig. 8a and Supplementary Table 2). The shared space-regulated DEGs between phyD and Ws, which is naturally deficient in PHYD, indicated a role of PHYD in regulating spaceflight-altered genes.
Discussion
Light conditions greatly influence growth, development, and environmental stress responses of plants33,34,35,36. While plant responses to terrestrial abiotic and biotic stresses can be strongly affected by both light intensity and quality36, there are also many key stress-associated genes that respond to the stress regardless of the lighting environment. The degree of induction or repression of these key stress responsive genes can vary among plants and instances, but these stress responsive genes typically show a consistent direction of response (induced or repressed) irrespective of the lighting status of the stress environment. Examples of this kind of key responsive genes are observed across multiple abiotic and biotic stress responses in terrestrial environments, such as HY5, CBF and COR genes in cold response37,38,39,40, PIF4 and HSP70 in heat response41,42, RAP2.4 in drought and salt responses43, and PR1 in response to tobacco mosaic virus44, etc. In contrast, there is no single set of genes showing universally consistent patterns of response to the spaceflight environment, and there is substantial variability among flight experiments, even when identical genotypes and tissues are sampled. Rather, there is a set of key categories of gene families and pathways that are commonly engaged in plant responses to the spaceflight environment. The genes comprising these families and pathways are widely observed in previous spaceflight experiments15,45, as well as in both the light and the dark in the CARA flight leaves (Fig. 9).
The major categories of spaceflight responsive genes that are differentially expressed in both the light and the dark in CARA represent pathways related to defense, oxidative stress response, light signaling/photosynthesis, and secondary metabolism. These categories of genes are very typical of the spaceflight response in plants and have been seen in experiments representing a wide variety of growth hardware and spaceflight environments. For example, among Arabidopsis spaceflight experiments grown on the ISS in diverse environments such as the BRIC (Biological Research in Canisters) hardware (both light- and dark-grown), the Vegetable production system (VEGGIE, directional light-grown), the Advanced Biological Research System (ABRS, directional light-grown), the European Modular Cultivation System (EMCS, controlled white, blue, or red light, or dark), and even on the ISS without plant-growth hardware (the bulkhead-grown CARA experiment presented here), these key gene categories are represented in the differentially expressed genes8,12,15,46,47,48,49,50,51,52,53,54,55,56. The connection can even be extended to completely different spaceflight platforms, which show that these key gene categories are also engaged by seedlings grown on the SJ-10 satellite (16 h light/8 h dark cycle) and the TG-2 orbital laboratory (light-grown)57,58. CARA with both light and dark growth conditions, therefore in many ways is typical of plant spaceflight responses.
The CARA experiment was, however, specifically designed to compare directly the impact of the lighting environment on spaceflight grown plants of three genotypes. CARA conducted parallel, concomitant light and dark growth conditions in the same flight experiment, thereby enabling side-by-side comparison of space-altered transcriptome in the light and in the dark. The data presented here suggest that variations in light conditions among spaceflight experiments have a substantial impact on the transcriptional patterns of key spaceflight-associated genes. In CARA leaves, the vast majority of genes differentially expressed in space (more than 99% of the DEGs in Col-0 and phyD, and more than 97% of the DEGs in Ws) have a distinct light-dependent response to spaceflight (Fig. 2b). Significant differential expression of key space-responsive gene families, such as LHC, were prominently represented in the CARA leaf transcriptomes (Fig. 4a), but the representatives from the key gene families are regulated in opposite directions with respect to the lighting environment; in most cases, if a key gene is up-regulated in the light, it is down-regulated or not significantly regulated in the dark, and vice versa (Fig. 4b). This trend is also observed for most pathway categorizations that are enriched in both the light and the dark in response to spaceflight (center column, Fig. 9); again, if the common pathway shows up-regulation in the light, it shows down-regulation in the dark (Fig. 9).
The nature of the lighting environment may also play a role in spaceflight associated gene expression. In the CARA experiment, plants were grown in the ambient, indirect lighting of the Destiny module on the ISS. The diffuse ambient lighting would provide general light signaling cues to plants, but might not serve as a powerful directional cue compared to plant growth facilities with strong and directional lighting17. The combination of an environment lacking a directional cue for gravity with a diminished directional cue for light may have contributed to the complexity of the leaf transcriptome in CARA compared to the leaf spaceflight transcriptomes for Ws and Col-0 plants grown in habitats with strong directional lighting8,54,55,56. The transcriptomes from dark-grown plants, which lack both gravity and light cues, are substantially different than the transcriptomes from light-grown leaves. In addition to the opposite direction of regulation in the key set of spaceflight-associated genes in light versus dark plants (Fig. 4), there were also far more genes differentially expressed in the light than in the dark (Figs. 1a, 3a), which might indicate the sensitivity of leaves to small variation in ambient light. These DEGs represented a larger number of metabolic pathways under light condition (Fig. 9). In the light, more stress-responsive signaling pathways were activated, and more pathways related to cell wall and cell cycle were repressed. In the dark, more genes involved in ethylene and jasmonic acid signaling were induced, while more ion transport and chemical homeostasis pathways were down-regulated in the absence of both light and gravity. In contrast, for root tips that can be more sensitive to environmental cues of tropic growth, more DEGs were detected in the dark with neither light nor gravity cues in spaceflight compared with the 1 g condition on the ground.
Phytochromes play a key role in light sensing of plants during spaceflight on the ISS1,6,9,10,47, and the CARA data suggest that the phytochrome gene family may play a broader role in an environment that relies on light as the primary tropic cue. In addition to modulating light-signaling responses, the phyD mutation influenced the space-altered modulation of defense and oxidative stress in both leaves and root tips, and desiccation response, cell wall metabolism and secondary metabolic process in leaves (Fig. 9). Thus, phytochrome D appeared to be involved in the modulatory network of stress responsive signaling and metabolic processes in response to spaceflight, in which the regulatory patterns of downstream genes are related to light conditions. The loss-of-function mutation of PHYD in Col-0 or the natural deficiency of PHYD in Ws did not severely compromise processes engaged in physiological adaptation of Arabidopsis to spaceflight. The elimination or adjustment of some of these genes may not compromise the plant health in space but can potentially reduce the metabolic cost. Given the largely altered transcription pattern in response to spaceflight (Fig. 2a), there were still considerably overlapped DEGs in Col-0, phyD and Ws, especially in the light (Fig. 2d). The phyD mutant altered the manner in which light-grown leaves responded to spaceflight, and many DEGs associated with the physiological adaptation of Col-0 to spaceflight were absent in the phyD plants. The lack of PHYD created the necessity for the plants to adjust the regulatory network required to deploy similar signaling pathways to establish physiological adaptation (Fig. 3a, pathways labeled by colored bars of light yellow, light green and dark green on the left).
The key genes that were differentially expressed in CARA are typical of the Arabidopsis spaceflight response and represent pathways identified by the mega-analyses conducted by Barker and colleagues across a variety of spaceflight environments15. However, the unique experimental design of CARA added another layer of perspective, by providing direct tissue-specific, genotype-specific and lighting-specific expression patterns in response to spaceflight. There was a much stronger transcriptomic response to spaceflight in leaves than in root tips as measured by DEG numbers (Fig. 1b). Meanwhile, these two tissues shared some space-altered gene categories, such as genes from GST and WRKY families (Fig. 4a), and genes enriched in pathways of defense, cell wall metabolism, and oxidative stress response17. However, the DEGs of these common space-altered gene categories were not always coordinately expressed in the two tissues (Fig. 3a, Fig. 9). There were many ecotype-dependent patterns of gene expression associated with spaceflight adaptation. Col-0 and Ws both engaged pathways involved in developmental regulation and abiotic stress responses, but each expressed different genes representing these categories (Fig. 6, Fig. 7, Fig. 9). And all of these tissue-specific and ecotype-related responses to spaceflight exhibit distinctly different patterns of expression between light and dark environments. (Fig. 2c, Fig. 8).
The transcriptomic profiles of the CARA biology suggest a dominant role of the light environment in the plant physiological adaptation to spaceflight. This study illustrates the distinct behaviors of genes deployed in plant responses to spaceflight under light and dark environments. The presence or absence of light greatly influences the genome-wide regulation of transcription across plant tissues and ecotypes. Different plant tissues, ecotypes or mutants with genetic deficiency in certain genes, can engage different sets of genes from a series of key categories in response to spaceflight-associated habitats. The genes from these key categories would be significantly changed by spaceflight, but their expression patterns are dependent on the light conditions. In comparison, the light-dependent influence on plant transcriptomic responses in orbital spaceflight is more overwhelming than other effects from genotypes or ecotypes. Future experimental design with respect to genetic engineering or genome editing for improved adaptation of plants to spaceflight habitats will require coordination in the selection of genetically modified targets and the establishment of optimal light conditions.
Methods
CARA experiment operations
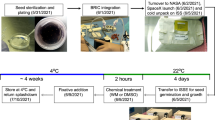
The CARA (Characterizing Arabidopsis Root Attractions) experiment performed on the ISS has been documented in Paul et al. 17. Briefly, the experiment launched on SpaceX CRS-3 under the NASA operations nomenclature (OpNom) of “Petri Plants” and was one of the ARK1 payloads flown by the Center for Advancement of Science in Space (CASIS) (https://www.nasa.gov/mission/station/research-explorer/investigation/?#id=1020).
Three lines of Arabidopsis thaliana (Arabidopsis) including two wild-types, Columbia-0 (Col-0) and Wassilewskija (Ws), and a Col-0 PHYD mutant (phyD, SALK_027956C), were grown in phytagel plates on orbit or at Kennedy Space Center (KSC) within the ISS Environment Simulator (ISSES) Chamber for 11 days. Six plates were used for each genotype and 11 seedlings were planted in each plate. Seedlings were grown in the ambient, diffuse light (4–6 μmol m–2 s−1) of the ISS cabin wall, or in the dark (plates were unwrapped for 4 h to activate germination, then re-wrapped by black-out cloth, which were also affixed to the wall). The ground control in the ISSES (ISS Environmental Simulation) chamber of KSC were performed 24 h later than spaceflight samples, allowing the manipulation of lighting and atmospheric composition for the ISSES to mimic the conditions that plants were experiencing on the ISS. The 11-day old plants were harvested into RNAlater in Kennedy Space Center Fixation Tubes (KFTs) and kept for 12–18 h at ambient temperature to ensure perfusion before being transferred to the MELFI freezer for storage on orbit. The KFTs were also kept frozen in transit to Earth and KSC, and during the transportation back to UF Space Plants Lab.
RNA extraction and sequencing
Seedlings frozen in RNAlater were thawed at 4 °C and then leaf tissues were dissected from the hypocotyl using a dissecting microscope and collected. The plants used in this study were from the same plates as those used for the collection of root tips in Paul et al. 17. All leaves from one plate were pooled for RNA extraction, thus one plate comprised a biological replicate. RNA extraction was conducted using RNeasy Plant Mini Kit (Qiagen, Germantown, USA). Three biological replicates were used for RNA-seq. RNA samples were multiplexed and sequenced in the NovaSeq 6000 platform with 2 × 150 bp reads at the Interdisciplinary Center for Biotechnology Research (ICBR), University of Florida, including Core of Gene Expression and Genotyping (RRID:SCR_019145) for library construction and Core of NextGen DNA sequencing (RRID:SCR_019152) for sequencing. More than 60 million paired-end reads were obtained per library.
Transcriptome data analysis
After demultiplexing, sequencing adaptors and low-quality bases with quality phred-like score <20 were trimmed using the cutadapt program59. Reads that were shorter than 40 bases were excluded from RNA-seq analysis. RNA-seq analysis was using the genome of Arabidopsis thaliana (version TAIR10.51) from TAIR (The Arabidopsis Information Resource) as the reference. The cleaned reads of each sample were mapped to the reference sequences using the read mapper of the STAR package (Spliced Transcripts Alignment to a Reference, v2.7.9a)60. The resulted mapping data were processed through HTSeq (High-Throughput Sequence Analysis in Python, v0.11.2)61, samtools, and scripts developed in house at ICBR to remove potential PCR duplicates, filter and count uniquely mapped reads for transcriptomic analysis62. The counted reads for each gene were analyzed by a DESeq2-based R pipeline. Differentially expressed genes (DEGs) with statistical significance were selected using a threshold of log2(fold-change) >1 or < −1, and FDR adjusted P value (Padj) < 0.05. (ICBR Bioinformatics Core, RRID:SCR_019120).
Process and pathway enrichment analysis was done using Metascape (https://metascape.org/gp/index.html#/main/step1)63. Ontology sources used in Metascape included GO Biological Processes, KEGG Pathways and WikiPathways. Gene family enrichment was performed using GenFam (https://www.mandadilab.com/genfam/)64. In these enrichment tests, P values were calculated and adjusted using default settings. The threshold for significant enrichment was Padj < 0.05. Venn diagrams were plotted using DeepVenn (http://www.deepvenn.com/).
Data availability
RNA-seq data of CARA leaves samples have been deposited with the accession number of PRJNA1069768 in BioProject (https://www.ncbi.nlm.nih.gov/bioproject/) of National Center for Biotechnology Information (NCBI) and with the OSD identifier of OSD-678 (DOI: 10.26030/65t6-7p29) in NASA GeneLab (https://genelab.nasa.gov/). CARA root tips transcriptome data and phenotype photos are deposited under OSD-120 (DOI: 10.26030/0w7t-3128) in NASA GeneLab.
Code availability
All codes used in this work have been described in methods section. No previously unpublished codes were used.
References
Vandenbrink, J. P., Kiss, J. Z., Herranz, R. & Medina, F. J. Light and gravity signals synergize in modulating plant development. Front. Plant Sci. 5, 563 (2014).
Yang, P. et al. Light modulates the gravitropic responses through organ-specific PIFs and HY5 regulation of LAZY4 expression in Arabidopsis. Proc. Natl. Acad. Sci. 117, 18840–18848 (2020).
Wheeler, R. M. Plants for human life support in space: From Myers to Mars. Gravitational Space Biol. 23, 25–36 (2010).
Zabel, P., Bamsey, M., Schubert, D. & Tajmar, M. Review and analysis of over 40 years of space plant growth systems. Life Sci. Space Res. 10, 1–16 (2016).
Paul, A.-L., Amalfitano, C. E. & Ferl, R. J. Plant growth strategies are remodeled by spaceflight. BMC Plant Biol. 12, 232 (2012).
Kiss, J. Z., Millar, K. D. L. & Edelmann, R. E. Phototropism of Arabidopsis thaliana in microgravity and fractional gravity on the International Space Station. Planta 236, 635–645 (2012).
Wu, Y., Xie, J., Wang, L. & Zheng, H. Circumnutation and growth of inflorescence stems of Arabidopsis thaliana in response to microgravity under different photoperiod conditions. Life 10, 26 (2020).
Paul, A.-L., Zupanska, A. K., Schultz, E. R. & Ferl, R. J. Organ-specific remodeling of the Arabidopsis transcriptome in response to spaceflight. BMC Plant Biol. 13, 112 (2013).
Vandenbrink, J. P., Herranz, R., Medina, F. J., Edelmann, R. E. & Kiss, J. Z. A novel blue-light phototropic response is revealed in roots of Arabidopsis thaliana in microgravity. Planta 244, 1201–1215 (2016).
Valbuena, M. A. et al. The combined effects of real or simulated microgravity and red-light photoactivation on plant root meristematic cells. Planta 248, 691–704 (2018).
Herranz, R. et al. RNAseq analysis of the response of Arabidopsis thaliana to fractional gravity under blue-light stimulation during spaceflight. Front. Plant Sci. 10, 1529 (2019).
Paul, A.-L. et al. Spaceflight transcriptomes: Unique responses to a novel environment. Astrobiology 12, 40–56 (2012).
Manzano, A., Carnero-Diaz, E., Herranz, R. & Medina, F. J. Recent transcriptomic studies to elucidate the plant adaptive response to spaceflight and to simulated space environments. iScience 25, 104687 (2022).
Hughes, A. M. & Kiss, J. Z. -Omics studies of plant biology in spaceflight: A critical review of recent experiments. Front. Astron. Space Sci. 9, 964657 (2022).
Barker, R. et al. Meta-analysis of the space flight and microgravity response of the Arabidopsis plant transcriptome. Npj Microgravity 9, 1–15 (2023).
Villacampa, A. et al. From spaceflight to Mars g-Levels: Adaptive Response of A. Thaliana seedlings in a reduced gravity environment is enhanced by red-light photostimulation. Int. J. Mol. Sci. 22, 899 (2021).
Paul, A.-L. et al. Genetic dissection of the Arabidopsis spaceflight transcriptome: Are some responses dispensable for the physiological adaptation of plants to spaceflight? PLOS ONE 12, e0180186 (2017).
Aukerman, M. J. et al. A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell 9, 1317–1326 (1997).
Rochaix, J.-D. & Bassi, R. LHC-like proteins involved in stress responses and biogenesis/repair of the photosynthetic apparatus. Biochem. J. 476, 581–593 (2019).
Paul, A.-L. et al. Arabidopsis gene expression patterns are altered during spaceflight. Adv. Space Res. 36, 1175–1181 (2005).
Sappl, P. G. et al. The Arabidopsis glutathione transferase gene family displays complex stress regulation and co-silencing multiple genes results in altered metabolic sensitivity to oxidative stress. Plant J. 58, 53–68 (2009).
Chen, X., Li, C., Wang, H. & Guo, Z. WRKY transcription factors: evolution, binding, and action. Phytopathol. Res. 1, 13 (2019).
Mochizuki, S., Sugimoto, K., Koeduka, T. & Matsui, K. Arabidopsis lipoxygenase 2 is essential for formation of green leaf volatiles and five-carbon volatiles. FEBS Lett. 590, 1017–1027 (2016).
Novoderezhkin, V. I. & Croce, R. The location of the low-energy states in Lhca1 favors excitation energy transfer to the core in the plant PSI-LHCI supercomplex. Photosynth. Res. 156, 59–74 (2023).
Jakoby, M. J. et al. Transcriptional profiling of mature arabidopsis trichomes reveals that NOECK encodes the MIXTA-like transcriptional regulator MYB106. Plant Physiol. 148, 1583–1602 (2008).
Hwang, J.-U. et al. Plant ABC transporters enable many unique aspects of a terrestrial plant’s lifestyle. Mol. Plant 9, 338–355 (2016).
Singh, S., Koyama, H., Bhati, K. K. & Alok, A. The biotechnological importance of the plant-specific NAC transcription factor family in crop improvement. J. Plant Res. 134, 475–495 (2021).
Alvarez, C., Calo, L., Romero, L. C., García, I. & Gotor, C. An O-acetylserine(thiol)lyase homolog with l-cysteine desulfhydrase activity regulates cysteine homeostasis in arabidopsis. Plant Physiol. 152, 656–669 (2010).
Alvarez, C. et al. Cysteine-generated sulfide in the cytosol negatively regulates autophagy and modulates the transcriptional profile in arabidopsis. Plant Cell 24, 4621–4634 (2012).
Zhou, M. Q., Shen, C., Wu, L. H., Tang, K. X. & Lin, J. CBF-dependent signaling pathway: A key responder to low temperature stress in plants. Crit. Rev. Biotechnol. 31, 186–192 (2011).
Riechmann, J. & Meyerowitz, E. The AP2/EREBP family of plant transcription factors. Biol. Chem. 379, 633–654 (1998).
Ji, J. et al. Plant SWEET family of sugar transporters: structure, evolution and biological functions. Biomolecules 12, 205 (2022).
Ma, L. et al. Light control of arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13, 2589–2607 (2001).
Sun, N. et al. Arabidopsis SAURs are critical for differential light regulation of the development of various organs. Proc. Natl. Acad. Sci. 113, 6071–6076 (2016).
Kumari, S. et al. Uncovering the molecular signature underlying the light intensity-dependent root development in Arabidopsis thaliana. BMC Genomics 20, 596 (2019).
Roeber, V. M., Bajaj, I., Rohde, M., Schmülling, T. & Cortleven, A. Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 44, 645–664 (2021).
Fowler, S. G., Cook, D. & Thomashow, M. F. Low temperature induction of arabidopsis CBF1, 2, and 3 is gated by the circadian clock. Plant Physiol. 137, 961–968 (2005).
Graham, C. A., Paajanen, P., Edwards, K. J. & Dodd, A. N. Genome-wide circadian gating of a cold temperature response in bread wheat. PLOS Genet 19, e1010947 (2023).
Soitamo, A. J., Piippo, M., Allahverdiyeva, Y., Battchikova, N. & Aro, E.-M. Light has a specific role in modulating Arabidopsis gene expression at low temperature. BMC Plant Biol. 8, 13 (2008).
Catalá, R., Medina, J. & Salinas, J. Integration of low temperature and light signaling during cold acclimation response in Arabidopsis. Proc. Natl Acad. Sci. 108, 16475–16480 (2011).
Tan, W. et al. The heat response regulators HSFA1s promote Arabidopsis thermomorphogenesis via stabilizing PIF4 during the day. Sci. Adv. 9, eadh1738 (2023).
Dickinson, P. J. et al. Chloroplast signaling gates thermotolerance in arabidopsis. Cell Rep. 22, 1657–1665 (2018).
Lin, R.-C., Park, H.-J. & Wang, H.-Y. Role of Arabidopsis RAP2.4 in regulating light- and ethylene-mediated developmental processes and drought stress tolerance. Mol. Plant 1, 42–57 (2008).
Chandra-Shekara, A. C. et al. Light-dependent hypersensitive response and resistance signaling against Turnip Crinkle Virus in Arabidopsis. Plant J. 45, 320–334 (2006).
Barker, R., Lombardino, J., Rasmussen, K. & Gilroy, S. Test of arabidopsis space transcriptome: A discovery environment to explore multiple plant biology spaceflight experiments. Front. Plant Sci. 11, 147 (2020).
Land, E. S., Sheppard, J., Doherty, C. J. & Perera, I. Y. Conserved plant transcriptional responses to microgravity from two consecutive spaceflight experiments. Front. Plant Sci. 14, 1308713 (2024).
Medina, F.-J., Manzano, A., Herranz, R. & Kiss, J. Z. Red light enhances plant adaptation to spaceflight and Mars g-levels. Life 12, 1484 (2022).
Olanrewaju, G. O. et al. Integrative transcriptomics and proteomics profiling of Arabidopsis thaliana elucidates novel mechanisms underlying spaceflight adaptation. Front. Plant Sci. 14, 1260429 (2023).
Kwon, T. et al. Transcriptional response of Arabidopsis seedlings during spaceflight reveals peroxidase and cell wall remodeling genes associated with root hair development. Am. J. Bot. 102, 21–35 (2015).
Johnson, C. M., Subramanian, A., Pattathil, S., Correll, M. J. & Kiss, J. Z. Comparative transcriptomics indicate changes in cell wall organization and stress response in seedlings during spaceflight. Am. J. Bot. 104, 1219–1231 (2017).
Choi, W.-G., Barker, R. J., Kim, S.-H., Swanson, S. J. & Gilroy, S. Variation in the transcriptome of different ecotypes of Arabidopsis thaliana reveals signatures of oxidative stress in plant responses to spaceflight. Am. J. Bot. 106, 123–136 (2019).
Kruse, C. P. S. et al. Spaceflight induces novel regulatory responses in Arabidopsis seedling as revealed by combined proteomic and transcriptomic analyses. BMC Plant Biol. 20, 237 (2020).
Angelos, E., Ko, D. K., Zemelis-Durfee, S. & Brandizzi, F. Relevance of the unfolded protein response to spaceflight-induced transcriptional reprogramming in arabidopsis. Astrobiology 21, 367–380 (2021).
Zhou, M., Sng, N. J., LeFrois, C. E., Paul, A.-L. & Ferl, R. J. Epigenomics in an extraterrestrial environment: organ-specific alteration of DNA methylation and gene expression elicited by spaceflight in Arabidopsis thaliana. BMC Genomics 20, 205 (2019).
Califar, B., Sng, N. J., Zupanska, A., Paul, A.-L. & Ferl, R. J. Root skewing-associated genes impact the spaceflight response of arabidopsis thaliana. Front. Plant Sci. 11, 239 (2020).
Paul, A.-L., Haveman, N., Califar, B. & Ferl, R. J. Epigenomic regulators elongator complex subunit 2 and methyltransferase 1 differentially condition the spaceflight response in arabidopsis. Front. Plant Sci. 12, 691790 (2021).
Xie, J., Wang, L. & Zheng, H. Molecular basis to integrate microgravity signals into the photoperiodic flowering pathway in arabidopsis thaliana under spaceflight condition. Int. J. Mol. Sci. 23, 63 (2021).
Wang, L. et al. Transcriptomic analysis of the interaction between FLOWERING LOCUS T induction and photoperiodic signaling in response to spaceflight. Front. Cell Dev. Biol. 9, 813246 (2022).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10–12 (2011).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Haveman, N. J. et al. Utilizing the KSC fixation tube to conduct human-tended plant biology experiments on a suborbital spaceflight. Life 12, 1871 (2022).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Bedre, R. & Mandadi, K. GenFam: A web application and database for gene family-based classification and functional enrichment analysis. Plant Direct 3, e00191 (2019).
Acknowledgements
This work was supported by grant number GA-2013-104, Center for Advancement of Science in Space (CASIS) awarded to A-LP and RJF. We thank the support from all members of UF Space Plants Lab, and members of UF ICBR cores of Gene Expression and Genotyping (RRID:SCR_019145), NextGen DNA sequencing (RRID:SCR_019152), and Bioinformatics (RRID:SCR_019120) for their support and services. The astronaut attending the experiment on the ISS was Expedition 39 Flight Engineer Steve Swanson.
Author information
Authors and Affiliations
Contributions
A.-L.P. and R.J.F. conceived and designed the CARA project. M.Z. performed RNA extraction and transcriptomic analysis. M.Z., A.-L.P., and R.J.F. wrote the manuscript. All authors approved the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, M., Ferl, R.J. & Paul, AL. Light has a principal role in the Arabidopsis transcriptomic response to the spaceflight environment. npj Microgravity 10, 82 (2024). https://doi.org/10.1038/s41526-024-00417-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41526-024-00417-0
- Springer Nature Limited