Abstract
Hydrogen in metals is a significant research area with far-reaching implications, encompassing diverse fields such as hydrogen storage, metal-insulator transitions, and the recently emerging phenomenon of room-temperature superconductivity under high pressure. Hydrogen atoms pose challenges in experiments as they are nearly invisible, and they are considered within ideal crystalline structures in theoretical predictions, which hampers research on the formation of metastable hydrides. Here, we propose pressure-induced hydrogen migration from tetrahedral (T-) site to octahedral (O-) site, forming \({{\rm{LaH}}}_{x}^{{\rm{O}}}{{\rm{H}}}_{2-x}^{{\rm{T}}}\) in cubic LaH2. Under decompression, it retains \({{\rm{H}}}_{x}^{{\rm{O}}}\) occupancy, and is dynamically stable even at ambient pressure, enabling a synthesis route of metastable dihydrides via compression-decompression process. We predict that the electron-phonon coupling strength of \({{\rm{LaH}}}_{x}^{{\rm{O}}}{{\rm{H}}}_{2-x}^{{\rm{T}}}\) is enhanced with increasing x, and the associated Tc reaches up to 10.8 K at ambient pressure. Furthermore, we calculated stoichiometric hydrogen migration threshold pressure (Pc) for various lanthanides dihydrides (RH2, where R = Y, Sc, Nd, and Lu), and found an inversely linear relation between Pc and ionic radii of R. We propose that the highest Tc in the face-centered-cubic dihydride system can be realized by optimizing the O/T-site occupancies.
Similar content being viewed by others
Introduction
Since Ashcroft suggested that hydrogen-dense materials could have high superconductivity transition temperature (Tc) at a relatively lower pressure than pure solid hydrogen by chemical precompression onto the hydrogen sublattice1, the hydrogen-rich materials have been intensively studied both in experimental and theoretical research2,3,4,5,6,7,8. The remarkable advances in accessible pressures in diamond anvil cells and the development of theoretical methods have accelerated the research on hydride superconductors. Among them, the superconductivity was observed in sulfur hydride with a maximum Tc of 203 K9. Most notably, rare-earth superhydride materials10,11,12 with hydrogen-rich clathrate sublattice are proposed to possess high Tc with finite electronic density of states (DOS) of hydrogen atoms at the Fermi level as well as strong electron-phonon coupling strength (λ). Eventually, experimental studies reported that LaH10 exhibits high-Tc superconductivity with Tc = 250 K at 300 GPa13,14.
While near-room-temperature superconductivity has been observed in rare-earth superhydrides under extremely high pressure, achieving superconducting properties at ambient pressure remains challenging due to the decomposition of the hydrogen sublattice into hydrogen molecules. Recently, there has been a strong research interest in finding ways to lower the transition pressure, making it more practical and feasible for technology. For example, metastable super hydrides derived from LaH10 have been proposed as candidates exhibiting superconductivity at lower pressures. Cataldo et al. proposed a ternary sodalite-like LaBH8 consisting of La-B scaffold and hydrogen sublattice15. They predicted that LaBH8 is thermodynamically stable above 100 GPa, and dynamically stable down to 40 GPa with Tc of 126 K. Zhang et al. suggested ternary hydrides with a fluorite-type backbone (XH8)16. They reported that the LaBeH8 is thermodynamically stable above 98 GPa with Tc of 192 K, and metastable under a pressure of 29 GPa with Tc of 193 K.
Rare-earth dihydride RH2 has a metallic ground state at ambient pressure. RH2 consists of face-centered-cubic (fcc)-R with hydrogen atoms intercalated at symmetric tetrahedral (T-) sites keeping the octahedral (O-) site empty17 (see Fig. 1a). When additional hydrogen atoms are introduced to RH2, they are located at O-site forming RH3. It is known that the Tcs in RH2 are negligible at ambient pressure18,19,20,21.
a The crystal structure of pure La (space group: \({Fm}3m\)) in the conventional unit cell displaying tetrahedral (T-) sites and octahedral (O-) sites corresponding open and filled circles, respectively. ELF on the [-110] plane of b pure La and c LaH2 d ELF isosurface of LaH2 with a cutoff of 0.5. e The relative formation enthalpy ΔH of \({\rm{La}}{{\rm{H}}}_{2}\) and \({\rm{LaH}}+{\rm{La}}{{\rm{H}}}_{3}\) as a function of pressure. The insets show the internal energy ΔE (upper right), and the pressure-volume term ΔPV (lower left) with pressure. f The variation of the energy along the reaction path between La\({{\rm{H}}}_{2}^{T}\) and \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) as a function of pressure. (The inset shows the migration path (black dashed arrow) of one hydrogen from T-site to O-site within the primitive cell.) The black arrow indicates the variation of the highest point of kinetic barriers with pressure.
Machida et al. reported that LaD2 undergoes disproportionation reaction to form both the D-deficient NaCl-type LaD and D-rich LaD3 above 11 GPa22. Furthermore, they also found that the LaD is recovered below 4 GPa by decompression processes23, and LaD3 was still observed at the measured lowest pressure (0.2 GPa), revealing the irreversibility of pressure-induced hydrogen migration. They mentioned that D atoms of LaD2 in the T-site are transferred into the O-site by compression. In experiments, metastable hydrides have partial occupation of the two interstitial sites.
Here, we focus on dynamical stable RH2 structure with partial occupation of the T-site and the O-site in \({{\rm{LaH}}}_{x}^{{\rm{O}}}{{\rm{H}}}_{2-x}^{{\rm{T}}}\) as another route to find superconductivity at ambient pressure. We studied the structural stability and superconductivity of \({{\rm{LaH}}}_{x}^{{\rm{O}}}{{\rm{H}}}_{2-x}^{{\rm{T}}}\), and demonstrated that \({{\rm{LaH}}}_{x}^{{\rm{O}}}{{\rm{H}}}_{2-x}^{{\rm{T}}}\) is dynamically stable at ambient pressure. Also, we obtained that λ of \({{\rm{LaH}}}_{x}^{{\rm{O}}}{{\rm{H}}}_{2-x}^{{\rm{T}}}\) becomes enhanced with increasing the occupation of O-site by H, which yields the increment of Tc up to 10.8 K at ambient pressure. Furthermore, we generalized the idea of superconductivity induced by partial occupancy of H atom to other lanthanides dihydrides (RH2, R: Y, Sc, Nd, and Lu). In the case of \({{\rm{LuH}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}\), we obtained Tc = 36.23 K with λ = 1.174 at 20 GPa.
Results
Metastability of LaH2
Figure 1a shows the crystal structure of pure La (space group: \({Fm}\bar{3}m\)) with two tetrahedral (T-) sites and one octahedral (O-) site. When hydrogen atoms are introduced into the pure La metal, they prefer the T-site to the O-site, forming LaH2 with ionic bonding. The Electron localization function24 (ELF) values of La are depicted in Fig. 1b. The values of ELF at the O-sites are approximately 0.7, higher than the homogenous electron gas (0.5), which is consistent with the previous reports25,26,27. Thus, in the pure La metal, the O-site possesses excessive localized anionic electrons, indicating the formation of electride and thus potentially hosting hydrogen atoms at the site. Similarly, the ELF value at the O-site in LaH2 is calculated to be above 0.5, as shown in Fig. 1c. We speculate that additional hydrogen atoms can be bound at the O-site.
We calculated the relative formation enthalpy (ΔH) of LaH + LaH3 with respect to LaH2 with pressure, as shown in Fig. 1e. ΔH is obtained from \([{H}_{{\rm{LaH}}}+\,{H}_{{\rm{La}}{{\rm{H}}}_{3}}]/2-\,{H}_{{\rm{La}}{{\rm{H}}}_{2}}\), where the enthalpy is defined as H = E0 + PV, and E0 is internal energy at a given pressure P and volume V. At ambient pressure, ΔH is approximately 0.32 eV/f.u., indicating stable LaH2 phase. With increasing pressure, ΔH is decreased due to the rapid change of ΔPV (see the lower left inset). Eventually, ΔH turns negative at the transition pressure (Pc) near 10 GPa. Thus, the stoichiometric disproportionation reaction from 2LaH2 to LaH and LaH3 is calculated to occurs at the pressure (Pc), which is consistent with the previous result22.
To understand the stability of H occupation at O-site within LaH2, we conducted nudged elastic band (NEB) calculations migrating one H atom located at the T-site (¼, ¼, ¼) into the O-site (½, ½, ½) in \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) as shown in Fig. 1f. \({\rm{La}}{{\rm{H}}}_{2}^{{\rm{T}}}\) is energetically stable against \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) configuration by 0.67 eV/f.u. at ambient pressure. However, one can easily find that there is a kinetic barrier between T- and O- sites, suggesting \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) can be a metastable phase. The peak of kinetic barrier shifts higher with pressure (i. e. the black arrow in Fig. 1e), further stabilizing \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) configuration. We also tested it in a supercell (see Supplementary Fig. S2) to check the size effect, and the results were consistent.
Interestingly, experimental reports indicate that the pressure-induced disproportionation reaction from LaD2 to LaD + LaD3 is irreversible under decompression22, which can be explained by our calculations. Therefore, the compression-decompression process can open a pathway to study metastable LaH2 with partial O-site occupancy at ambient pressure.
Electronic structure
Figure 2a–c shows the electronic band structures and the DOS along the high-symmetry k-points in the fcc-Brillouin-zone (BZ) for LaH, \({\rm{La}}{{\rm{H}}}_{2}^{{\rm{T}}}\), and \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\). The energy levels near the Fermi level (EF) are predominantly contributed by spd-bands of La, while f-bands of La are situated well above the EF. The s band of H in \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) is more dispersive than those of LaH and \({\rm{La}}{{\rm{H}}}_{2}^{{\rm{T}}}\) as shown in Fig. 2c due to its H-H shorter distance compared with others (LaH: 2.72 Å, \({\rm{La}}{{\rm{H}}}_{2}^{{\rm{T}}}\): 2.83 Å, \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\): 2.38 Å).
a–c Electronic band structures and DOS of LaH, \({\rm{La}}{{\rm{H}}}_{2}^{{\rm{T}}}\), and \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) with fat band plot along high-symmetry lines. The sizes of circles in the band dispersion are proportional to the contribution of each orbital. d–f Charge density plot of band energy from −1 eV to EF.
Note that the DOS near EF of \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) is much higher than that of LaH and \({\rm{La}}{{{\rm{H}}}_{2}^{{\rm{T}}}}\), which can be explained by the flat band around the W point in the Brillouin-zone. The DOS of HT and HO are superposed near EF. Figure 2d–f display the contour plot of charge density of the band energy from -1 eV up to EF for LaH, \({\rm{La}}{{\rm{H}}}_{2}^{T}\) and \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\), respectively. While the hydrogen atoms of LaH and \({\rm{La}}{{\rm{H}}}_{2}^{{\rm{T}}}\) are isolated, HO and HT in \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) are weakly bound. The reduced distance between HO-HT through hydrogen migration results in significant DOS overlap near EF, enhancing the charge carriers in the metastable dihydride, \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\,\).
Superconductivity properties
In order to investigate the structural stability, electron-phonon coupling strength, and superconducting transition temperature (Tc), we have calculated the phonon dispersion relation, projected phonon density of states (PDOS), Eliashberg spectral functions \(({\alpha }^{2}F(\omega ))\), and electron-phonon coupling constants (λqυ) of \({\rm{La}}{{\rm{H}}}_{1}^{{\rm{O}}},\) \({\rm{La}}{{\rm{H}}}_{2}^{{\rm{T}}}\) and \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) at ambient pressure as shown in Fig. 3a–c. In all cases, La atoms dominate the low-frequency region (0~5 meV), while H atoms contribute to the high-frequency region. The phonon frequencies of H in \({\rm{La}}{{\rm{H}}}_{2}^{{\rm{T}}}\) (~110 meV) are higher than that of \({\rm{La}}{{\rm{H}}}_{1}^{{\rm{O}}}\) (~90 meV) due to the shorter bonding distances between La and H. In \({\rm{La}}{{\rm{H}}}_{1}^{{\rm{O}}}\) and \({\rm{La}}{{\rm{H}}}_{2}^{{\rm{T}}}\), the contributions to the spectral function \({\alpha }^{2}F(\omega )\) come from both La and H. However, their electron-phonon couplings (λtot) are mainly contributed by La due to the small DOS of H atoms at EF, as shown in Fig. 2a, b. Accordingly, their total λtots are quite small (0.28 for \({\rm{La}}{{\rm{H}}}_{1}^{{\rm{O}}}\) and 0.20 for \({\rm{La}}{{\rm{H}}}_{2}^{{\rm{T}}}\)), which results in negligibly small Tc ~ 0 K for both hydrides.
The phonon dispersion, PDOS, \({\alpha }^{2}F(\omega )\), and λqυ(ω) of a \({\rm{La}}{{\rm{H}}}_{1}^{{\rm{O}}}\), b \({\rm{La}}{{\rm{H}}}_{2}^{{\rm{T}}}\) and c \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\). The color code in the phonon dispersion represents the magnitude of λqυ. The values of d the total DOS at EF, e ωlog, f λtot, and g superconductivity Tc as the function of x in \({{\rm{LaH}}}_{x}^{{\rm{O}}}{{\rm{H}}}_{2-x}^{{\rm{T}}}\).
Interestingly, \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) shows a quite different phonon dispersion relations compared to \({\rm{La}}{{\rm{H}}}_{1}^{{\rm{O}}}\) and \({\rm{La}}{{\rm{H}}}_{2}^{{\rm{T}}}\). The phonon dispersion and the PDOS reveals the interaction between HT and HO manifesting the peaks near 60 meV. At those ranges, \({\alpha }^{2}F(\omega )\) possesses strong peaks unlike LaHO and \({\rm{La}}{{\rm{H}}}_{2}^{{\rm{T}}}\) with the enhancement of λ(ω), which is the feature of the metastable dihydride. In our normal mode analysis (see Supplementary Fig. S3) at the Γ and K points, we found that the eigenvectors near 60 meV exhibit vibrational modes along the line connecting HO and HT, confirming the coupling motion.
In Fig. 3 (d) to (g), we computed the DOS at EF, the logarithmic average phonon frequency (ωlog), total λtot, and Tc, varying the O-site occupancy (x) of hydrogen atoms (see Supplementary Fig. S4). We observed an enhancement in electron density at EF for x = 0.25 and 0.5, with a minor decrease at x = 0.75. However, a significant enhancement in DOS is evident when the migration reaches x = 1. Since the Tc is obtained from McMillan equation28,29: \(\{{\omega }_{\log }/1.2\}\left\{\exp \left[-1.04\left(1+\lambda \right)/\lambda \left(1-0.62{\mu }^{* }\right)-{\mu }^{* }\right]\right\}\), it depends on both ωlog and λtot. While ωlog fluctuates, Tcs are monotonically enhanced due to the exponential dependence on λtot as x approaches 1, as shown in Fig. 3g Thus, we conclude that Tc reaches its maximum value when the occupation of HO and HT are evenly distributed.
Accordingly, the Tc of \({\rm{La}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) exhibits 10.8 K with λtot = 0.778. This study highlights that the metastable structure of LaH2, with a change in the site occupancy of O-site/T-site, can increase Tc dramatically, providing a mechanism to enhance superconductivity transition temperature at ambient pressure.
Generalized metastable RH2
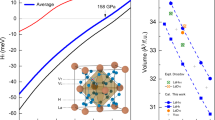
It is also straightforward to expect that this mechanism could also be applicable to other rare-earth hydrides with the same structural frame such as fcc-RH2 (R: La, Nd, Y, Lu, and Sc). Figure 4a shows the relative formation enthalpies (ΔH) of RH2 as a function of pressure. The disproportionation reaction for stoichiometric change from T-site to O-site (RH2 → RH + RH3) happens at ~10 GPa for La and ~75 GPa for Sc. In Fig. 4b, we plotted Pc vs effective ionic radius of studied compounds and interestingly found a linear relation: the smaller the ionic size, the higher the Pc required. The lanthanide series shows a gradual decrease in ionic radius due to similarities in electronic properties between the trivalent cations of lanthanides and the shielding effect of 5 s and 5p electrons on 4 f orbitals. Consequently, smaller lanthanide metals need compression for equivalent bonding with hydrogen atoms to form dihydrides, compared with larger lanthanide metals. In LaH2, our result is in a good match with experimental data (see the star in Fig. 4b). Thus, Fig. 4b can be a guideline for experimental synthesis of metastable dihydrides superconductors.
a Relative formation enthalpy plots of \(\frac{1}{2}(R{\rm{H}}+R{{\rm{H}}}_{3})\) with respect to the RH2 with pressure. b The critical pressure (Pc) required for the disproportionation reaction into RH + RH3 with respect to the ionic size. (experimental result22 for La with the star symbol).
Discussion
Very recently, Nathan et al. reported that \({\rm{Lu}}{{\rm{H}}}_{3-\delta }{{\rm{N}}}_{\delta }\left({Fm}\bar{3}m\right)\), one of lanthanide hydrides, exhibits maximum Tc of 296 K at 1 GPa, which nearly reaches the room-temperature superconductivity at ambient conditions30. It is quite striking because the result is against our conventional wisdom about high-Tc superhydrides and it is not consistent with previous experimental data for LuH3 reporting only 12.4 K at 122 GPa31. We need to note that this discovery 30 hasn’t been reproduced by other groups yet, several follow-up studies claim absence of superconductivity 32,33. The reported process of synthesizing \({\rm{Lu}}{{\rm{H}}}_{3-\delta }{{\rm{N}}}_{\delta }\) seems to depend on pressure and temperature conditions, possibly forming metastable hydrides. We have calculated the superconducting properties of lutetium hydride (\({\rm{Lu}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\)) as shown in Fig. S5. At ambient pressure, \({\rm{Lu}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) shows phonon instability (imaginary phonon frequency) as shown in Supplementary Fig. S3a. Pc for \({\rm{Lu}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) is predicted to be ~55 GPa and thus the stable phonon can be obtained at relatively high pressure. As shown in Supplementary Fig. S3b, our result shows that \({\rm{Lu}}{{{\rm{H}}}_{1}^{{\rm{O}}}{\rm{H}}}_{1}^{{\rm{T}}}\) becomes stable at pressures above 10 GPa. Also, we obtained that Tc of \({\rm{Lu}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) is 36.23 K with λ = 1.174 while Tc of \({\rm{Lu}}{{\rm{H}}}_{2}^{{\rm{T}}}\) is 0.09 with λ = 0.29, exhibiting nontrivial enhancement of electron-phonon coupling. Nitrogen-doping may increase the Tc by shifting EF, however, we cannot find any clue to reach the recently observed room-temperature superconductivity.
In summary, we studied hydrogen migration between T- and O- sites in LaH2. Pressure stabilizes O-site and eventually disproportionation reaction happens at the threshold pressure (Pc), which induces migration of hydrogen positions from \({{\rm{LaH}}}_{2}^{{\rm{T}}}\) at ambient pressure to \({{\rm{LaH}}}_{x}^{{\rm{O}}}{{\rm{H}}}_{2-x}^{{\rm{T}}}\) at high pressure. Lanthanum dihydride with partial hydrogen atoms at octahedral sites (\({{\rm{LaH}}}_{x}^{{\rm{O}}}{{\rm{H}}}_{2-x}^{{\rm{T}}})\) is dynamically stable at ambient pressure, and it possesses enhanced electron-phonon coupling strength to exhibit finite superconductivity transition temperature. We also generalized the investigation to other lanthanides dihydrides (RH2, where R = Y, Sc, Nd, and Lu) and found that they follow the same trend as LaH2. Moreover, we found a simple connectivity between Pc and ionic radius of lanthanides, providing a guideline to further experiments. We also obtained that \({\rm{Lu}}{{{\rm{H}}}_{1}^{{\rm{O}}}{{\rm{H}}}_{1}^{{\rm{T}}}}\) has Tc = 36.23 K with λ = 1.174 at 20 GPa.
Methods
Electronic structure calculations
For the band structure calculations, we employed the pseudopotential method in Vienna Ab initio Simulation Package34,35. The electronic charge density was evaluated up to the kinetic energy cutoff of 350 eV. The face-centered-cubic (fcc) Brillouin zone integration for the self-consistent calculations was carried out with a 12 × 12 × 12 k points grid with Γ centered Monkhorst-Pack scheme. The convergence tests of ENMAX and k points grid are shown in the Supplementary Fig. S1. To calculate the barrier from tetrahedron site of hydrogen to octahedron site, we performed nudged elastic band calculations36. We used seven images of the intermediate configurations connecting the two most stable structures. Each image was relaxed until the force on each atom was smaller in magnitude than 0.05 eV/ Å-1.
Superconducting properties
Phonon dispersion and electron-phonon coupling constants were obtained by using the density-functional perturbation theory implemented in the Quantum ESPRESSO package37 with PAW-PBE pseudopotential of PSlibrary 38. The plane-wave basis energy cutoff and charge density cutoff were chosen as 90 Ry and 360 Ry, respectively. We used a 12 × 12 × 12 k-points grid with the Γ-centered Monkhorst-Pack scheme and an 8 × 8 × 8 q-points grid for the primitive unit cell, as well as a 2 × 2 × 2 q-points grid for the conventional unit cell. The structural optimization to the pressure values of interest was conducted by using the Broyden-Fletcher-Goldfarb-Shannon quasi-Newtonian algorithm39. We employed linear interpolation40 to generate the electron-phonon coupling matrix with a denser grid of 24 × 24 × 24 k -points for the primitive unit cell and 12 × 12 × 12 k-points for the conventional unit cell. The sigma broadening parameter used was 0.02 Ry. The convergence test results of cutoff and q-grid are explained in detail (see Supplementary Fig. S1).
Note added during revision
Ref. 30 by Dasenbrock-Gammon, N. et al., titled “Evidence of near-ambient superconductivity in an N-doped lutetium hydride”, Nature 615, 244–250 (2023) was retracted on 07 November 2023.
Data availability
The data supporting the findings are available in the main text, Supplementary Information file, and upon reasonable request from the corresponding authors. Source data are included in the paper.
References
Ashcroft, N. W. Hydrogen dominant metallic alloys: high temperature superconductors? Phys. Rev. Lett. 92, 187002 (2004).
Duan, D. F. et al. Structure and superconductivity of hydrides at high pressures. Natl. Sci. Rev. 4, 121 (2017).
Flores-Livas, J. A. et al. A perspective on conventional high-temperature superconductors at high pressure: methods and materials. Phys. Rep. 856, 1–78 (2020).
Pickard, C. J., Errea, I. & Eremets, M. I. Superconducting hydrides under pressure. Annu. Rev. Condens. Matter Phys. 11, 57–76 (2019).
Li, Y. et al. Guangtian, superconductivity at ∼100 K in dense SiH4(H2)2 predicted by first principles. Proc. Natl Acad. Sci. USA 107, 15708 (2010).
Gao, G. et al. Guangtian Superconducting high pressure phase of germane. Phys. Rev. Lett. 101, 107002 (2008).
Martinez-Canales, M. et al. Novel structures and superconductivity of silane under pressure. Phys. Rev. Lett. 102, 087005 (2009).
Gao, G. et al. High-pressure crystal structures and superconductivity of Stannane (SnH4). Proc. Natl Acad. Sci. USA 107, 1317–1320 (2010).
Drozdov, A. P., Eremets, M. I., Troyan, I. A., Ksenofontov, V. & Shylin, S. I. Conventional superconductivity at 203 Kelvin at high pressures in the sulfur hydride system. Nature 525, 73–76 (2015).
Li, Y. et al. Pressure-stabilized superconductive yttrium hydrides. Sci. Rep. 5, 9948 (2015).
Wang, H., Tse, J. S., Tanaka, K., Iitaka, T. & Ma, Y. Superconductive sodalite-like clathrate calcium hydride at high pressures. Proc. Natl Acad. Sci. USA 109, 6463–6466 (2010).
Peng, F. et al. Hydrogen clathrate structures in rare earth hydrides at high pressures: possible route to room-temperature superconductivity. Phys. Rev. Lett. 119, 107001 (2017).
Drozdov, A. P. et al. Superconductivity at 250 K in lanthanum hydride under high pressures. Nature 569, 528–531 (2019).
Somayazulu, M. et al. Evidence for superconductivity above 260 K in lanthanum superhydride at megabar pressures. Phys. Rev. Lett. 122, 027001 (2019).
Di Cataldo, S., Heil, C., von der Linden, W. & Boeri, L. LaBH8: towards high- low-pressure superconductivity in ternary superhydrides. Phys. Rev. B 104, L020511 (2021).
Zhang, Z. et al. Design principles for high-temperature superconductors with a hydrogen-based alloy backbone at moderate pressure. Phys. Rev. Lett. 128, 047001 (2022).
Holley, C. E., Mulford, R. N. R., Ellinger, F. H., Koehier, W. C. & Zachariasen, W. H. The crystal structure of some rare earth hydrides. J. Phys. Chem. 59, 1226–1228 (1955).
Villa-Cortés, S. & De La Peña-Seaman, O. Electron- and hole-doping on ScH2 and YH2: effects on superconductivity without applied pressure. J. Phys. Condens. Matter 33, 425401 (2021).
Kai, K., Gschneidner, K. A., Beaudry, B. J. & Peterson, D. T. Heat capacities of LaDx and LaHx (1.9 ≤ x ≥ 3.0) from 1 to 300 K. Phys. Rev. B 40, 6591 (2021).
Gupta, S. K. & Jha, P. K. Dynamical stability of the lanthanum dihydride under high pressure: a density functional lattice dynamics approach. Int. J. Hydrog. Energy 38, 4654–4663 (2013).
Fisk, Z. & Johnston, D. C. Isotope effect in the resistivity of scandium hydride. Phys. Lett. 53, 39–49 (1975).
Machida, A. et al. Formation of NaCl-type monodeuteride LaD by the disproportionation reaction of LaD2. Phys. Rev. Lett. 108, 205501 (2012).
Machida, A., Watanuki, T., Kawana, D. & Aoki, K. Disproportionation reaction of LaH2 at high pressure and low temperature. J. Phys. Ser. 500, 022001 (2014).
Savin, A., Nesper, R., Wengert, R. S. & Fässler, T. F. ELF: the electron localization function. Angew. Chem. Int. Ed. Engl. 36, 1808–1832 (1997).
Mizoguchi, H. et al. Hydride-based electride material, LnH2 (Ln = La, Ce, or Y). Inorg. Chem. 55, 8833–8838 (2016).
Yi, S., Wang, C., Jeon, H. & Cho, J. H. Stability and bonding nature of clathrate H cages in a near-room-temperature superconductor LaH10. Phys. Rev. Mater. 5, 024801 (2021).
Sun, Y. & Miao, M. Chemical templates that assemble the metal superhydrides. Chem 9, 443–459 (2023).
Allen, P. B. & Dynes, R. C. Transition temperature of strong-coupled superconductors reanalyzed. Phys. Rev. B 12, 905 (1975).
McMillan, W. L. Transition temperature of strong-coupled superconductors. Phys. Rev. 167, 331 (1968).
Dasenbrock-Gammon, N. et al. Evidence of near-ambient superconductivity in a N-doped lutetium hydride. Nature 615, 244–250 (2023).
Shao, M. et al. Superconducting ScH3and LuH3 at megabar pressures. Inorg. Chem. 60, 15330–15335 (2021).
Xing, X. et al. Observation of non-superconducting phase changes in nitrogen doped lutetium hydrides. Nat. comm. 14, 5991 (2023).
Ming, X. et al. Absence of near-ambient superconductivity in LuH2±xNy. Nature 620, 72–77 (2023).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Kresse, G. & Furthmiiller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15 (1996).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Baroni, S., De Gironcoli, S., Corso, A. D. & Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 73, 515–562 (2001).
Dal Corso, A. Pseudopotentials periodic table: from H to Pu. Comput. Mater. Sci. 95, 337–350 (2014).
Head, J. D. & Zerner, M. C. A broyden-fletcher-goldfarb-Shanno optimization procedure for molecular geometries. Chem. Phys. Lett. 122, 264–270 (1985).
Wierzbowska, M., de Gironcoli, S. & Giannozzi, P. Origins of low-and high pressure discontinuities of Tc in niobium. arXiv Preprint at https://arxiv.org/pdf/cond-mat/0504077 (2006).
Acknowledgements
This work was supported by the National Research Foundation (NRF) Korea (Grant No. 2020R1A5A1019141, 2021R1C1C2011276, RS-2023-00220471, RS-2023-00272090, RS-2024-00406755). D.Y.K. acknowledges the support from the National Natural Science Foundation of China (11774015).
Author information
Authors and Affiliations
Contributions
H.K. performed the calculations and data analysis, and D.Y.K. conceived and supervised the project. I.P. and J.H.S. performed data analysis and review. All authors contributed to the discussion of the results and participated in preparing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, H., Park, I., Shim, J.H. et al. Superconductivity of metastable dihydrides at ambient pressure. npj Comput Mater 10, 173 (2024). https://doi.org/10.1038/s41524-024-01359-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41524-024-01359-7
- Springer Nature Limited