Abstract
In situ growth of pyrochlore iridate thin films has been a long-standing challenge due to the low reactivity of Ir at low temperatures and the vaporization of volatile gas species such as IrO3(g) and IrO2(g) at high temperatures and high PO2. To address this challenge, we combine thermodynamic analysis of the Pr-Ir-O2 system with experimental results from the conventional physical vapor deposition (PVD) technique of co-sputtering. Our results indicate that only high growth temperatures yield films with crystallinity sufficient for utilizing and tailoring the desired topological electronic properties and the in situ synthesis of Pr2Ir2O7 thin films is fettered by the inability to grow with PO2 on the order of 10 Torr at high temperatures, a limitation inherent to the PVD process. Thus, we suggest techniques capable of supplying high partial pressure of key species during deposition, in particular chemical vapor deposition (CVD), as a route to synthesis of Pr2Ir2O7.
Similar content being viewed by others
Introduction
Complex 5d oxide systems, such as pyrochlore iridates (RE2Ir2O7 with RE = Rare Earth), have an unusual combination of magnetic and structural features that makes them ideal systems for the formation of non-trivial topological phases arising from geometric frustration combined with strong spin–orbit coupling. The spin–orbit-coupled Ir states in RE2Ir2O7 dominate conduction as the Iridium-Oxygen network is much more covalent than the praseodymium-oxygen network1,2,3,4,5,6,7,8,9. The high entanglement among crystal lattice, strong spin–orbital coupling, and electronic correlation makes the system enticing for thin-film engineering, but this requires high-quality films both in terms of crystallinity and stoichiometry. Synthesis of requisite films has long been impeded due to the volatility of Iridium-Oxygen compounds at temperatures suitable for crystalline growth.
Attempts at in situ growth via physical vapor deposition (PVD) such as magnetron sputtering, pulsed laser deposition (PLD), and molecular beam epitaxy have failed to yield phase-pure epitaxial thin films, leaving most experimental studies limited to bulk systems. In situ synthesis via PVD techniques, such as PLD, shows that this difficultly comes from the low reactivity between Ir and RE oxides combined with the high volatility of the gas phase of Ir oxides such as IrO3(g)10,11. To overcome this difficulty, solid-phase epitaxy has been successfully used to synthesize RE2Ir2O7 epitaxial thin films10,12,13,14,15. This method circumvents the IrO3(g) volatility dilemma through first depositing amorphous phase at conditions suitable for a stoichiometric balance of elements, then post-annealing at high temperatures in air to induce crystallization. The high temperatures necessary for ex situ crystallization inherently induce surface roughening16 and intermixing between adjacent heterostructure layers17, limiting the study of RE2Ir2O7 based on heterostructure design. Advancing the study of electronic properties is thus contingent upon success of in situ synthesis to obtain simultaneous stoichiometric growth with near-perfect crystallinity.
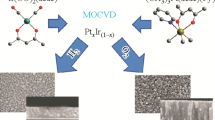
The most common methods for synthesizing high-quality ceramic oxide films all employ PVD. PVD requires that the constituents of the film be physically moved from a source material to the substrate, upon which they deposit. The substrate temperature and chamber partial pressures are controlled to create thermodynamic conditions for the formation of gas species involved in film deposition when the source materials propagate towards the substrate surface. Governed by thermodynamic properties and growth conditions, the gas species near the substrate surface for deposition can be different from the species in the flux from the source materials11, illustrated in Fig. 1a. Although many materials have been synthesized successfully via the PVD methods, including some iridates with oxygen pressures close to the upper limit for PVD18,19, we show that Pr2Ir2O7 synthesis is thwarted due to the surprising stability of a similar Pr3IrO7 phase, as shown in Fig. 1b. Our experimental sampling of the thermodynamic space yielded only the Pr3IrO7 phase and other species of the constituents, but not Pr2Ir2O7. Seeking to circumvent the formation of this pernicious Pr3IrO7 phase, we calculate the phase diagram for the Pr-Ir-O2 ternary system using the Calculation of Phase Diagrams (CALPHAD) approach20,21,22 for insight into the thermodynamics of Pr2Ir2O7 synthesis. Our results indicate that the presence of volatile IrO3(g) is vital to the formation of the desired Pr2Ir2O7 phase and thus indicate that future attempts for in situ growth should be performed under high deposition pressure, utilizing high-pressure sputtering or chemical vapor deposition (CVD).
In the actual experiment, Pr2Ir2O7 and IrO2 targets are simultaneously sputtered, (a). The details are included in Supplementary Fig. 2. The condensation of the vapor species is key to the thin-film synthesis, (b). By synthesizing epitaxial Pr2Ir2O7 thin films, we can tailor the properties based on breaking the cubic symmetry. The strong spin–orbital coupling, originating from the Ir, opens paths toward a new generation of spintronics based on frustrated antiferromagnetic (AFM) conductors.
Results
As the volatilization of the iridium-oxygen compounds, specifically IrO3, is the prime suspect for Ir deficiency in the synthesis of Pr2Ir2O7, we use computational thermodynamics to predict the vapor pressures of the Ir-O and Pr-O species at conditions relevant to PVD. There are nine relevant gas species in the Pr-Ir-O system based on the Scientific Group Thermodata Europe (SGTE) substance database (SSUB5)23: Ir, IrO, IrO2, IrO3, O, O2, O3, PrO, and Pr. The results reveal that IrO3(g) is the dominant gas species, which has a partial pressure up to 1000 times greater than all other gas species in the system, except for molecular oxygen (O2(g)). We then compute the isothermal phase diagram to elucidate how the relative proportion of supplied constituents from the source materials will impact the compounds that crystallize on the substrate. In the isothermal ternary phase diagram, we identify the Pr3IrO7 compound, which is Ir deficient relative to the desired Pr2Ir2O7, yet structurally similar. As our primary growth control is via the selection of the gas partial pressures, and almost all iridium in the system oxidizes into IrO3(g), we plot potential phase diagrams of partial pressures of gas species O2(g) and IrO3(g) for various stable compounds from the ternary phase diagram. The relationship between O2(g), IrO3(g), and the solid-phase compounds serves as a guide to changing growth conditions for Pr2Ir2O7 synthesis. The computational calculations are confirmed by thin-film deposition using a range of conditions throughout the relevant thermodynamic windows. Overall, we find that Pr2Ir2O7 can only form when both O2(g) and IrO3(g) partial pressures are high, indicating that IrO3(g) appears to be the principal species by which hybridized Ir becomes incorporated into the film crystal. In particular, the IrO3(g) partial pressure, the dynamic equilibrium value in the gas phase, must be at or above the equilibrium value at the substrate growth temperature, indicated in Fig. 2, and shown over a wider range in Supplementary Fig. 1. We note that the O : Ir ratio of 3.5 in Pr2Ir2O7 is very close to the ratio in IrO3(g), and that solid-phase Ir(s) forms in films deposited under conditions of high Ir and low-oxygen partial pressures. These results point to deposition techniques that can support high deposition pressure up to 9 Torr as promising routes toward successful in situ synthesis.
In thermodynamic calculations, the ratios of the components of Ir : O2 and Pr : O2 were fixed at 1 : 1 and 2 : 1.5, to represent the compositions of IrO2 and Pr2O3, respectively. The vertical dot-dashed black line indicates the growth temperature (1163 K) and the dot-dashed horizontal lines refer to the partial pressure of IrO3(g), IrO2(g), and Pr(g) gas species, respectively. More information is shown in Supplementary Fig. 1.
Vapor pressures of the binary systems IrO2 and Pr2O3
As the final film composition is determined by the equilibrium between solid and vapor phases at the substrate temperature, we start by considering the partial pressure vs. temperature relationship for the binary Ir-O and Pr-O systems. As shown in Fig. 2, IrO3(g) has the highest partial pressure by at least three orders of magnitude of the species, except Oxygen, throughout the range of temperatures considered. At our growth temperature of 1163 K, the partial pressure of IrO3(g) is 2 × 10−3 Torr. With O2(g) in the growth chamber, regardless of the Ir flux type (Ir metal vapor or Ir-O binary compounds, or Ir precursor) provided from the source materials, the dominant Ir flux to the substrate surface is gaseous IrO3(g). The partial pressure of IrO3(g) depends on the equilibrium of species in gas phase such as O2(g) and Ir(g), and is not an independent thermodynamic variable nor an independent experimental growth parameter. This high vapor pressure of IrO3(g) explains the common Ir deficiency of thin films24.
By comparison, the most volatile Pr-O species is Pr(g), followed by PrO(g), both of which have partial pressures more than 104 times lower than IrO3(g) at our growth temperature. As such a low partial pressure is easily satisfied by the available O2(g) and has minimal contribution to total pressure, the Pr-containing compounds condense on the substrate at a much higher rate than the Ir-containing compounds. Our attempt to compensate for this effect, adding a separate IrO2(s) target as shown in Supplementary Fig. 2, proved insufficient on its own to synthesize in situ Pr2Ir2O7.
Isothermal phase diagram of the Pr-Ir-O2 system at 1163 K
Having determined the main factors controlling element proportions of gaseous species above the substrate, we utilize a ternary isothermal phase diagram to study which compounds form when different stoichiometries are present on the substrate. We base our calculations on the SSUB5 database23 for the binary oxides and the formation energies of Pr2Ir2O7 (227) and Pr3IrO7 (317) from first-principles calculations, which were further refined by experimental partial pressure of oxygen. The calculated isothermal Pr-Ir-O2 phase diagram at our deposition temperature of 1163 K and 760 Torr is plotted in Fig. 3 with the stoichiometric phases shown by points, and the two- and three-phase regions shown by lines and areas, respectively. Although the gas phase is dominated by O2, many other species are present as shown in Fig. 2. We think of the gas phase as necessary to transport constituents to the substrate surface, making the praseodymates important in addition to the higher-partial pressure iridates. First, the calculations do not consider any stable phases along the Ir-Pr binary system axis, because this binary system has not yet been modeled by the CALPHAD method. Possible Ir-Pr phases are not critical for the present work due to the current interest in oxides with much higher stability than the compounds between Ir and Pr under experimental conditions. It can be seen in Fig. 3 that, in addition to the desired Pr2Ir2O7 phase, Pr3IrO7 becomes stable at lower Ir concentrations. As a result, we expect that Iridium deficiency will lead to some amount of Pr3IrO7 in our films. The Pr2Ir2O7 (227) and Pr3IrO7 (317) phases are in equilibrium with the gas phase, forming a three-phase invariant equilibrium region of “317 + 227 + gas.” Figure 3 also shows that Pr2Ir2O7(s) is in equilibrium with the solid Ir(s) and oxides IrO2(s), whereas Pr3IrO7(s) is additionally in equilibrium with Pr7O12(s). These results indicate that Ir(s) and oxides IrO2(s), Pr3IrO7(s), and Pr7O12(s) are possible secondary phases during the synthesis of Pr2Ir2O7(s).
A single point represents the stable condensed phase, the line connecting two points represents the coexistence of the two corresponding phases, and the triangular area connected by three points indicates three-phase invariant equilibria. In the three-phase equilibrium regime around the right bottom corner, the dominant gas species is O2(g) with other gas species plotted in Supplementary Fig. 1.
Potential phase diagram of the Pr-Ir-O2 system at 1163 K
Figure 4 illustrates the calculated isothermal Gibbs-potential-derived phase diagram of the Pr-Ir-O2 system at 1163 K and 760 Torr as a function of the partial pressures of O2(g) and IrO3(g)—the two dominant gas species (Fig. 2). The phase diagram shows the species, which minimize the Gibbs thermodynamic potential at each condition. In this potential phase diagram, the cross-points indicate three-phase invariant equilibria, the lines show the two-phase equilibria, and the areas are the single-phase regions. It is understood that when the partial pressure of O2(g) equals to the total pressure of 760 Torr, the region is a single gas phase. When analyzing the potential phase diagram for growth considerations, we consider the plotted partial pressures as representative of the conditions immediately above the substrate during growth.
Calculated potential phase diagram of the Pr-IrO3-O2 system at 1163 K (890 °C) and total pressure of 1 atm (760 Torr) with the partial pressures of O2 (PO2) and IrO3 (PIrO3) plotted (a), the zoomed-in diagram and the experimental comparison from the co-sputtering results (b). In the calculated phase diagram, the points represent three-phase equilibrium, the lines two-phase equilibrium, and the areas one-phase equilibrium. Here, 227 represents Pr2Ir2O7 and 317 represents Pr3IrO7. The cross-point of “317 + Pr7O12 + Ir” is located at [2 × 10−2, 9 × 10−8] Torr with respect to PO2 and PIrO3, respectively, whereas the minimum partial pressures are [9, 8 × 10−4] Torr of the equilibria of “317 + 227 + Ir” in order to form Pr2Ir2O7. It is worth noting that the gas single-phase boundary here located at 760 Torr reflects the 1 atm pressure condition of our calculation.
The most striking aspect of the potential phase diagram is that the compounds with more Ir become stable with lower PO2 for a given PIrO3, even ones with lower oxygen-to-cation ratios. Although this is apparent for pure-phase Ir(s), it is less so for the sequence of IrO2 to Pr2Ir2O7, to Pr3IrO7 at high PIrO3 with increasing PO2. We attribute this trend to the volatilization of Ir in the presence of O2(g) as a higher PO2 requires a lower PIr to maintain the same PIrO3, stabilizing the phases with lower Ir concentration. At low PIrO3, PO2 increases with the amount of oxygen towards Pr7O12(s). Furthermore, pure Ir(s) corresponds to exceedingly large PIrO3 partial pressure at any PO2 within the total pressure constraint. These phase relationships highlight the delicate balance of needing enough oxygen for Ir(s) to hybridize into the film crystal but not too much that it forms a high vapor pressure in equilibrium with the desired phase. Furthermore, as PIrO3 decreases at the same PO2 value, the proportion of Pr present in the solid increases, as seen in the “227” stability adjacent to the IrO2 region, which has the same Ir oxidation state. When the O2(g) partial pressure reaches the total pressure of 760 Torr, only the gas phase, dominated by O2, is stable, as everything oxidizes to volatile compounds.
Experimentally, we grew thin films via PVD co-sputtering and compared the stabilized phases to the potential phase diagram. We determined which phases we stabilized using X-ray diffraction, as shown in Supplementary Fig. 3. For comparison, the thermodynamic oxygen partial pressure corresponds to the oxygen partial pressure set in the chamber far from the substrate. We consider the Ir flux to be IrO3(g), because the increase of PIr proportionally increases PIrO3 under constant PO2. The number of Ir atoms approximated from the growth rate is plotted in Fig. 4b as the corresponding IrO3 pressure. By comparing these thin-film growths with thermodynamic calculations (Fig. 4b), it can be seen that the equilibrium-phase dependences on IrO3(g) and O2(g) are in agreement. Our experimental observation of the “317 + Pr7O12 + Ir” three-phase invariant equilibrium at higher IrO3(g) pressure and lower O2 validates the shape of our CALPHAD results for the Pr-Ir-O2 system, especially regarding the vital role of oxygen for bonded-Ir incorporation in the resultant thin film.
Due to the pressure limitations inherent in PVD, our experimental films were only able to test the low-pressure portion (≤40 mTorr) of the phase diagram. We were unable to synthesize Pr2Ir2O7 in situ. We used the “317 + Pr7O12 + Ir” three-phase invariant equilibrium point as a reference to quantitatively compare the current experiments and determined the experimental pressures to be 2 × 10−2 Torr and 9 × 10−8 Torr for PO2 and PIrO3, respectively. According to this comparison, the minimum PO2 and PIrO3 values needed to stabilize Pr2Ir2O7 are 9 Torr and 8 × 10−4 Torr, respectively, which correspond to the “317 + 227 + Ir” three-phase invariant equilibrium. The necessity of high PO2 and PIrO3 partial pressures makes synthesis of this system ideal for growth techniques that can support high-pressure thin-film growth, such as CVD.
Effect of the growth temperature on the phase relationships
We sought to enlarge the Pr2Ir2O7 stabilization window by changing the temperature, as, in accordance with Fig. 2, a lower temperature allows for much lower IrO3 partial pressure. The previously discussed growth temperature of 1163 K was chosen to optimize the kinetic aspects of film growth to ensure single-crystalline films for heterostructure engineering. Thermodynamic calculations, however, indicate that lower growth temperatures could reduce the impact of the requirements for high Ir-component pressures. In addition, thermostability experiments, shown in Supplementary Fig. 4, show Pr2Ir2O7 is more stable at 1273 K than at 1373 K. The vapor pressure calculations in Fig. 2 indicate that the equilibrium partial pressure of IrO3 drops off by orders of magnitude if the growth temperature is lowered to 1000 K. Figure 5 illustrates the effects of the temperature on the phase relationships in the Pr-Ir-O2 system. Calculations at 1000 K indicate that the Pr2Ir2O7 phase forms with PIrO3 as low as 9 × 10−7 Torr, which is almost 1000 times lower than that at 1163 K, and that the minimum PO2 value decreased by 70 times from 9.2 Torr at 1163 K to 0.13 Torr at 1000 K. The same relationship between PIrO3 and PO2 to maintain Pr2Ir2O7 phase stability occurs at 1000 K; notably, the interplay of increasing PIrO3 coinciding with increasing PO2. These results also indicate that phase stability at fixed partial pressures is very sensitive to temperature, resulting in a narrow growth window for Pr2Ir2O7.
Unfortunately, our experimental films grown below 1073 K showed poor crystallinity, negating the benefits of the Pr2Ir2O7 phase for heterostructuring applications that demand high-quality films, forcing us to pursue high-pressure in situ growth such as CVD in the future.
Discussion
In summary, we utilized the CALPHAD modeling technique to explore thermodynamic properties of the Pr-Ir-O2 system and find that Pr2Ir2O7 can only form high-quality crystals above 1073 K and under oxygen partial pressures much higher (PO2 > 9 Torr). These conditions cannot be achieved with conventional PVD methods and provide insights into why PVD is an inadequate synthesis pathway. However, these conditions can be accessed in CVD growth. Our study, therefore, suggests exploring CVD growth for high-quality Pr2Ir2O7 films.
Methods
Details of thermodynamic calculations
Thermodynamic calculations of the Pr-Ir-O2 system were performed by the Thermo-Calc software25 in terms of the SSUB5 database23. Structural space groups from the Materials project, used for phonon calculations, are shown in Supplementary Table 1. The thermodynamic properties of ternary compounds of interest, i.e., Pr2Ir2O7 and Pr3IrO7, absent in the SSUB5 database, can be estimated with respect to binary oxides as follows,
In the present work, the reaction entropies for Eqs. (1) and (2) are assumed to be zero, because there is no net change of gas species involved26. The reaction Gibbs energies, ΔGreaction, contain only the reaction enthalpies and are determined by the following two experimental data: (i) the partial pressure of O2, PO2 = 22 ± 6 mTorr (16 and 28 mTorr, see Fig. 4b), for the invariant equilibrium of “Pr3IrO7 + Ir + Pr7O12” at 1163 K and (ii) the Pr2Ir2O7 (227) phase decomposes at around 1400 K. The details are described in the Supplementary Information. It is noteworthy that the decomposition temperature of Pr3IrO7 is unknown. The Gibbs free energy differences ΔGreaction = −8.72 kJ mol−1 atom−1 for Pr2Ir2O7 (see Eq. (1)) and ΔGreaction = −13.30 kJ mol−1 atom−1 for Pr3IrO7 (see Eq. (2)) were obtained in the present work. These two ΔGreaction values are lower than those from the density functional theory (DFT) based first-principles calculations; see details in Supplementary Table 2 and Supplementary Methods in the Supplementary Information. However, these ΔGreaction values from experiments agree with the observation that the DFT-based results of enthalpy of formation are in general higher than experimental data by, e.g., 10–40%27; see the comparisons in Supplementary Table 2 in the Supplementary Information.
The influence of interfacial energy and strain energy on the phase diagram were not included in the calculation28,29. These contributions, which are widely used to tailor the growth of thin films, are controlled mainly by the gas phase under deposition conditions. For bulk films, interfacial energy and strain energy play limited roles. The strain energy can expand some phase regions and shrink other phase regions. In most cases of epitaxial thin films, the substrate is chosen to favor the phase of interest, thus expanding its stability window. The growth windows from our thermodynamic calculations thus work well in most cases. Thermodynamic predictions of the regime for stable phase formation has been demonstrated for a number of cases such as the in situ epitaxial thin films of MgB230,31 and Sr3SnO32, and the adsorption-controlled epitaxial thin films of SrRuO3 and CaRuO333, La-doped BaSnO334, PbTiO335, LuFe2O436, BiMnO337, and BiFeO338. In addition, the calculations of phase diagrams and potential diagrams (Figs. 2–5) in the present work were performed using Thermo-Calc25; see more details in our previous work11.
Experimental details of co-sputtering
We used co-sputtering for the experimental study of phase formation in the Pr-Ir-O2 system, a conventional PVD method. In our co-sputtering deposition system (detailed geometry included in the Supplementary Information), the flux of Pr2Ir2O7 and IrO2 can be controlled separately by the voltage applied to each radiofrequency magnetron sputtering gun. The simultaneous sputtering from both Pr2Ir2O7 and IrO2 targets gives the extra tunability of partial pressures of Iridium and its gaseous species. The controllability of these parameters guarantees a direct comparison of the conditions between experiments and thermodynamic predictions.
Data availability
The data that support the findings of the work are in the manuscript’s main text and Supplementary Information. Additional data are available from the corresponding author upon reasonable request.
Code availability
The commercial codes, Thermo-Calc (www.thermocalc.com) and VASP (www.vasp.at), were used in the present work.
References
Matsuhira, K., Wakeshima, M., Hinatsu, Y. & Takagi, S. Metal–insulator transitions in pyrochlore oxides Ln2Ir2O7. J. Phys. Soc. Jpn 80, 094701 (2011).
Nakayama, M. et al. Slater to Mott crossover in the metal to insulator transition of Nd2Ir2O7. Phys. Rev. Lett. 117, 056403 (2016).
Ueda, K. et al. Variation of charge dynamics in the course of metal-insulator transition for pyrochlore-type Nd2Ir2O7. Phys. Rev. Lett. 109, 136402 (2012).
Nakatsuji, S. et al. Metallic spin-liquid behavior of the geometrically frustrated Kondo lattice Pr2Ir2O7. Phys. Rev. Lett. 96, 087204 (2006).
Tomiyasu, K. et al. Emergence of magnetic long-range order in frustrated pyrochlore Nd2Ir2O7 with metal–insulator transition. J. Phys. Soc. Jpn 81, 034709 (2012).
Shapiro, M. C. et al. Structure and magnetic properties of the pyrochlore iridate Y2Ir2O7. Phys. Rev. B 85, 214434 (2012).
Disseler, S. M. et al. Magnetic order in the pyrochlore iridates A2Ir2O7 (A = Y, Yb). Phys. Rev. B 86, 14428 (2012).
Donnerer, C. et al. All-in–all-out magnetic order and propagating spin waves in Sm2Ir2O7. Phys. Rev. Lett. 117, 037201 (2016).
Sagayama, H. et al. Determination of long-range all-in-all-out ordering of Ir4+ moments in a pyrochlore iridate Eu2Ir2O7 by resonant x-ray diffraction. Phys. Rev. B Condens. Matter Mater. Phys. 87, 100403 (2013).
Fujita, T. C. et al. Odd-parity magnetoresistance in pyrochlore iridate thin films with broken time-reversal symmetry. Sci. Rep. 5, 9711 (2015).
Adkison, K. M. K. M. et al. Suitability of binary oxides for molecular-beam epitaxy source materials: a comprehensive thermodynamic analysis. APL Mater. 8, 081110 (2020).
Gallagher, J. C. et al. Epitaxial growth of iridate pyrochlore Nd2Ir2O7 films. Sci. Rep. 6, 22282 (2016).
Ohtsuki, T. et al. Strain-induced spontaneous Hall effect in an epitaxial thin film of a Luttinger semimetal. Proc. Natl Acad. Sci. USA 116, 8803–8808 (2019).
Ohtsuki, T., Tian, Z., Halim, M., Nakatsuji, S. & Lippmaa, M. Growth of Pr2Ir2O7 thin films using solid phase epitaxy. J. Appl. Phys. 127, 035303 (2020).
Guo, L. et al. Spontaneous Hall effect enhanced by local Ir moments in epitaxial Pr2Ir2O7 thin films. Phys. Rev. B 101, 104405 (2020).
Gao, H. & Nix, W. D. Surface roughening of heteroepitaxial thin films. Annu. Rev. Mater. Sci. 29, 173–209 (1999).
Hulko, O., Thompson, D. A. & Simmons, J. G. Semiconductor science and technology quantitative compositional profiles of enhanced intermixing in GaAs/AlGaAs quantum well heterostructures annealed with and without a SiO2 cap layer. Semicond. Sci. Technol. 24, 045015 (2009).
Anderson, T. J. et al. Metastable honeycomb SrTiO3/SrIrO3 heterostructures. Appl. Phys. Lett. 108, 151604 (2016).
Anderson, T. J. et al. Interfacial B-site atomic configuration in polar (111) and non-polar (001) SrIrO3/SrTiO3 heterostructures. APL Mater. 5, 096110 (2017).
Liu, Z. K. First-principles calculations and CALPHAD modeling of thermodynamics. J. Phase Equilibria Diffus. 30, 517–534 (2009).
Saunders, N. & Miodownik, A. P. CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide (Pergamon, 1998).
Liu, Z. K. Computational thermodynamics and its applications. Acta Mater. 200, 745–792 (2020).
Scientific Group Thermodata Europe (SGTE), Thermodynamic Properties of Inorganic Materials. Landolt-Boernstein New Series, Group IV. Vol. 19 (Springer, 1999).
Kim, W. J., Ko, E. K., Kim, S. Y., Kim, B. & Noh, T. W. In-operando spectroscopic ellipsometry studies of IrO2 dynamic instabilities: guide to in-situ growth of pyrochlore iridate thin films. Curr. Appl. Phys. 19, 400–405 (2019).
Andersson, J. O. et al. Thermo-Calc & Dictra, computational tools for materials science. Calphad 26, 273–312 (2002).
Liu, Z. K. & Wang, Y. Computational Thermodynamics of Materials (Cambridge Univ. Press, 2016).
Shang, S.-L., Wang, Y., Anderson, T. J. & Liu, Z.-K. Achieving accurate energetics beyond (semi-)local density functional theory: illustrated with transition metal disulfides, Cu2ZnSnS4, and Na3PS4 related semiconductors. Phys. Rev. Mater. 3, 015401 (2019).
Shen, J. Y., Johnston, S., Shang, S. L. & Anderson, T. Calculated strain energy of hexagonal epitaxial thin films. J. Cryst. Growth 240, 6–13 (2002).
Shang, S.-L. et al. Lateral versus vertical growth of two-dimensional layered transition-metal dichalcogenides: thermodynamic insight into MoS2. Nano Lett. 16, 5742–5750 (2016).
Liu, Z. K., Schlom, D. G., Li, Q. & Xi, X. X. Thermodynamics of the Mg–B system: implications for the deposition of MgB2 thin films. Appl. Phys. Lett. 78, 3678 (2001).
Zeng, X. H. et al. In situ epitaxial MgB2 thin films for superconducting electronics. Nat. Mater. 1, 35–38 (2002).
Ma, Y. et al. Realization of epitaxial thin films of the topological crystalline insulator Sr3SnO. Adv. Mater. 32, 2000809 (2020).
Nair, H. P. et al. Synthesis science of SrRuO3 and CaRuO3 epitaxial films with high residual resistivity ratios. APL Mater. 6, 046101 (2018).
Paik, H. et al. Adsorption-controlled growth of La-doped BaSnO3 by molecular-beam epitaxy. APL Mater. 5, 116107 (2017).
Smith, E. H. et al. Exploiting kinetics and thermodynamics to grow phase-pure complex oxides by molecular-beam epitaxy under continuous codeposition. Phys. Rev. Mater. 1, 023403 (2017).
Brooks, C. M. et al. The adsorption-controlled growth of LuFe2O4 by molecular-beam epitaxy. Appl. Phys. Lett. 101, 132907 (2012).
Lee, J. H. et al. Adsorption-controlled growth of BiMnO3 films by molecular-beam epitaxy. Appl. Phys. Lett. 96, 262905 (2010).
Ihlefeld, J. F. et al. Adsorption-controlled growth of BiFeO3 by MBE and integration with wide band. IEEE Trans. Ultrason. Ferroelectr. Frequency Control 56, 1528–1533 (2009).
Acknowledgements
Synthesis of thin films at the University of Wisconsin-Madison was supported by NSF through the University of Wisconsin Materials Research Science and Engineering Center (DMR-1720415), the Gordon and Betty Moore Foundation’s EPiQS Initiative, grant GBMF9065 to C.B.E., and Vannevar Bush Faculty Fellowship (N00014-20-1-2844). Thin-film characterizations at the University of Wisconsin-Madison was supported by the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, under award number DEFG02-06ER46327. S.L.S. and Z.K.L. acknowledge partial financial support from the National Science Foundation (NSF) through Grant number CMMI-1825538 and the Dorothy Pate Enright Professorship. First-principles calculations were carried out partially on the ACI clusters at the Pennsylvania State University, partially on the resources of the National Energy Research Scientific Computing Center (NERSC) supported by the U.S. Department of Energy Office of Science User Facility operated under Contract number DE-AC02-05CH11231, and partially on the resources of the Extreme Science and Engineering Discovery Environment (XSEDE) supported by National Science Foundation with Grant number ACI-1548562. We thank T. Nan, A. Edgeton, J.W. Lee, and Y. Yao for helpful discussion.
Author information
Authors and Affiliations
Contributions
L.G. and C.B.E. conceived the project. C.B.E., M.S.R., and P.G.E. supervised experimental work and Z.K.L. supervised thermodynamic calculations. L.G. performed the films growth and structural characterizations. S.L.S. performed thermodynamic calculations. L.G., N.G.C., and S.L.S. wrote the manuscript. C.B.E. directed the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, L., Shang, SL., Campbell, N. et al. Searching for a route to synthesize in situ epitaxial Pr2Ir2O7 thin films with thermodynamic methods. npj Comput Mater 7, 144 (2021). https://doi.org/10.1038/s41524-021-00610-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41524-021-00610-9
- Springer Nature Limited