Abstract
BRCA1 and BRCA2 pathogenic variant carriers develop breast cancers with distinct pathological characteristics and mutational signatures that may result in differential response to chemotherapy. We compared rates of pathologic complete response (pCR) after NAC between BRCA1/2 variant carriers and noncarriers in a cohort of 1426 women (92 [6.5%] BRCA1 and 73 [5.1%] BRCA2) with clinical stage I–III breast cancer treated with NAC followed by surgery from 11/2013 to 01/2022 at Memorial Sloan Kettering Cancer Center. The majority received doxorubicin/cyclophosphamide/paclitaxel therapy (93%); BRCA1/2 carriers were more likely to receive carboplatin (p < 0.001). Overall, pCR was achieved in 42% of BRCA1 carriers, 21% of BRCA2 carriers, and 26% of noncarriers (p = 0.001). Among clinically node-positive (cN+) patients, nodal pCR was more frequent in BRCA1/2 carriers compared to noncarriers (53/96 [55%] vs. 371/856 [43%], p = 0.015). This difference was seen in HR+/HER2− (36% vs. 20% of noncarriers; p = 0.027) and TN subtypes (79% vs. 45% of noncarriers; p < 0.001). In a multivariable analysis of the overall cohort, BRCA1 status, and TN and HER2+ subtypes were independently associated with pCR. These data indicate that BRCA1 carriers may be more likely to achieve overall and nodal pCR in response to NAC compared with BRCA2 carriers and patients with sporadic disease. Further studies with a larger cohort of BRCA1/2 mutation carriers are needed, as a small sample size may have a restricted ability to detect a significant association between mutational status and pCR in sensitivity analyses stratified by subtype and adjusted for clinically relevant factors.
Similar content being viewed by others
Introduction
BRCA1 and BRCA2 are the most commonly mutated cancer susceptibility genes in hereditary breast cancers1; affected carriers have a 2–3% annual risk of developing disease2. The epidemiology of breast cancers arising in the context of a BRCA1 or BRCA2 mutation is different than that of sporadic cancers. These cancers tend to be higher grade and, in the case of BRCA1 mutations, are more likely to be triple negative3. Whether this variability leads to differences in response to systemic therapy between carriers and noncarriers remains unclear4,5,6,7,8.
Certain functions of BRCA1 and BRCA2 proteins, such as their role in DNA repair, directly impact cellular response to chemotherapeutic agents9,10. Additionally, tumors associated with BRCA1/2 mutations display distinct mutational signatures and gene expression profiles3. These factors may affect response to the standard chemotherapy regimen traditionally given to patients with sporadic breast cancers11,12,13.
In the case of neoadjuvant chemotherapy (NAC), patients with BRCA1 pathogenic mutations have been shown to have high pathologic complete response (pCR) rates, ranging from 24% to 90%14,15,16,17,18. Patients with BRCA2 pathogenic mutations, conversely, seem to have similar pCR rates (13%–53%) to those with sporadic breast cancer1,19. However, the small sample sizes of these studies prevented investigation of differences in response to NAC between BRCA1 vs. BRCA2 carriers. We describe a robust single-institutional experience that addresses this gap in knowledge by distinguishing between rates of pCR after NAC in BRCA1 compared to BRCA2 carriers and noncarriers.
Results
Of 1426 patients included in this study, 92 (6.5%) and 73 (5.1%) were BRCA1 and BRCA2 pathogenic variant carriers, respectively. Compared to noncarriers, BRCA1 and BRCA2 carriers were younger (p < 0.001), more frequently presented with clinical T1 disease (p = 0.002), and more frequently underwent bilateral mastectomy (p < 0.001). Regarding molecular subtype, 73% (67/92) of BRCA1 carriers presented with TNBC, and 60% (44/73) of BRCA2 carriers presented with HR+ disease. Among noncarriers who received NAC, 38% (481/1261) were HER2+ and 25% (317/1261) had TNBC. BRCA1-associated tumors were more frequently poorly differentiated compared to BRCA2-associated and sporadic cancers (p = 0.001). Almost all patients received doxorubicin/cyclophosphamide/paclitaxel therapy (93%); BRCA1 carriers were more likely to receive carboplatin (p < 0.001) (Table 1).
Response to NAC in overall cohort
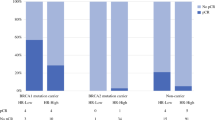
Overall pCR occurred in 42% (39/92) of BRCA1 carriers, 21% (15/73) of BRCA2 carriers, and 26% (327/1261) of noncarriers (p = 0.001). Among clinically node-positive (cN+) patients, nodal pCR was more frequent in BRCA1 carriers (65% [31/48]) compared to BRCA2 carriers (46% [22/48]) and noncarriers (43% [371/856]) (p = 0.015; Table 2). Lower cT stage (p = 0.028), cN0 (p = 0.010), BRCA1 status (p = 0.001), poorly differentiated tumors (p < 0.001), ductal histology (p < 0.001), TN and HER2+ subtypes (p < 0.001), carboplatin receipt (p = 0.041), and absence of lymphovascular invasion (LVI) (p < 0.001), were associated with overall pCR on univariate analysis. After adjusting for differentiation and subtype, BRCA1 carrier status remained independently associated with pCR (OR 2.37 [95% CI 1.32–4.18]; p = 0.003). Relative to HR+/HER2− tumors, HR+/HER2+, HR−/HER2+, and TNBC had higher odds of pCR (Table 3). Of patients with TNBC who received carboplatin, 11/24 (46%) of BRCA1 carriers achieved pCR compared with 3/9 (33%) BRCA2 carriers and 23/77 (30%) of noncarriers.
Response to NAC in TN disease
Of the 67 BRCA1 patients with TN disease, 31 (46%) experienced an overall pCR compared to 11/27 (41%) of BRCA2 mutation carriers, and 91/317 (29%) of patients without a mutation. Younger age, BRCA status, and absence of LVI were associated with overall pCR on univariate analysis. In the adjusted analysis, age (OR 0.97, 95% CI 0.95, 1.00, p = 0.024) and LVI (OR 0.44, 95% CI 0.22, 0.83, p = 0.014) remained inversely associated with overall pCR but BRCA mutation status did not. Nodal pCR occurred in 26/34 (76%) of BRCA1 carriers, 11/13 (85%) of BRCA2 carriers, and 87/191 (46%) of noncarriers who presented with cN+ disease. Factors that were significantly associated with nodal pCR in TN disease included younger age (p < 0.001), Asian race (p = 0.026), BRCA mutation status (p < 0.001), and absence of LVI (p < 0.001). On multivariable analysis, younger age remained associated with nodal pCR (median [IQR] 52 [43, 60]) among patients without nodal pCR vs. 49 (36, 57) among patients with nodal pCR). LVI was less likely to be associated with pCR in this analysis (OR 0.29, 95% CI 0.13, 0.62; p = 0.002) (Table 4).
Response to NAC in HR+/HER2− disease
Overall pCR occurred in 8/21 (38%) of BRCA1 mutation carriers, 2/38 (5%) of BRCA2 mutation carriers, and 30/463 (7%) of non-mutation carriers. Among patients with HR+/HER2-negative disease, BRCA1 status (p < 0.001), poorly differentiated disease (p < 0.001), ductal histology (p = 0.012), HR-negative disease (p < 0.001), carboplatin use (p = 0.007), and absence of LVI (p < 0.001) were associated with pCR on univariate analysis. On multivariable analysis, BRCA1 status remained significantly associated with pCR (Table 5). Compared to poorly differentiated disease, well-differentiated tumors were less likely to be associated with pCR (OR 0.05, 95% CI 0.01, 0.16; p < 0.001). Nodal pCR occurred in 5/12 (42%) of BRCA1 mutation carriers, 10/30 (33%) of BRCA2 mutation carriers, and 73/370 (20%) of non-mutation carriers. BRCA status (p = 0.040), poorly differentiated disease (<0.001), ductal histology (p = 0.022), carboplatin administration (p = 0.006), and absence of LVI (p < 0.001) were associated with nodal pCR on univariate analysis. On multivariable analysis, while BRCA status did not predict nodal pCR, well-differentiated disease, and LVI were inversely associated with nodal pCR.
Discussion
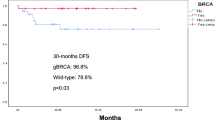
This study compares rates of pCR between BRCA1 and BRCA2 variant carriers and noncarriers in, to our knowledge, one of the largest cohorts analyzed in this manner to date. Both overall pCR and nodal pCR more frequently occurred in BRCA1 carriers compared to BRCA2 carriers and noncarriers. When stratified by receptor subtype, we observed higher nodal pCR rates among BRCA1 and BRCA2 carriers compared to noncarriers within both subtype cohorts, including sufficient patients, namely HR+/HER2− and TNBC. In sensitivity analyses by subtype, BRCA1 status was significantly associated with overall pCR among patients with HR+/HER2− disease.
The characteristics of BRCA-associated tumors were consistent with those in existing literature4. While 75% of BRCA2-associated breast cancers are reported to be HR+, approximately 70% of BRCA1-associated breast cancers are TNBC20,21,22,23. In this study, a similar rate of HR+ tumors was seen in BRCA2 carriers and noncarriers, and BRCA1 carriers more frequently had TNBC compared to BRCA2 carriers and those with sporadic disease. Poorly differentiated tumors were more common in patients with BRCA1 mutations. This is in line with reports that 66–100% of tumors in BRCA1 mutation carriers exhibit grade 3 histology compared to 16–57% and 15–55% of disease in BRCA2 carriers and sporadic breast cancers, respectively24.
Our finding that BRCA1 carriers had a higher rate of pCR compared to BRCA2 carriers and noncarriers supports previous studies that have assessed response to NAC in BRCA1/2 mutation carriers. Arun et al. demonstrated higher odds of pCR in BRCA1 carriers (26/57; 46%) than BRCA2 carriers (3/23; 13%) and noncarriers (53/237; 22%)14. In another study, Wunderle et al. observed that among patients treated with anthracycline-based NAC, pCR occurred more frequently in BRCA1/2 carriers (11/25 [44%] BRCA1 carriers and 4/13 [30%] BRCA2 carriers) compared to noncarriers (30/230 [13%])18. When stratified by subtype, the proportion of patients with luminal A/B tumors who achieved pCR was highest among BRCA1 carriers. In patients with TNBC, pCR rates were higher in BRCA1/2 carriers compared to noncarriers. Together, these studies and ours corroborate preclinical data indicating differential response to chemotherapy by mutation status3. We were not, however, able to appreciate an association between BRCA status and overall pCR for TNBC or nodal pCR for TNBC or HR+/HER2− subtypes in adjusted sensitivity analysis. Although we postulate that this may have been due to limited sample size, our observation that BRCA1 mutation status was significantly associated with overall pCR among those with HR+/HER2− disease after adjusting for clinically relevant factors merits further investigation.
Patients in this study uniformly received ACT. Addition of carboplatin was more common among BRCA1/2 carriers compared to noncarriers. Many studies have demonstrated increased pCR rates with the addition of platinum to ACT in TNBC25,26,27. While carboplatin was significantly associated with pCR on univariate analysis, this significance dissipated after adjusting for other factors, likely due to sample size and the small number of pCR events, and was not included in our multivariable model. However, in this study, we observed that BRCA1 patients with TNBC who received carboplatin had a pCR rate higher than that reported in existing literature for the general population21,22,23. These data are discordant with published literature from the subgroup analysis of the BrighTNess and GeparSixto trials27,28. These trials demonstrated that although pCR rates were increased among patients with TNBC who received neoadjuvant carboplatin in addition to ACT, pCR rates were not enhanced among those with germline BRCA1/2 mutations compared to those without BRCA1/2 mutations. These conflicting results may have been due to differences in sample size and analytic strategy. Compared to the GeparSixto and BrighTNess trials, we included a larger cohort of mutation carriers and analyzed data from BRCA1 and BRCA2 carriers separately. Future investigations are needed to determine how changes to standard NAC regimens will contribute to differences in pCR between BRCA1/2 carriers and noncarriers, especially in those with TNBC who now receive chemoimmunotherapy given the findings of KEYNOTE-52229.
NAC chemotherapy importantly provides prognostic data and informs adjuvant treatment recommendations that improve overall survival. NAC is also frequently used to downstage locally advanced disease for the purposes of surgical de-escalation30. However, women with BRCA1/2 mutations may prefer bilateral mastectomy for risk reduction, regardless of eligibility for breast conservation31,32. Accordingly, 90% of BRCA1/2 carriers in this study underwent bilateral mastectomy. While management of in-breast disease may not be altered by NAC in mutation carriers, axillary downstaging after NAC remains a consideration. We report that nearly two-thirds of BRCA1 carriers who had cN+ disease prior to NAC achieved pCR in their axilla. The nodal pCR rate among BRCA1 carriers with HR+/HER2− disease was significantly higher than that reported in the literature for cases of sporadic cancer (7.5%–16.2%)33. As changes in the surgical management of the axilla during the time period of this study have made a larger proportion of women eligible for less invasive approaches, especially in HR+/HER2− disease, further study is needed to assess how germline genetics affect rates of axillary downstaging.
This study has several limitations. The small sample size, particularly for patients with HER2+ disease, precluded further subgroup analysis by receptor status. Additionally, where subset analysis was performed for TN and HR+/HER2− subtypes, a small sample size limits the ability for granular analysis. This study includes patients with cN+ disease who received NAC based on previously accepted guidelines and for whom systemic chemotherapy may no longer be the standard of care based on the RxPONDER trial34. Although a small proportion of patients may have received preoperative chemoimmunotherapy according to the KEYNOTE-522 regimen35, which became incorporated into our institutional practice in August 2021, we were not able to specifically account for the role of immunotherapy in our analysis of pCR. Additionally, we cannot account for bias that may have resulted in variability from clinician practices regarding patient selection for neoadjuvant treatment. In this study, patients were classified as noncarriers if they had negative genetic testing or did not meet the criteria for genetic testing based on NCCN guidelines; inaccurate assessment of mutational status may have influenced findings. We could not examine the association of pCR with survival because of short follow-up. As the majority of patients received ACT-based chemotherapy, our data provide little insight into treatment response to alternative regimens. Although our findings are consistent with existing literature, the single-institution nature of this study limits generalizability.
We conclude that BRCA1-associated breast cancer has a higher rate of overall and nodal pCR than both BRCA2-associated and sporadic disease. We demonstrate that pCR outcomes vary by BRCA1 compared to BRCA2 mutational status, and the importance of analyzing these groups separately in future studies. Lastly, these differences in pCR were observed in HR+/HER2− cancers that traditionally have lower response rates to NAC.
Methods
Study characteristics
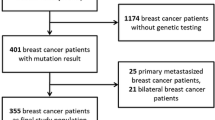
After approval from the Memorial Sloan Kettering Cancer Center institutional review board, we identified consecutive women who were diagnosed with clinical stage I-III breast cancer and treated with NAC followed by surgery between November 2013 and January 2022. The need for informed consent was waived based on the retrospective nature of the study. Patients who qualified for genetic testing based on NCCN guidelines were referred for genetic counseling and evaluation for BRCA1 and BRCA2 germline variants36. Patients with BRCA variants of uncertain significance, non-BRCA deleterious pathogenic mutations, or missing pathology data were excluded. Genetic testing was either performed at our institution or outside the facility, and results were reviewed by the treating physicians.
Demographic, tumor, and treatment characteristics were abstracted from the electronic medical record. The tumor molecular subtype was defined as hormone receptor-positive (HR+) if ≥1% of tumor cells stained positive for estrogen receptor (ER) or progesterone receptor (PR) by immunohistochemistry37. HER2 overexpression was defined by immunohistochemistry or amplification based on fluorescence in situ hybridization according to ASCO/CAP guidelines38. In this study, clinically node-positive (cN+) patients were those who had evidence of malignant cells in ≥1 lymph node by fine needle aspiration or core needle biopsy at the time of diagnosis. PCR was defined as the absence of residual invasive tumor in the breast and ipsilateral axillary lymph nodes (ypT0/is ypN0).
Baseline disease characteristics were compared between BRCA1 and BRCA2 carriers and noncarriers using the Kruskal–Wallis rank sum test for continuous variables, and Fisher’s exact test or chi-square test for categorical variables. Logistic regression was used to evaluate the association between BRCA1 or BRCA2 mutation and pCR (i.e., ypT0/is pN0), adjusting for variables selected using backward elimination. BRCA status, clinical stage, differentiation, tumor subtype, NAC regimen, histology, and LVI were chosen a priori as clinically relevant variables and included for consideration by backward elimination. Given that studies have demonstrated pCR differs by tumor molecular subtype39, sensitivity analyses were performed to evaluate factors associated with pCR by subtype except in HER2+ disease, given the small sample size of BRCA1/2 patients with HER2+ tumors. All analyses were performed using R 4.2 with a two-sided type I error rate (α) set to 0.05.
We have complied with all relevant ethical regulations, including the Declaration of Helsinki.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Begg, C. B. et al. Variation of breast cancer risk among BRCA1/2 carriers. JAMA 299, 194–201 (2008).
Rebbeck, T. R. & Domchek, S. M. Variation in breast cancer risk in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 10, 108 (2008).
Roy, R., Chun, J. & Powell, S. N. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat. Rev. Cancer 12, 68–78 (2011).
Cortesi, L. et al. Favourable ten-year overall survival in a Caucasian population with high probability of hereditary breast cancer. BMC Cancer 10, 90 (2010).
Foulkes, W. D. et al. Primary node negative breast cancer in BRCA1 mutation carriers has a poor outcome. Ann. Oncol. 11, 307–313, (2000).
Goodwin, P. J. et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an international prospective breast cancer family registry population-based cohort study. J. Clin. Oncol. 30, 19–26 (2012).
Huzarski, T. et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J. Clin. Oncol. 31, 3191–3196 (2013).
Zhong, Q., Peng, H. L., Zhao, X., Zhang, L. & Hwang, W. T. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin. Cancer Res. 21, 211–220 (2015).
Chappuis, P. O. et al. A significant response to neoadjuvant chemotherapy in BRCA1/2 related breast cancer. J. Med. Genet. 39, 608–610 (2002).
Egawa, C. et al. Increased expression of BRCA1 mRNA predicts favorable response to anthracycline-containing chemotherapy in breast cancers. Breast Cancer Res. Treat. 78, 45–50 (2003).
Hedenfalk, I. et al. Gene-expression profiles in hereditary breast cancer. N. Engl. J. Med. 344, 539–548 (2001).
Kennedy, R. D., Quinn, J. E., Mullan, P. B., Johnston, P. G. & Harkin, D. P. The role of BRCA1 in the cellular response to chemotherapy. J. Natl. Cancer Inst. 96, 1659–1668, (2004).
Lakhani, S. R. et al. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J. Natl. Cancer Inst. 90, 1138–1145 (1998).
Arun, B. et al. Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J. Clin. Oncol. 29, 3739–3746 (2011).
Byrski, T. et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J. Clin. Oncol. 28, 375–379 (2010).
Byrski, T. et al. Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res. Treat. 115, 359–363 (2009).
Byrski, T. et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res. Treat. 147, 401–405 (2014).
Wunderle, M. et al. BRCA mutations and their influence on pathological complete response and prognosis in a clinical cohort of neoadjuvantly treated breast cancer patients. Breast Cancer Res. Treat. 171, 85–94 (2018).
Rennert, G. et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N. Engl. J. Med. 357, 115–123 (2007).
Atchley, D. P. et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J. Clin. Oncol. 26, 4282–4288 (2008).
Incorvaia, L. et al. BRCA1/2 pathogenic variants in triple-negative versus luminal-like breast cancers: genotype-phenotype correlation in a cohort of 531 patients. Ther. Adv. Med. Oncol. 12, 1758835920975326 (2020).
Mavaddat, N. et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol. Biomark. Prev. 21, 134–147 (2012).
National Cancer Institute Surveillance, E. & End Results Program. Cancer stat facts: female breast cancer subtypes 2023. https://seer.cancer.gov/statfacts/html/breast-subtypes.html (Accessed June 28, 2024).
Honrado, E., Benítez, J. & Palacios, J. Histopathology of BRCA1- and BRCA2-associated breast cancer. Crit. Rev. Oncol. Hematol. 59, 27–39 (2006).
Caramelo, O. et al. Efficacy of different neoadjuvant treatment regimens in BRCA-mutated triple negative breast cancer: a systematic review and meta-analysis. Hered. Cancer Clin. Pract. 20, 34 (2022).
Geyer, C. E. et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann. Oncol. 33, 384–394 (2022).
Metzger-Filho, O. et al. Matched cohort study of germline BRCA mutation carriers with triple negative breast cancer in brightness. NPJ Breast Cancer 7, 142 (2021).
Hahnen, E. et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol. 3, 1378–1385 (2017).
Schmid, P. et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 382, 810–821 (2020).
Heil, J. et al. Eliminating the breast cancer surgery paradigm after neoadjuvant systemic therapy: current evidence and future challenges. Ann. Oncol. 31, 61–71 (2020).
Ramaswami, R., Morrow, M. & Jagsi, R. Contralateral prophylactic mastectomy. N. Engl. J. Med. 377, 1288–1291 (2017).
Wang, C. et al. Breast-conserving therapy for breast cancer with BRCA mutations: a meta-analysis. Breast Cancer 29, 314–323 (2022).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Kalinsky, K. et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N. Engl. J. Med. 385, 2336–2347 (2021).
Schmid, P. et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N. Engl. J. Med. 386, 556–567 (2022).
Daly, M. B. et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, Version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl Compr. Canc. Netw. 19, 77–102 (2021).
Hammond, M. E. et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 28, 2784–2795 (2010).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. Arch. Pathol. Lab. Med. 142, 1364–1382 (2018).
Myers, S. P. et al. Association of tumor molecular subtype and stage with breast and axillary pathologic complete response after neoadjuvant chemotherapy for breast cancer. Ann. Surg. Oncol. 28, 8636–8642 (2021).
Acknowledgements
The authors would like to thank Paula Garcia and Tiana Le for their assistance in creating and maintaining the study database. This study was supported in part by NCI Cancer Center Support Grant P30 CA008748, which funds the institution’s core resources.
Author information
Authors and Affiliations
Contributions
S.P.M. was responsible for collecting, analyzing, and interpreting data, as well as drafting the manuscript. V.S. was responsible for analyzing and interpreting data. A.V.B., A.B.T., A.M., and M.E.R. took part in data interpretation and drafting of the manuscript. M.M. and M.L.K. were responsible for study conceptualization, study oversight, and data interpretation. All authors critically reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Myers, S.P., Sevilimedu, V., Barrio, A.V. et al. Pathologic complete response after neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers. npj Breast Cancer 10, 63 (2024). https://doi.org/10.1038/s41523-024-00674-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-024-00674-y
- Springer Nature Limited