Abstract
Ciprofloxacin (CIP) is used to treat Pseudomonas aeruginosa biofilm infections. We showed that the pathways of CIP-resistance development during exposure of biofilms and planktonic P. aeruginosa populations to subinhibitory levels of CIP depend on the mode of growth. In the present study, we analyzed CIP-resistant isolates obtained from previous evolution experiments, and we report a variety of evolved phenotypic and genotypic changes that occurred in parallel with the evolution of CIP-resistance. Cross-resistance to beta-lactam antibiotics was associated with mutations in genes involved in cell-wall recycling (ftsZ, murG); and could also be explained by mutations in the TCA cycle (sdhA) genes and in genes involved in arginine catabolism. We found that CIP-exposed isolates that lacked mutations in quorum-sensing genes and acquired mutations in type IV pili genes maintained swarming motility and lost twitching motility, respectively. Evolved CIP-resistant isolates showed high fitness cost in planktonic competition experiments, yet persisted in the biofilm under control conditions, compared with ancestor isolates and had an advantage when exposed to CIP. Their persistence in biofilm competition experiments in spite of their fitness cost in planktonic growth could be explained by their prolonged lag-phase. Interestingly, the set of mutated genes that we identified in these in vitro-evolved CIP-resistant colonies, overlap with a large number of patho-adaptive genes previously reported in P. aeruginosa isolates from cystic fibrosis (CF) patients. This suggests that the antibiotic stress is contributing to the bacterial evolution in vivo, and that adaptive laboratory evolution can be used to predict the in vivo evolutionary trajectories.
Similar content being viewed by others
Introduction
Sublethal concentrations of antibiotics are present in clinical settings at the infection site in certain tissues as a consequence of limited accessibility as well as in the environment. It has been shown that resistant mutants, even with high MICs can be selected at sublethal antibiotic concentrations1,2,3. In addition, antibiotics at sublethal concentrations are responsible for increasing genetic variation4 by means of different pathways involving oxidative stress5, by inducing error-prone polymerases mediated by the SOS response6, by misbalancing nucleotide metabolism or acting directly on DNA7. In this respect, quinolones have been known for years to be mutagenic in bacteria8. This implies that antibiotic therapy, in some cases, may have the detrimental side-effect of accelerating the adaptation of pathogens as well as of the commensal strains (suggested to be a major reservoir of resistance).
We have previously reported in a planktonic experimental evolution study conducted on more than 900 generations of Pseudomonas aeruginosa, that high-level CIP-resistant mutants with cross-resistance to beta-lactams developed quickly in the presence of a subinhibitory concentration of ciprofloxacin9, and a phenotypic shift of the P. aeruginosa populations evolved under constant exposure to subinhibitory concentrations of CIP10. Compared to the controls (CTRL), the populations evolved in the presence of sublethal concentrations of CIP showed decreased protease activity and swimming motility, higher levels of quorum-sensing (QS) signal molecules and upregulation of denitrification genes10. These observations suggested that evolution in the presence of sublethal concentrations of CIP has pleiotropic effects on the bacterial phenotypes and this might promote persistence of the resistant bacterial population.
Persistent infections are caused by biofilm-embedded bacteria which are characterized by multifactorial intrinsic tolerance (phenotypic resistance) to antibiotics11. However, several recent studies have demonstrated the supplementary role played by mutational resistance in the survival of biofilms to antibiotic treatment12,13.
We have recently investigated the evolution of mutational resistance in P. aeruginosa biofilms exposed to subinhibitory concentrations of CIP and showed that the biofilm mode of growth promotes development of low-level mutational resistance14 and that this process was accelerated during evolution of a P. aeruginosa catalase mutant, as a surrogate of the oxidative stress environment present at the site of chronic infections15.
The phenotypic shifts that we have previously observed in the experimental evolution of planktonic cultures exposed to sublethal levels of CIP10 encouraged us to investigate, the phenotypic and genomic evolution associated with CIP-resistance in biofilms14,15. The experimental set-up of the investigation of the phenotypic and genotypic landscape of evolution under exposure to subinhibitory levels of CIP is presented in Fig. 1.
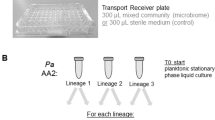
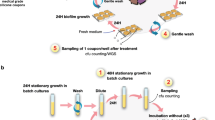
a Experimental evolution in colony biofilm and stationary-phase planktonic culture. A colony biofilm was formed on polycarbonate membrane from an overnight culture of P. aeruginosa (WT PAO1 or ΔkatA). In the step called passage 0 (P0), membranes containing 48-h colony biofilms were transferred to fresh LB plates with either CIP or without CIP for 48 h (CTRL). Every 48 h, the colony biofilms were disrupted, and the bacterial populations were used to start new biofilms. The bacterial populations of disrupted CIP biofilms were used to start new colony-biofilms on plates with CIP and the bacterial populations of disrupted CTRL biofilms were used to start new colony-biofilms on LB plates. This was repeated for six passages (P1–P6). b Evolved biofilm and planktonic populations (CIP and CTRL) were plated on concentrations higher than the minimum inhibitory concentrations (MIC) of ciprofloxacin. The resistant colonies were collected for phenotypic and genotypic analysis to elucidate the evolutionary trajectories under exposure to subinhibitory concentrations of ciprofloxacin.
We show here that prolonged exposure of P. aeruginosa biofilms to sub-MIC CIP led to mutations which were associated with extensive phenotypic changes. Cross-resistance between CIP and beta-lactam antibiotics such as aztreonam and ceftazidime, differential swarming and twitching motility compared to CTRL, growth curves with a prolonged lag phase, and metabolic shifts toward anaerobic respiration were observed. A fitness cost of CIP resistance in biofilms was observed in the absence of antibiotics. However, non-surprisingly, CIP-resistant colonies had increased fitness when the competition experiments were conducted in the presence of CIP. Overall, our data show that under antibiotic stress, mutational resistance to CIP associates with mutations in several genes that probably play a role in the persistence of biofilms during antibiotic treatment. Interestingly, the set of mutated genes that we identified in these in vitro-evolved CIP-resistant colonies, overlap with a large number of patho-adaptive genes previously reported in P. aeruginosa isolates from cystic fibrosis (CF) patients. This is identifying antibiotic stress as an important environmental factor that shapes the bacterial evolution in vivo. It can thus be speculated that this, together with the inflammatory response leads to the so-called chronic phenotype. Chronic phenotype occurs when bacteria become well-adapted to the conditions of the respiratory tract of CF patients and, therefore, persists in spite of antibiotic therapy.
Results
CIP exposure selects for CIP-resistant variants with prolonged lag phase
Growth curves of 92 CIP-resistant isolates (47 WT PAO1 and 45 ΔkatA) representing 57 isolates recovered from CIP-evolved populations and 35 isolates recovered from CTRL-evolved populations were analyzed.
Three parameters of bacterial growth were calculated: maximum growth density, doubling time, and time to initiation of the exponential phase (lag time).
CIP-resistant colonies from CIP-evolution had significantly extended lag phase compared to CTRL (p = 0.002). For WT PAO1, the lag phase (median (range) in h) was 5.36 (3.5–13) vs. 3.5 (3.5–9.7) while for ΔkatA it was 6.84 (4.2–14) vs. 5.2 (4.8–6.5) (Fig. 2). The extended lag phase was more frequent in ΔkatA colonies compared to WT PAO1, and it occurred also in some of the CTRL colonies suggesting adaptation to growth in media during the evolution experiment.
a Significant longer lag phase was observed in CIP-evolved compared to CTRL-evolved PAO1 colonies (in red) (p = 0.002) and ΔkatA (in blue) (p = 0.002). The lag phase of the ancestor colonies is represented by the red (PAO1) and the blue (ΔkatA) dot. Mann–Whitney test for nonparametric data was used to compare the different groups.
For CIP-resistant colonies of the WT PAO1, a statistically significant (p = 0.0007) increase in the doubling time was found for the colonies isolates from CIP evolved compared to CTRL populations. The doubling time (median (range) in min) was 97.5 (71–893) vs. 69 (69–104). This was not observed for colonies isolated from the planktonic evolution experiment
The fitness cost of CIP-resistance development
To determine the fitness cost of the CIP resistance, the fitness index (FI) of 42 CIP-resistant colonies (24 colonies from CIP-evolved populations and 18 colonies from CTRL-evolved populations) (Supplementary Table 1) were investigated in competition experiments started at ratio 1:1 between the evolved CIP-resistant and the ancestor colonies in both planktonic and colony-biofilm cultures. The competition in colony-biofilm cultures was conducted in the presence and absence of 0.1 mg/L CIP.
The FI was expressed as the ratio between the doubling- time of the CIP-resistant colony and the ancestor colony after 24 h incubation (see Supplementary methods).
In planktonic competition studies, the CIP-resistant colonies showed decreased fitness compared to the WT ancestors (FI < 1), with the exception of CIP_Pl_10, CIP_Pl_11, and CIP_Pl_14. The highest fitness cost was observed for the CIP-resistant colonies isolated from the planktonic evolution of ΔkatA and the lowest for CIP-resistant colonies isolated from planktonic evolution of PAO1.
An inverse correlation (R2 = 0.53) between the lag time and the planktonic FI was observed: the shorter the lag time, the better the fitness (Fig. 3a).
The FI in a 24 h colony-biofilm without exposure or with 24 h exposure to 0.1 mg/L CIP are shown in Supplementary Table 2. In colony-biofilm competition studies, few CIP-resistant isolates were identified in the mixed 48 h biofilm population. However, this was dramatically changed when a 24 h-old biofilm was exposed to 0.1 mg/L ciprofloxacin for 24 h. In the latter case, the CIP-resistant colonies had a significant (p = 0.0005), growth advantage compared to ancestor colonies (Fig. 3b) (Supplementary Table 2).
CIP exposure selects for CIP-resistant colonies with impaired twitching but maintained swarming motility
The swimming, swarming and twitching motility of 92 CIP-resistant isolates were analyzed (57 CIP and 35 CTRL) (Fig. 4).
For comparisons, the motilities of the WT PAO1 are presented by black symbols in the figure. A significant lower twitching motility (p = 0.03) and higher swarming motility (p = 0.0001) was observed in CIP-resistant colonies isolated from CIP compared to and CTRL biofilms. Mann–Whitney test was used for statistical analysis.
No significant difference in swimming motility between the CIP and CTRL isolates was observed, though for both groups the CIP-resistant isolates were impaired in swimming compared to ancestor colonies.
CIP-resistant colonies from CTRL-evolved populations had significantly lower swarming motility (p = 0.0001) and higher twitching motility (p = 0.03) compared to CIP-evolved populations.
Cross-resistance to beta-lactam antibiotics
The MIC of ciprofloxacin, ceftazidime, aztreonam, meropenem, tobramycin, and colistin in CIP-resistant (24 colonies from CIP-evolved populations and 18 colonies from CTRL-evolved populations) were determined and are presented in Fig. 5a, b and Supplementary Table 3.
Heat map showing the changes in the resistance levels to six classes of antibiotics. Results shown are the log2 fold change in the minimum inhibitory concentrations (MIC) (mg/L) in the evolved CIP-resistant isolates compared to the sensitive ancestral strain. a The relative changes in the MIC levels in CIP-resistant isolates recovered from CIP-evolved populations. b The relative changes in the MIC levels in CIP-resistant isolates recovered from CTRL-evolved populations. CIP ciprofloxacin, CEF ceftazidime, MER meropenem, AZT aztreonam, TOB tobramycin, COL colistin.
The level of resistance was higher in the CIP-resistant colonies from CIP-exposed populations compared to CTRL population and simultaneous resistance to beta-lactams such as aztreonam and ceftazidime was observed, in many cases with MICs above the clinically resistance breakpoints (Supplementary Table 2). However, CIP-resistant isolates showed decreased MICs to tobramycin and colistin antibiotics.
No correlations between the CIP MIC levels and the fitness index, lag period or doubling time were observed.
Growth of resistant subpopulations in the inhibition zone of the antibiotic strip were observed for all the CIP-resistant colonies with hypermutable phenotype (Supplementary Table 3) but also in other populations of single CIP-resistant colonies suggesting a hetero-resistant phenotype, especially to meropenem.
Genomic mutations of the evolved-resistant isolates that could explain the observed phenotypes
To elucidate the genetic mechanisms underlying the antibiotic resistance and the evolved phenotypes in the evolved CIP-resistant colonies, we have sequenced 24 CIP-resistant colonies recovered from CIP-evolved populations (16 colonies isolated from biofilm populations and 8 colonies isolated from planktonic populations) and 18 CIP-resistant colonies recovered from CTRL-evolved populations (11 colonies isolated from biofilm populations and 7 colonies isolated from planktonic populations) for both WT PAO1 and ΔkatA. The genomic DNA of original ancestors PAO1 and ΔkatA were sequenced as well. The identification and phenotypes of the sequenced colonies are presented in Supplementary Tables 1 and 2a, b.
Mutations with frequencies above 80% were prioritized for determining the number of mutations and calculating the ratio between nonsynonymous and synonymous mutations dN/dS (Table 1). In CIP-evolved populations the ratio dN/dS is higher than in CTRL populations suggesting a positive selection. To get more insights into the mutational landscape, we also considered mutations with frequencies below 80% and higher than 10%. (Supplementary Data Set).
Gene mutations which are repeatedly observed after independent exposures to a condition provide strong evidence for adaptive evolution. To identify common genes that were mutated under evolution at sub-MIC CIP, we focused on genes which were recurrently mutated in several of the CIP-resistant colonies16. A map showing the presence or absence of the mutated genes in different functional classes related to the observed phenotypic shifts is presented in Fig. 6.
The blue boxes indicate the genes with nonsynonymous mutations identified in the individual CIP-resistant colonies recovered from CIP- and CTRL-evolved biofilm and planktonic (PLA) populations. The identity number of the colonies is shown in Supplementary Table 1.
Mutations in CIP-resistant colonies from CIP-evolved populations
Mutations in antibiotic resistance determinants
Common evolutionary trajectories among CIP-resistant colonies leading to resistance to CIP were observed.
In CIP-resistant colonies from WT PAO1 planktonic populations, simultaneous mutations in gyrase gyrA and efflux-pump regulators of MexAB-OprM (mexR, nalC, and nalD) at high frequencies might explain the high-MIC values to ciprofloxacin. In addition, in one of the colonies CIP_Pl_10, mutations in PA3228 gene that encodes for a putative ATP-binding/permease fusion ABC transporter that may function as a multidrug transporter was observed with high frequencies (>80%).
In the CIP-resistant colonies from WT PAO1 biofilm populations, nfxB gene, a negative regulator of efflux-pump MexCD-OprJ was repeatedly mutated as well as other efflux-pump regulator genes, such as mexR and mexS, at high frequencies. The MIC ciprofloxacin levels in these colonies are lower than in planktonic colonies with the exception of CIP_BF_5 (Supplementary Tables 1, 3) with high MIC of CIP of 32 mg/L in which an additional mutation in PA3228 gene encoding an ABC transporter was observed at low frequencies.
In CIP-resistant colonies from ΔkatA planktonic populations, simultaneous mutations in mexR or nalD at high frequencies and parC at low frequencies can explain the CIP-resistant phenotype. In one colony CIP_Pl_25 (Supplementary Tables 1, 3) with a subpopulation with MIC to ciprofloxacin of 6 mg/L, an additional mutation in PA3228 was also identified at low frequencies.
In CIP-resistant colonies of ΔkatA biofilm populations, three colonies (CIP_BF_15, CIP_BF_16, and CIP_BF_18) had a 28 nucleotide insertion in mutL causing a hypermutable phenotype (Supplementary Data Set). In the hypermutable colonies simultaneous mutations in gyrase gyrB, parC, as well as efflux-pump regulators such as nfxB at high frequencies might explain the high-MIC values to ciprofloxacin. In one of the colonies CIP_BF_15, mutations in the ABC transporter PA3228 gene were found at high frequencies (>80%).
A list of mutated genes that might explain for the observed cross-resistance of CIP-resistant colonies to beta-lactams, and especially aztreonam is presented in Fig. 6.
In CIP-resistant colonies isolated from evolution under CIP exposure, mutations in efflux pumps regulators (nfxB, nalC, nalD, and mexR) were identified. These mutations cause upregulation of the MexCD-OprJ and MexAB-OprM pumps which, besides ciprofloxacin, also accommodate beta-lactams. Interestingly, in addition we detected mutations in genes encoding for proteins involved in cell division (the divisome) (for example, ftsI, ftsZ, and murG), penicillin-binding protein pbpAC and amp genes involved in the regulation of AmpC beta-lactamase (Fig. 6).
Mutations in genes of metabolic pathways
In CIP-resistant colonies there were mutations at frequencies higher than 80% in different genes related to the arginine metabolism and transport such as argS and aotQ in colonies CIP_BF_4, CIP_BF_8 of the WT PAO1 and CIP_BF_15 of ΔkatA. While at frequencies lower than 80%, mutations in aruB, aruD, aruI, speA, argC, aotJ and hutE were repeatedly detected in CIP-resistant colonies of the WT PAO1 and ΔkatA CIP-resistant colonies (Fig. 6).
We have also detected mutations in genes related to the metabolism and transport of polyamines, as arginine is one of the precursors for polyamine synthesis. Mutated genes, such as aphA, potA, and potB were detected in several colonies (Fig. 6).
In CIP-resistant colonies isolated from biofilm, mutations in PA1300 encoding for σ factor 70 and in rpoN encoding for σ factor 54 were observed repeatedly and this might correlate to the observed prolonged lag phase observed in these colonies.
In addition, mutations in rpoS gene that codes for stress response regulator RpoS were detected in five CIP-resistant colonies (colonies no. CIP_BF_3, CIP_BF_5, CIP_BF_6, and CIP_Pl_26). It is noteworthy that these mutations are not present in WT CTRL-resistant colonies. These mutations might explain the increased doubling time of the CIP-resistant colonies.
In some of the CIP-resistant colonies, mutations in genes related to TCA cycle were observed. In colonies no. CIP_BF_7, CIP_BF_21, and CIP_Pl_25, the sdhA gene that encodes succinate dehydrogenase was mutated at frequencies higher than 80%. In addition, poxB and PA2290 genes that encodes pyruvate and glucose dehydrogenases were also found to be mutated.
In the ΔkatA-hypermutable CIP-resistant colonies, the genes related to iron storage and transport were mutated such as fecA, fecI, hasR, and PA2911 that encodes tonB dependent receptors at frequencies above 80% (Supplementary Data Set).
Mutations in CIP-resistant colonies from CTRL-evolved populations
Surprisingly, in CIP-resistant colonies from CTRL populations, nfxB gene was also frequently mutated in the majority of CTRL-resistant colonies showing that population with decreased susceptibility to CIP can arise in biofilm and planktonic growth modes without exposure to antibiotics.
Quorum-sensing genes such as lasR and prmC were mutated in CTRL –resistant colonies of both WT PAO1 and ΔkatA in colonies CTRL_BF_3, CTRL_BF_4, CTRL_BF_5, CTRL_Pl_17, and CTRL_Pl_18. This might explain the impaired swarming motility observed in CTRL CIP-resistant colonies compared to CIP-exposed colonies.
Mutations in genes considered patho-adaptive in chronic infections
We queried if the mutated genes identified during in vitro experimental evolution were of relevance for the in vivo evolution, and we looked for common genes with the patho-adaptive genes found to be mutated in several P. aeruginosa isolates from chronic lung infections of patients with CF17,18. A list of common genes encountered during in vivo and in vitro evolution is presented in Supplementary Table 4 and Fig. 7.
This diagram shows the mutated genes in the evolved-resistant isolates recovered from the in vitro experimental evolution of P. aeruginosa that are correlated with the patho-adaptive genes detected in naturally evolved clinical isolates inside the CF lungs18,33,39,43. The genes that are only mutated in CIP-evolved colonies are in black, those mutated only in CTRL-evolved colonies are highlighted in red while the genes that are mutated in both CTRL- and CIP-evolved colonies are highlighted in blue. The threshold of the frequency of the mutated genes in evolved isolates is higher than 10%.
Discussion
Extensive phenotypic and genotypic changes compared to the ancestor colonies have been observed in the CIP-resistant colonies isolated from P. aeruginosa WT and ΔkatA biofilm and planktonic evolution experiments in the presence of sub-MIC concentration of CIP. The evolution in the presence of subinhibitory concentrations of CIP selected for CIP-resistant mutants with distinct phenotypes compared to those isolated from CTRL populations.
We show that pathways of CIP-resistance development depend on of the bacterial life-style. High-level CIP-resistant mutants were isolated especially from evolution in planktonic populations while low-level CIP-resistant mutants were isolated from evolution in biofilm. The genetic basis for the two distinct phenotypes were simultaneous mutations in quinolone resistance determinants gyrA, parC, and efflux-pump regulators in high CIP-resistant mutants from planktonic populations as repeatedly published9,14,15,19 and mutations in efflux-pump regulators for CIP-resistant mutants evolved in biofilms. Similar observations have been recently published in Acinetobacter baumanii20 suggesting that this is a general characteristic of evolution in biofilms.
Low-level CIP-resistant mutants due to mutations in mexZ, a negative regulator of MexXY-OprM efflux-pump associated to active efflux of aminoglycosides and ciprofloxacin were reported in CF P. aeruginosa isolates from the initial phase of the chronic lung infection, suggesting that this may contribute to in vivo persistence21.
Association between resistance to CIP and beta-lactams was observed in this study (Supplementary Table 3). Here, we detected many genes especially involved with cell division and TCA cycle responsible for high resistance to ceftazidime and aztreonam antibiotics, confirming previous findings22,23 (Figs 5 and 6). Cross-resistance between CIP and beta-lactam antibiotics was also found in the planktonic experimental evolution study of P. aeruginosa9. In addition, our data show that CIP-resistant colonies displayed collateral increased sensitivity to both tobramycin and colistin. Previous studies24 showed that CIP-resistant isolates of P. aeruginosa are susceptible to aminoglycosides. This might be explained by the mutations in nfxB which has been shown to correlate to decreased activity of the MexXY-OprM and collateral sensitivity to aminoglycosides25. However, the minimal increase in the sensitivity to aminoglycosides and colistin of nfxB mutants has probably no or limited impact on the clinical efficacy of the treatment with these antibiotics since clinical treatments use concentrations that are far above these MICs. An extended lag phase and a reduction in max growth in LB were observed in CIP-evolved colonies. This is in accordance to recent studies investigating the metabolic functionality of experimentally evolved antibiotic-resistant P. aeruginosa which showed that CIP-evolved lineages exhibited longer lag phases and longer doubling times26.
The importance of the lag phase in the bacterial response to antibiotics has been previously shown by Fridman et al.27, who reported that “tolerance by lag” allows bacteria to survive under high antibiotic concentrations, and may facilitate the subsequent development of resistance. However, in our studies, the extension of the lag phase is not an adaptive response to antibiotics as the growth curves of the CIP-resistant colonies were conducted in the absence of the antibiotic, and the CIP-resistant colonies have been passed twice in antibiotic-free media after isolation from the population analysis plates. The mutations identified in the different metabolic pathways and RNA polymerase sigma factors might explain this phenotype.
Competition experiments in planktonic cultures showed that CIP resistance was associated with a fitness cost. However, in biofilm competition studies the resistant colonies persisted as a very small subpopulation and overgrew the ancestor colony in the presence of 0.1 mg/L CIP. Persistence of CIP-resistant colonies in biofilms in spite of their fitness cost suggest that growth rates are less important for survival in this mode of growth which probably allows maintenance of resistance for longer period of time in the absence of selective pressure.
Swarming, which is QS regulated28,29, was maintained in CIP-resistant colonies evolved under selection with sub-MIC CIP while CTRL CIP-resistant colonies lost the swarming. The mutations in the QS genes found in CTRL CIP-resistant colonies can explain this phenotype. This is in accordance with a recent published study confirming the lack of selection of QS mutants (lasR) during evolution experiments in the presence of antibiotics30.We have previously shown in planktonic experimental evolution studies that higher levels of QS molecules in P. aeruginosa populations evolved under sub-MIC ciprofloxacin compared to CTRL10.
It has been shown that swarming P. aeruginosa exerts adaptive resistance to a number of antibiotics, including CIP, which might explain the maintenance of the swarming motility during evolution in the presence of CIP28.
The loss of twitching motility is correlated to the frequent mutations in pil genes encoding for type IV pili identified in CIP-resistant colonies. The association between CIP resistance and mutations in pil genes has been reported by other groups but the reason for this association is not completely clear24.
Mutations in genes responsible for the catabolism of arginine such as aruL involved in the arginine decarboxylase and arcA involved in the arginine deaminase catabolic pathways were identified. Impairment of these pathways promotes the utilization of arginine by nitric oxide synthase to convert it to nitric oxide31,32. Nitric oxide will promote anaerobic respiration, and this is in agreement with previous studies that showed that anaerobic respiration is promoted in P. aeruginosa under CIP treatment10,33. Nitric oxide has been shown to be involved in the biofilm disruption34, but if this would occur as a consequence of the metabolic rewiring in CIP-treated biofilms remains to be investigated.
Antibiotic-resistant P. aeruginosa mutants overexpressing efflux pumps were shown to rewire their metabolism to avoid fitness cost including increased expression of the anaerobic nitrate respiratory chain when cells are growing under aerobic conditions35.
In accordance with the metabolic rewiring in the presence of ciprofloxacin, we have detected mutations in the enzymes involved in the TCA cycle in CIP-resistant colonies. This is in agreement with studies5 showing that mutants with impaired activity of TCA cycle enzymes were more tolerant to antibiotics due to decreased levels of bactericidal hydroxyl-radicals36.
Interestingly, mutations in the enzymes of the TCA cycle have been reported in clinical P. aeruginosa isolates that have evolved in the lung of CF patients37 and associated to the impaired catabolic capacity of the isolates. We have previously reported that metabolic reduction is a common adaptive trait of the P. aeruginosa nonmucoid isolates in the CF lung38 which has probably an impact on the persistence of the bacteria in the lung, in spite of the antibiotic treatment.
In addition, RNA polymerase σ54 (rpoN) and σS (rpoS) were found to be mutated in some CIP-biofilm resistant colonies and ΔkatA planktonic-resistant colonies and associated to impaired growth of the isolates. However, mutations in these genes were not detected in WT PAO1 planktonic-CIP and either of the CTRL-resistant colonies. Interestingly, rpoN mutants were found in P. aeruginosa from chronically infected patients17,39.
A comparison of the identified mutated genes in CIP-resistant colonies from in vitro-evolved populations with a list of patho-adaptive genes found to be mutated in several P. aeruginosa isolates from chronic lung infections of patients with CF17,18 revealed a large degree of overlap (Fig. 7; Supplementary Table 4) suggesting the important role played by antibiotic exposure of biofilm-growing bacteria in shaping the adaptation in vivo. This is in agreement with previous published results showing that, in vitro evolution can be used to predict in vivo adaptive changes in the presence of antibiotics24. However, some patho-adaptive genes, for example those involved in conversion to mucoidity and biofilm formation (mucA, algU, algG, pelA, and rbd A) were only mutated in clinical isolates suggesting the role of the immune system in the selection of these phenotypes, in accordance to our previous publication40.
In the future, supplementation of the present results with gene expression data will improve the understanding of evolution processes in biofilms.
In conclusion, our data on the phenotypic and mutagenic evolution in P. aeruginosa biofilms support the mutagenic potential of CIP at subinhibitory concentrations41. Mutations in CIP-resistance determinants were associated with mutations conferring phenotypes supporting a general survival advantage in the presence of antibiotics, as they were affecting some of the common mechanisms of action described for antibiotics, such as switch of the metabolic pathway to anaerobic respiration or affecting the TCA cycle. Phenotypes such as increased lag phases will also impair the effect of other antibacterial agents, while loss of twitching might impair biofilm dispersal42. Overall, our data show that CIP-resistant mutants acquire several other mutations that might confer survival advantages during antibiotic treatment of biofilms.
Methods
Strains
The wild-type (WT) P. aeruginosa PAO1 and its catalase mutant (ΔkatA) (MIC CIP = 0.125 µg/ml) have been used previously to start biofilm and planktonic evolution experiments in the presence of 0.1 mg/L ciprofloxacin (CIP) or without selection pressure (CTRL) for seven passages, as previously published14,15. The evolved populations were plated on LB plates containing ciprofloxacin (0.5, 1, and 2 mg/L) (population analysis) and three colonies from the plates with the highest ciprofloxacin concentration which allowed growth were isolated, passed twice on antibiotic-free media and stored at −80 oC for further analysis (see Fig. 1a). CIP-resistant colonies isolated after the last passage of the biofilm and planktonic evolution experiments were used for further investigations in this study.
For growth curves and motility assays all the available CIP-resistant colonies from CIP and CTRL evolution experiments, which are in total 92 isolates, were investigated.
For whole-genome sequencing, susceptibility testing to antipseudomonal antibiotics and fitness cost determinations in both planktonic and biofilm growth, a total of 42 colonies were investigated; 24 colonies from CIP-evolved populations (16 colonies isolated from biofilm populations and 8 colonies isolated from planktonic populations) and 18 colonies from CTRL-evolved populations (11 colonies isolated from biofilm populations and 7 colonies isolated from planktonic populations). The selection was randomly done and aimed at including colonies representing the different replicate lineages (Supplementary Table 1). Four of the ΔkatA CIP-resistant colonies had hypermutable phenotype, as indicated in Supplementary Table 1.
Phenotypic characterizations
Growth curves, MIC determinations, fitness cost determination in planktonic and biofilm cultures and motilities (swimming, swarming, and twitching) were performed, as described in Supplementary methods.
WGS and analysis
The whole-genome sequencing was performed for 42 CIP-resistant colonies (24 isolated from CIP-evolved population and 18 from CTRL-evolved populations) as well as for WT PAO1 and ΔkatA ancestor colonies. Genomic DNA was extracted from the colonies using Gentra puregene yeast/bacteria DNA purification kit (Qiagen). The DNA was prepared for sequencing using the Illumina TruSeq DNA Nano kit and sequenced on an Illumina MiSeq yielding a coverage of approximately 120×. Sequencing reads were mapped to the reference genome of P. aeruginosa PAO1 (GenBank accession. NC_002516), and single and multiple nucleotide variants (SNVs and MNVs) were called using CLC genomic workbench (Qiagen). Large indels and structural variants were also detected using using CLC genomic workbench. Mutations present in the CIP-resistant colonies were filtered out from the genome of the sequenced WT PAO1 and ΔkatA ancestor colonies using customized Linux and AWK scripts. R (version 3.2.5) was used for further statistical analysis of the mutations detected in colonies and all mutations occurring in >10% of the reads and at least 10 unique reads were included in the analysis.
The Pseudomonas Genome Database was used for gene function analysis. dN/dS, the ratio of the rate of nonsynonymous substitutions (dN) to the rate of the synonymous substitutions (dS), was calculated as a measure of the selection pressure acting on the protein-coding genome, as previously described, assuming that 25% of all single-nucleotide polymorphisms (SNP) result in synonymous changes33. dN/dS is expected to be >1 if natural selection promotes changes in protein sequences and <1 if natural selection suppresses changes.
Statistical analysis
Graphs and statistical analysis were performed using GraphPad Prism 7, Statview software and R (version 3.2.5). Student t-test and Mann–Whitney for nonparametric were used for comparisons among resistant isolates for growth rates and motilities (comparing CIP populations to CTRL populations and CIP-biofilm populations to CIP planktonic populations). The differences were considered significant when the p value was ≤0.05.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The datasets generated or analyzed during this study are available and included in this publication (and its published supplementary files). The project information is accessible with the following link http://www.ncbi.nlm.nih.gov/bioproject/643668.
References
Andersson, D. I. & Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 12, 465–478 (2014).
Hughes, D. & Andersson, D. I. Selection of resistance at lethal and non-lethal antibiotic concentrations. Curr. Opin. Microbiol. 15, 555–560 (2012).
Gullberg, E. et al. Selection of resistant bacteria at very low antibiotic concentrations. PLoS. Pathog. 7, e1002158 (2011).
Blazquez, J., Rodriguez-Beltran, J. & Matic, I. Antibiotic-induced genetic variation: how it arises and how it can be prevented. Annu. Rev. Microbiol. 72, 209–230 (2018).
Kohanski, M. A., DePristo, M. A. & Collins, J. J. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol. Cell 37, 311–320 (2010).
Cirz, R. T., O’Neill, B. M., Hammond, J. A., Head, S. R. & Romesberg, F. E. Defining the Pseudomonas aeruginosa SOS response and its role in the global response to the antibiotic ciprofloxacin. J. Bacteriol. 188, 7101–7110 (2006).
Blazquez, J., Couce, A., Rodriguez-Beltran, J. & Rodriguez-Rojas, A. Antimicrobials as promoters of genetic variation. Curr. Opin. Microbiol. 15, 561–569 (2012).
Gocke, E. Mechanism of quinolone mutagenicity in bacteria. Mutat. Res. 248, 135–143 (1991).
Jørgensen, K. M. et al. Sublethal ciprofloxacin treatment leads to rapid development of high-level ciprofloxacin resistance during long-term experimental evolution of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 57, 4215–4221 (2013).
Wassermann, T. et al. The phenotypic evolution of Pseudomonas aeruginosa populations changes in the presence of subinhibitory concentrations of ciprofloxacin. Microbiology 162, 865–875 (2016).
Hoiby, N. et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 21, S1–S25 (2015).
Rojo-Molinero, E., Macia, M. D. & Oliver, A. Social behavior of antibiotic resistant mutants within pseudomonas aeruginosa biofilm communities. Front. Microbiol. 10, 570 (2019).
Macia, M. D., Perez, J. L., Molin, S. & Oliver, A. Dynamics of mutator and antibiotic-resistant populations in a pharmacokinetic/pharmacodynamic model of Pseudomonas aeruginosa biofilm treatment. Antimicrob. Agents Chemother. 55, 5230–5237 (2011).
Ahmed, M. N., Porse, A., Sommer, M. O. A., Hoiby, N. & Ciofu, O. Evolution of antibiotic resistance in biofilm and planktonic Pseudomonas aeruginosa populations exposed to subinhibitory levels of ciprofloxacin. Antimicrob. Agents Chemother. 62, e00320–18 (2018).
Ahmed, M. N. et al. Lack of the major multifunctional catalase KatA in Pseudomonas aeruginosa accelerates evolution of antibiotic resistance in ciprofloxacin-treated biofilms. Antimicrob. Agents Chemother. 63, e00766–19 (2019).
Huse, H. K. et al. Parallel evolution in Pseudomonas aeruginosa over 39,000 generations in vivo. MBio 1(4), e00199–10 (2010).
Marvig, R. L., Sommer, L. M., Jelsbak, L., Molin, S. & Johansen, H. K. Evolutionary insight from whole-genome sequencing of Pseudomonas aeruginosa from cystic fibrosis patients. Fut. Microbiol. 10, 599–611 (2015).
Winstanley, C., O’Brien, S. & Brockhurst, M. A. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol. 24, 327–337 (2016).
Rehman, A., Patrick, W. M. & Lamont, I. L. Mechanisms of ciprofloxacin resistance in Pseudomonas aeruginosa: new approaches to an old problem. J. Med. Microbiol. 68, 1–10 (2019).
Santos-Lopez, A., Marshall, C. W., Scribner, M. R., Snyder, D. J., & Cooper, V. S. Evolutionary pathways to antibiotic resistance are dependent upon environmental structure and bacterial lifestyle. Elife. 8, e47612 (2019).
Frimodt-Moller, J. et al. Mutations causing low level antibiotic resistance ensure bacterial survival in antibiotic-treated hosts. Sci. Rep. 8, 12512 (2018).
Jorth, P. et al. Evolved aztreonam resistance is multifactorial and can produce hypervirulence in Pseudomonas aeruginosa. MBio 8, e00517–17 (2017).
Lopez-Causape, C., Cabot, G., Del Barrio-Tofino, E. & Oliver, A. The versatile mutational resistome of Pseudomonas aeruginosa. Front Microbiol. 9, 685 (2018).
Wardell, S. J. T. et al. A large-scale whole-genome comparison shows that experimental evolution in response to antibiotics predicts changes in naturally evolved clinical Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 63, e01619–19 (2019).
Imamovic, L. et al. Drug-driven phenotypic convergence supports rational treatment strategies of chronic infections. Cell 172, 121–134 (2018).
Dunphy, L. J., Yen, P. & Papin, J. A. Integrated experimental and computational analyses reveal differential metabolic functionality in antibiotic-resistant Pseudomonas aeruginosa. Cell Syst. 8, 3–14 (2019).
Fridman, O., Goldberg, A., Ronin, I., Shoresh, N. & Balaban, N. Q. Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature 513, 418–421 (2014).
Overhage, J., Bains, M., Brazas, M. D. & Hancock, R. E. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J. Bacteriol. 190, 2671–2679 (2008).
Ha, D. G., Kuchma, S. L. & O’Toole, G. A. Plate-based assay for swarming motility in Pseudomonas aeruginosa. Methods Mol. Biol. 1149, 67–72 (2014).
Hernando-Amado, S., Sanz-Garcia, F. & Martinez, J. L. Antibiotic resistance evolution is contingent on the quorum-sensing response in Pseudomonas aeruginosa. Mol. Biol. Evol. 36, 2238–2251 (2019).
Cunin, R., Glansdorff, N., Pierard, A. & Stalon, V. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50, 314–352 (1986).
Xiong, L. et al. Arginine metabolism in bacterial pathogenesis and cancer therapy. Int. J. Mol. Sci. 17, 363 (2016).
Yang, L. et al. Evolutionary dynamics of bacteria in a human host environment. Proc. Natl Acad. Sci. USA 108, 7481–7486 (2011).
Barraud, N. et al. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J. Bacteriol. 191, 7333–7342 (2009).
Olivares, P. J., Alvarez-Ortega, C., Alcalde,R. M., & Martinez, J. L. Metabolic compensation of fitness costs is a general outcome for antibiotic-resistant Pseudomonas aeruginosa mutants overexpressing efflux pumps. MBio 8, e00500–17 (2017).
Crabbe, A., Jensen, P. O., Bjarnsholt, T. & Coenye, T. Antimicrobial tolerance and metabolic adaptations in microbial biofilms. Trends Microbiol. 27, 850–863 (2019).
Marvig, R. L. et al. Within-host microevolution of Pseudomonas aeruginosa in Italian cystic fibrosis patients. BMC Microbiol. 15, 218 (2015).
Jørgensen, K. M. et al. Diversity of metabolic profiles of cystic fibrosis Pseudomonas aeruginosa during the early stages of lung infection. Microbiology 161, 1447–1462 (2015).
Smith, E. E. et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl Acad. Sci. USA 103, 8487–8492 (2006).
Mathee, K. et al. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology 145, 1349–1357 (1999).
Pribis, J. P. et al. Gamblers: an antibiotic-induced evolvable cell subpopulation differentiated by reactive-oxygen-induced general stress response. Mol. Cell 74, 785–800 (2019).
Kassen, R. & Rainey, P. B. The ecology and genetics of microbial diversity. Annu. Rev. Microbiol. 58, 207–231 (2004).
Marvig, R. L., Sommer, L. M., Molin, S. & Johansen, H. K. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat. Genet. 47, 57–64 (2015).
Acknowledgements
A.P. and M.O.A.S. acknowledge the Novo Nordisk Foundation under NFF grant no. NNF10CC1016517, the European Union H2020 (ERC-2014-STG) under Grant Agreement 638902, Limit MDR and the Danish Council for Independent Research, Sapere Aude Program DFF 4004-00213. The financial support of M.A. from the Egyptian Ministry of Higher Education is acknowledged.
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of the data: M.A., A.A., T.W., A.P., J.B., M.S., N.H., and O.C. Drafting the work or revising it critically for important intellectual content: M.A., A.A., T.W., A.P., J.B., M.S., N.H., and O.C. Final approval of the submitted version: M.A., A.A., T.W., A.P., J.B., M.S., N.H., O.C. Accountability for all aspects of the work are appropriately investigated and resolved: M.A., A.A., T.W., A.P., J.B., M.S., N.H., and O.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, M.N., Abdelsamad, A., Wassermann, T. et al. The evolutionary trajectories of P. aeruginosa in biofilm and planktonic growth modes exposed to ciprofloxacin: beyond selection of antibiotic resistance. npj Biofilms Microbiomes 6, 28 (2020). https://doi.org/10.1038/s41522-020-00138-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-020-00138-8
- Springer Nature Limited
This article is cited by
-
Siderophores promote cooperative interspecies and intraspecies cross-protection against antibiotics in vitro
Nature Microbiology (2024)
-
Intermittent antibiotic treatment of bacterial biofilms favors the rapid evolution of resistance
Communications Biology (2023)
-
Tolerance and resistance of microbial biofilms
Nature Reviews Microbiology (2022)
-
Biofilm antimicrobial susceptibility through an experimental evolutionary lens
npj Biofilms and Microbiomes (2022)
-
rpoS-mutation variants are selected in Pseudomonas aeruginosa biofilms under imipenem pressure
Cell & Bioscience (2021)