Abstract
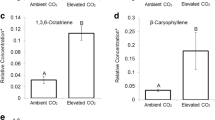
Recent carbon dioxide (CO2) concentrations promoted higher parthenin concentrations in an invasive Parthenium hysterophorus biotype. Mean concentrations of parthenin, an allelopathic and defensive sesquiterpene lactone, were 49% higher at recent (~400 ppm) than at mid-twentieth-century (~300 ppm) CO2 concentrations, but did not vary in a non-invasive biotype, suggesting that recent increases in atmospheric CO2 may have already altered the chemistry of this destructive weed, potentially contributing to its invasive success.

Similar content being viewed by others
Data availability
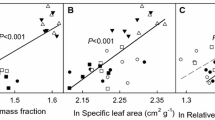
All data collected can be viewed in the Supplementary Information, including plant dry weights over time (Supplementary Table 3) and concentrations of parthenin (Supplementary Table 4).
References
Climate Change: Vital Signs of the Planet (NASA, 2021); https://climate.nasa.gov/vital-signs/carbon-dioxide
Kimball, B. A. Crop responses to elevated CO2 and interactions with H2O, N, and temperature. Curr. Opin. Plant Biol. 31, 36–43 (2016).
Leakey, A. D. B. et al. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J. Exp. Bot. 60, 2859–2876 (2009).
Lee, T. D., Barrott, S. H. & Reich, P. B. Photosynthetic responses of 13 grassland species across 11 years of free-air CO2 enrichment is modest, consistent and independent of N supply. Glob. Change Biol. 17, 2893–2904 (2011).
Ainsworth, E. A. & Long, S. P. 30 years of free-air carbon dioxide enrichment (FACE): what have we learned about future crop productivity and its potential for adaptation? Glob. Change Biol. 27, 27–49 (2021).
Dusenge, M. E., Duarte, A. G. & Way, D. A. Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 221, 32–49 (2019).
Sardans, J. et al. Ecometabolomics for a better understanding of plant responses and acclimation to abiotic factors linked to global change. Metabolites 10, 239 (2020).
Poorter, H. & Navas, M.-L. Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytol. 157, 175–198 (2003).
Parmesan, C. & Hanley, M. E. Plants and climate change: complexities and surprises. Ann. Bot. 116, 849–864 (2015).
Ode, P. J., Johnson, S. N. & Moore, B. D. Atmospheric change and induced plant secondary metabolites—are we reshaping the building blocks of multi-trophic interactions? Curr. Opin. Insect Sci. 5, 57–65 (2014).
Robinson, E. A., Ryan, G. D. & Newman, J. A. A meta-analytical review of the effects of elevated CO2 on plant–arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 194, 321–336 (2012).
Willeit, M., Ganopolski, A., Calov, R. & Brovkin, V. Mid-Pleistocene transition in glacial cycles explained by declining CO2 and regolith removal. Sci. Adv. 5, eaav7337 (2019).
Busch, F. A. & Sage, R. F. The sensitivity of photosynthesis to O2 and CO2 concentration identifies strong Rubisco control above the thermal optimum. New Phytol. 213, 1036–1051 (2017).
Drake, B. G., Gonzàlez-Meler, M. A. & Long, S. P. More efficient plants: a consequence of rising atmospheric CO2? Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 609–639 (1997).
Ziska, L. H., Sicher, R. C., George, K. & Mohan, J. E. Rising atmospheric carbon dioxide and potential impacts on the growth and toxicity of poison ivy (Toxicodendron radicans). Weed Sci. 55, 288–292 (2007).
Ziska, L. H. & Caulfield, F. A. Rising CO2 and pollen production of common ragweed (Ambrosia artemisiifolia L.), a known allergy-inducing species: implications for public health. Funct. Plant Biol. 27, 893 (2000).
Ziska, L. H., Panicker, S. & Wojno, H. L. Recent and projected increases in atmospheric carbon dioxide and the potential impacts on growth and alkaloid production in wild poppy (Papaver setigerum DC.). Clim. Change 91, 395 (2008).
Del Fabbro, C. & Prati, D. The relative importance of immediate allelopathy and allelopathic legacy in invasive plant species. Basic Appl. Ecol. 16, 28–35 (2015).
Ni, G. et al. Exploring the novel weapons hypothesis with invasive plant species in China. Allelopath. J. 29, 199–214 (2012).
Peñuelas, J. et al. Higher allocation to low cost chemical defenses in Iinvasive species of Hawaii. J. Chem. Ecol. 36, 1255–1270 (2010).
Bajwa, A. A., McClay, A. & Adkins, S. W. in Parthenium Weed: Biology, Ecology and Management (eds Adkins, S., Shabbir, A. et al.) 7–39 (CABI, 2019).
Adkins, S. & Shabbir, A. Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.): management of parthenium weed. Pest Manag. Sci. 70, 1023–1029 (2014).
Niranjan, A. et al. Identification and quantification of heterologous compounds parthenin and organic acids in Parthenium hysterophorus L. using HPLC-PDA-MS-MS. Anal. Lett. 46, 48–59 (2013).
Belz, R. G., van der Laan, M., Reinhardt, C. F. & Hurle, K. Soil degradation of parthenin—does it contradict the role of allelopathy in the invasive weed Parthenium hysterophorus L.? J. Chem. Ecol. 35, 1137–1150 (2009).
Hanif, Z., Adkins, S. W., Prentis, P. J., Navie, S. C. & O’Donnell, C. J. Characterization of the reproductive behaviour and invasive potential of parthenium weed in Australia. Pak. J. Weed Sci. Res. 18, 767–774 (2012).
Bajwa, A. A., Chauhan, B. S. & Adkins, S. Morphological, physiological and biochemical responses of two Australian biotypes of Parthenium hysterophorus to different soil moisture regimes. Environ. Sci. Pollut. Res. 24, 16186–16194 (2017).
Nguyen, T., Bajwa, A. A., Navie, S., O’Donnell, C. & Adkins, S. Parthenium weed (Parthenium hysterophorus L.) and climate change: the effect of CO2 concentration, temperature, and water deficit on growth and reproduction of two biotypes. Environ. Sci. Pollut. Res. 24, 10727–10739 (2017).
Chadwick, M., Trewin, H., Gawthrop, F. & Wagstaff, C. Sesquiterpenoids lactones: benefits to plants and people. Int. J. Mol. Sci. 14, 12780–12805 (2013).
Ojija, F., Arnold, S. E. J. & Treydte, A. C. Impacts of alien invasive Parthenium hysterophorus on flower visitation by insects to co-flowering plants. Arthropod Plant Interact. 13, 719–734 (2019).
Bajwa, A. A., Chauhan, B. S. & Adkins, S. W. Germination ecology of two Australian biotypes of ragweed parthenium (Parthenium hysterophorus) relates to their invasiveness. Weed Sci. 66, 62–70 (2018).
Bajwa, A. A. et al. Toxic potential and metabolic profiling of two Australian biotypes of the invasive plant parthenium weed (Parthenium hysterophorus L.). Toxins 12, 447 (2020).
Grime, J. P. Plant Strategies, Vegetation Processes, and Ecosystem Properties (Wiley, 2001).
Grime, J. P. in Plant Evolutionary Biology (eds Gottlieb, L. D. & Jain, S. K.) 371–393 (Springer, 1988).
Craine, J. M. Reconciling plant strategy theories of Grime and Tilman. J. Ecol. 93, 1041–1052 (2005).
Bae, J. et al. Effect of elevated atmospheric carbon dioxide on the allelopathic potential of common ragweed. J. Ecol. Environ. 43, 21 (2019).
Wang, R.-L. et al. Responses of Mikania micrantha, an invasive weed to elevated CO2: induction of β-caryophyllene synthase, changes in emission capability and allelopathic potential of β-caryophyllene. J. Chem. Ecol. 36, 1076–1082 (2010).
Robinson, J. M. Photosynthetic carbon metabolism in leaves and isolated chloroplasts from spinach plants grown under short and intermediate photosynthetic periods. Plant Physiol. 75, 397–409 (1984).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019).
Filion, M., Dutilleul, P. & Potvin, C. Optimum experimental design for Free-Air Carbon dioxide Enrichment (FACE) studies. Glob. Change Biol. 6, 843–854 (2000).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. https://www.jstatsoft.org/article/view/v067i01 (2015).
Searle, S. R., Speed, F. M. & Milliken, G. A. Population marginal means in the linear model: an alternative to least squares means. Am. Stat. 34, 216–221 (1980).
Acknowledgements
We thank R. Belz for sharing the parthenin standard, without which this study would not have been possible. M. Kramer of USDA’s Northeast Area Statistics Group provided invaluable assistance for which we are grateful. A.A.B. and S.W.A. acknowledge financial support from the University of Queensland, Australia, making possible A.A.B.’s trip to USDA-ARS Beltsville, which laid the foundation of this collaborative project.
Author information
Authors and Affiliations
Contributions
The study was initially conceived by A.A.B., L.H.Z. and C.R. Study design and methods were developed by J.W., S.M.A., L.H.Z., A.A.B., D.H.F. and C.R., with guidance from S.W.A. The experiment was carried out by J.W. and S.M.A. J.W. extracted parthenin and C.R. quantified it. J.W. analysed the data and wrote the first draft, which was expanded and reviewed by S.W.A., D.H.F., L.H.Z., A.A.B. and C.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Paul Ode and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–4.
Rights and permissions
About this article
Cite this article
Rice, C., Wolf, J., Fleisher, D.H. et al. Recent CO2 levels promote increased production of the toxin parthenin in an invasive Parthenium hysterophorus biotype. Nat. Plants 7, 725–729 (2021). https://doi.org/10.1038/s41477-021-00938-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-00938-6
- Springer Nature Limited