Abstract
CRISPR homing gene drives can suppress pest populations by targeting female fertility genes, converting wild-type alleles into drive alleles in the germline of drive heterozygotes. fsRIDL (female-specific Release of Insects carrying a Dominant Lethal) is a self-limiting population suppression strategy involving continual release of transgenic males carrying female lethal alleles. Here, we propose an improved pest suppression system called “Release of Insects carrying a Dominant-sterile Drive” (RIDD), combining performance characteristics of homing drive and fsRIDL. We construct a split RIDD system in Drosophila melanogaster by creating a 3-gRNA drive disrupting the doublesex female exon. Drive alleles bias their inheritance in males, while drive alleles and resistance alleles formed by end-joining cause dominant female sterility. Weekly releases of RIDD males progressively suppressed and eventually eliminated cage populations. Modeling shows that RIDD is substantially stronger than SIT and fsRIDL. RIDD is also self-limiting, potentially allowing targeted population suppression.
Similar content being viewed by others
Introduction
Insect pests cause vast damage to agriculture and pose a threat to public health1,2. Chemical pesticides have been utilized to suppress pests for decades, but increasing development of pesticide-resistance and harmful environment effects prevent this from being sustainable3,4,5. Thus, effective and ecologically friendly pest control techniques are urgently required. In response to this growing need, a number of pest control technologies have developed in recent years based on genetic engineering biotechnology6,7,8,9,10,11. Some of these strategies have successfully suppressed target populations in laboratory trials, which can potentially lead to deployment in the future7,12,13,14,15.
Sterile insect technique (SIT), an environmental-friendly and species-specific pest control method, was the first genetic pest control system16. It aims to reduce local reproductive potential and thereby reduce the population. A large number of male insects sterilized by radiation or other methods are released to the wild, mating with wild females, which then produce no or fewer offspring6,10,16. If sufficient sterile insects are released repeatedly, the local population will be reduced or even eliminated. SIT programs have been conducted with varying degrees of success against screwworm, tsetse fly, and medfly6,10,17,18. However, despite its attractive features, exposing insects to sterilizing doses of radiation or chemicals reduces their longevity and mating competitiveness19. This means that in many scenarios, it may be impractical to release enough insects to cause population suppression.
With the development of genetic engineering, transgenic pest control strategies were proposed to improve and complement conventional SIT. Female-specific release of insects carrying a dominant lethal gene (fsRIDL) system is similar to SIT, avoiding the side effects of irradiated sterilization by using genetic manipulation18,19,20,21. Dominant lethal transgenes specifically expressed in females yield several advantages compared with SIT. The transgenic allele can be transmitted by released transgenic males, rendering the female offspring nonviable. The heterozygous male offspring carrying the transgenic allele can continue to help suppress the population by transmitting it to half of their daughters. Thus, not only do fsRIDL males potentially have a fitness advantage over SIT males (due to lack of harmful sterilization technique), but they also can cause effects over multiple generations, making the technique substantially more powerful than SIT22,23. It avoids the need for sex separation and radiation/chemosterilization as well, though rearing may still be more expensive than SIT due to the need to use a ligand to repress the female-lethal allele. fsRIDL has been utilized to successfully suppress fruit flies24, mosquitos19,25,26,27,28, and moths29,30 in cage or field trials. A similar strategy was also proposed in non-insect species, called daughterless technology for invasive carp elimination31,32,33. Nevertheless, fsRIDL still relies on mass-rearing technology, which is costly and demanding in some species14,25,34,35,36,37,38, so it may still often lack the power necessary for success.
At the frontier of pest control, gene drive shows great promise for blocking disease transmission or directly suppressing pest populations7,8,12,15,39. Gene drives are alleles that can bias their own inheritance, increasing in frequency and eventually spreading through a population along40. The most common engineered gene drive system is the CRISPR homing drive, which converts wild-type alleles to drive alleles in the germline of drive heterozygotes. Specifically, the target site is cleaved by Cas9 and a guide RNA (gRNA), and the DNA break then undergoes homology-directed repair (HDR).
To accomplish population suppression, CRISPR homing drive can be designed to target a haplosufficient but essential gene, still allowing drive conversion to take place in healthy heterozygotes while reducing the fertility or viability of homozygous individuals41. One potential target is doublesex (dsx), which is a central nexus for sex determination in insects22,42,43,44. With alternative splicing, sex-specific dsx transcripts are necessary for sexual differentiation45,46,47. Females that are homozygous for a disrupted dsx allele at the female-specific exon (by insertion of drive allele or a nonfunctional resistance allele) lose reproductive capability43,48. Kyrou et al. successfully suppressed a cage population of Anopheles gambiae by targeting the intron 4/exon 5 junction of dsx43. Recently, Yadav et al. also reported a population suppression design in agricultural pest Drosophila suzukii by targeting the female exon of dsx49. Interestingly, they reported that in some lines, the drive was dominant and sterile. Rapid emergence of resistance alleles presents a large challenge for CRISPR homing drives50,51,52, but this can be mitigated by a multiplexed gRNAs strategy53,54.
High efficiency homing drive enables population elimination with a small initial release if the drive is sufficiently efficient. However, this is a double-edged sword: the zero-introduction threshold implies that the drive cannot be confined to a target population because even small numbers of migrants will spread the drive to other even weakly connected populations, which may raise sociopolitical concerns or be undesired from an ecological point of view9,39,55. There are two commonly used forms of CRISPR-based gene drive constructs. The first is “complete drive”, with Cas9 and gRNA are integrated together as one drive unit at a single genomic site41,56. The second is “split drive”, where the gRNAs cassette can only function in combination with a separated source of Cas9, which does not have biased inheritance49,57,58. Split drives have been considered flexible safeguarding strategies for gene drive experiments, as well as confinement strategies for localized release purposes57,58,59. To open up new possibilities for the safe and effective control of harmful species, it has been proposed to combine fsRIDL with drive20,60,61. Modeling showed that this system would have higher efficiency compared to bisexual RIDL and female-specific RIDL. A similar system could involve a standard homing suppression drive with a somatic expression that makes the drive dominant female sterile, though the experimental demonstration suffered from functional resistance alleles41.
We here propose a similar strategy, the Release of Insects carrying a Dominant sterile Drive (RIDD), which combines the advantages of homing gene drive and fsRIDL. We designed a 3-gRNA dsx-based split RIDD system in Drosophila melanogaster. The gRNAs target the dsx female-specific exon, while Cas9 is positioned in a separate genetic locus. We found that both drive and resistance alleles were dominant and sterile in females. Dominant sterility and intersex phenotype from dsx mutations in female D. melanogaster was reported decades ago62,63, but our design consistently achieves this with most resistance alleles. We attempted to harness this property in our drive system. The drive has no apparent impact on males, and drive conversion can take place in the germline of male heterozygotes. Thus, when drive males are released to the wild population, the drive allele can spread when they mate with wild-type females. The male offspring that carry a drive allele can continue gene drive in the next generation, while female offspring with a drive allele or resistance allele, which together compose all transmitted alleles, will be sterile. Repeatedly releasing RIDD males into cage populations resulted in a slow increase in drive allele frequency and, eventually, population elimination after several generations. Modeling was used to support the cage study, estimating drive and other parameters. We constructed a general model to show that RIDD is substantially more efficient than SIT and fsRIDL while remaining self-limiting, suggesting its potential use in future pest control.
Results
Homing drive design targeting dsx
We aimed to develop a homing suppression drive in D. melanogaster by targeting doublesex. Null mutations of dsx result in female recessive sterility but show no effects on males, according to the previous drive study in Anopheles mosquitoes43. dsx is a crucial central “nexus”, responding to upstream sex determination signals as well as regulating thousands of downstream sexual differentiation cascades22,42. A highly conserved female-specific alternative splicing acceptor site is near the boundary of intron 3 and exon 4, resulting in different dsx transcripts in males and females64,65 (Fig. 2a).
Our drive is inserted between the leftmost and rightmost gRNA target sites of dsx (Fig. 1a). DsRed driven by 3xP3 promoter is included as a fluorescent marker to indicate the presence of a drive allele. The drive also contains three gRNAs, all expressed by the U6:3 promoter and multiplexed by tRNA sequences, avoiding repetitive gRNA promoter elements. The gRNAs target near the boundary between intron 3 and exon 4, with all cut sites within the exon (though the first cut site is only two nucleotides into the exon). Multiplexed gRNA targets ensure that frameshift mutations or disruption of sexual alternative splicing of the female-specific exon would firmly disrupt the gene’s function in females, avoiding the formation of function resistance alleles53. Our gRNA sites were also chosen to avoid strong off-target sites.
a Our gene drive construct consists of a DsRed fluorescent marker driven by the 3xP3 promoter and three gRNAs driven by the U6:3 promoter that are separated by tRNAs. Exon-4 of dsx on chromosome 3 R is a female-specific exon. gRNAs target the intron 3/exon 4 boundary within the exon. In a drive heterozygote’s germline, Cas9 and gRNA cleaves the wild-type allele. Repair of the cleaved chromosome through homologous directed repair (HDR) leads to the copying of the drive allele (called “drive conversion” or “homing”). b The split Cas9 construct is on chromosome 2 R. Cas9 is under the control of one of the following 5’UTR/promotors: nanos, rcd-1r and CG4415. The 3’ UTR is either Nanos or shu. A 3xP3:EGFP cassette is used to indicate the presence of the Cas9 allele. c The drive inheritance rate of the progeny of dsx drive heterozygous males with heterozygous or homozygous Cas9 (marked by homo. or heter.) controlled by the indicated regulatory elements. Each dot represents progeny from a single drive male in one vial, and the size of each spot shows the number of individuals phenotyped. The mean inheritance rate (± s.e.m.) is shown. Source data are provided in Supplementary Data Set 1.
The Cas9 element, required for drive activity, is placed on chromosome 2 R and provided through a separate line carrying Cas9 and EGFP with the 3xP3 promoter. To assess the effect of Cas9 regulation on drive performance, we tested our drive with several Cas9 constructs (Fig. 1b), which are comprised of different 5’UTR and 3’UTR. The main Cas9 line used nanos regulatory elements57, but we also tested combinations of CG4415 and rcd-1r promotor/5’UTR elements with nanos and shu 3’UTR elements, which were recently shown to support high drive performance66. The drive will only be active in individuals both containing Cas9 and drive alleles in this split drive system57.
Drive performance and morphological analysis of the dsx drive
To obtain active drive individuals for performance tests, we crossed dsx-drive male heterozygotes with five different Cas9 lines females, differing in Cas9 regulatory elements (Fig.1a, b). The offspring with both green and red fluorescent eyes, indicating that they were heterozygous for the drive allele and Cas9 allele, were used for drive conversion efficiency and morphology assessment by crossing them to w1118 females.
For males, the dsx drive shows no impact on their morphology and fertility. Drive conversion (also called homing) takes place in the germline of heterozygotes, where the wild-type allele can be converted to a drive allele by CRISPR cleavage and homology-directed repair (Fig. 1c). The drive allele was inherited at a high rate, up to 84.8% among progeny of individuals with homozygous nanos-Cas9-nanos allele. It was slightly lower in heterozygotes at 81%, and lower still for the CG4415 Cas9 promoter (70.0% with shu 3’UTR and 73.3% with nanos 3’UTR). For the rcd-1r promotor, drive inheritance was also good (85.5% with Nanos 3’UTR, 81.8% with shu 3’UTR) (Fig. 1c). These drive inheritance rates were all significantly higher than the Mendelian inheritance rate of 50% (Binomial test, P < 0.0001) and thus indicative of strong drive activity in the male germline. For homozygous Cas9 regulated by the nanos promoter/Nanos 3’UTR combination, the drive conversion rate was 69.6%, assuming that all genotypes had the same viability.
The DsRed in the eyes of drive individuals was observed to have two discrete levels of brightness. Bright DsRed individuals and faint individuals gave rise to progeny of the same brightness in the absence of Cas9. When Cas9 was present, faint individuals still had only faint offspring, but bright-eyed individuals had approximately one-third of progeny that were faint eye, implying that these may have been the result of drive conversion. However, we found no sequence difference between the drive construct and the surrounding insertion site. For drive performance, we found that the faint eye males, 75.7% drive inheritance with nanos-Cas9, representing a small but significant reduction (Fisher’s exact test, P < 0.0004, Supplementary Fig. 2). However, this apparently reduced drive inheritance may be partially due to the difficulty in phenotyping faint eye flies in the presence of EGFP.
Surprisingly, we found that all drive females were sterile, regardless of the presence of any Cas9 allele, indicating that the drive was dominant-sterile for females. Such females had a (partially) masculine phenotype, which is different from the dsx-target drive in A. gambiae, where the drive allele was recessive sterile, and heterozygous females lacked an abnormal phenotype, with only homozygous females showing a strong masculine phenotype43. Examination of external sexually dimorphic structures in drive females showed phenotypic abnormalities, including a larger dark stripe at the end of the abdomen and male-like genitalia (Fig. 2b and Supplementary Fig. 1). To determine the molecular mechanism causing female-specific dominant sterility, we designed sex-specific primers and used cDNA as template for PCR (Fig. 2a). We found that female- and male-specific versions of dsx transcripts were both expressed in drive females (Fig. 2c). One possible reason is that the drive allele insertion disrupts the splice acceptor site of female-specific exon 4, preventing female-specific splicing from recognizing the site and recruiting splicing machinery. The next splice acceptor is then used instead, which is specific for the male version of the dsx transcript and results in incorrect sex development. This result is analogous to a similar dominant female-sterile construct in D. suzukii49.
a Schematic representation of the male- and female-specific dsx transcripts. Red arrows are primers designed for male- or female-specific transcript diagnostic PCR. The PCR product for the female dsx transcript is 713 bp, and the product for the male transcript is 352 bp. b Pictures of wild-type (+/+) flies, female drive carriers (D/+), and nonfunctional (r2) resistance allele females are shown. dominant r2/+ refers to dominant sterile r2 individuals, recessive r2/+ refers to recessive sterile r2 individuals. D/+, dominant r2/+, and recessive r2/+ females were homozygous for the nanos-Cas9-nanos allele. c Diagnostic PCR on cDNA using female-specific primers (top) and male-specific primers (bottom).
We also tested combinations of drives with different Cas9 lines varying in the amount of somatic expression66. Such somatic expression could give drive heterozygous females a similar phenotype to drive homozygous females, which could not be obtained because the drive is dominant female-sterile. The addition of Cas9 driven by the nanos promoter had no effect on the phenotype, as expected due to the very low somatic expression of nanos-Cas9. Compared to drive females carrying nanos-Cas9, drive females with Cas9 expressed by the CG4415 and rcd-1r promotors often had stronger masculine phenotypes (Supplementary Figs. 1, 3).
Resistance allele formation and classification
To identify resistance alleles that prevent Cas9 cleavage, we monitored the occurrence of mutations at the drive target site in the progeny of drive males with nano-Cas9. Though mutated dsx transcripts lose function, the dsx female transcript is haplosufficient. Yet strikingly, all resistance alleles had a dominant visible phenotype, which was an uncommon type of allele in a previous study62 (Fig. 2b). In terms of morphological analysis and fertility, three types of nonfunctional (r2) resistance allele females were observed in our experiments: (1) dominant sterile r2 alleles with the same intersex/masculinized phenotype as drive females, which is the most common type of r2. (2) dominant sterile r2 alleles with a stronger intersex phenotype, representing about 8.3% of dominant r2 alleles (Supplementary Data Set 1), and (3) recessive sterile r2 alleles with mild intersex phenotype, but which were fertile when heterozygous for the r2 allele and a wild-type allele. The frequency of recessive sterile r2 alleles is about 5.3% of all resistance alleles (2 of 38 in our r2 female fertility test, Supplementary Data Set 1). Females that were homozygous for this last class of r2 alleles displayed stronger intersex phenotypes. No functional/r1 resistance alleles were detected (wild-type progeny of drives males all had wild-type alleles), likely because the dsx target exon site was highly conserved and targeted by three gRNAs. According to the fraction of masculine non-drive female offspring of male drive heterozygotes, germline resistance alleles forming in 97.5% of wild-type alleles that failed to undergo drive conversion for homozygous nanos-Cas9 and 99.6% for heterozygous nanos-Cas9.
Diagnostic PCR suggested that male form dsx transcripts were generated both in dominant sterile as well as recessive sterile r2 females, but the male transcript expression level may be reduced in the latter (Fig. 2c). In all but the second class of r2 allele sequences, the region between the outermost gRNA cut sites were deleted. For r2 females with stronger intersex phenotypes, we saw a diverse mix of sequences, including full deletions between the outmost sites, individual indels that formed sequentially at each of the three gRNA target sites, and two sequences with an indel at the leftmost gRNA target site and incomplete homology-directed repair of the drive after a simultaneous cleavage of the middle and rightmost gRNA target site (Fig. 3). Remarkably, the AG in the 3’ intron, the splice acceptor site, was preserved in all but one sequence for females with moderate intersex/ masculinized phenotype, while AG was partially or fully deleted in females with stronger intersex phenotypes. Thus, it is possible that the disruption of the splicing acceptor site in female-specific exon 4 increased the possibility of generating more male-specific dsx products. In contrast, the preservation of the splice acceptor site enabled (disrupted) female dsx products to be generated in spite of mutations64, reducing but not eliminating the chance that splicing skipped directly to the male-specific exon.
Each sequence originates from one female carrying a resistance allele with the listed phenotype. Vertical dashed lines indicate the boundaries of the female-specific exon’s coding sequence. Orange highlighting shows the gRNA target sequences, and yellow shows the gRNA PAM sequences.—indicates deletion. “Insertion” refers to a 311 bp region from the right side of the drive that was copied by incomplete homology-directed repair.
New pest control strategy RIDD system
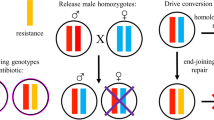
Based on the dominant-sterile property of dsx drive in Drosophila, the largely dominant-sterile resistance alleles (~ 95%), and the high total germline cut rates in males (> 99%, Supplementary Fig. 3), we put forward a new pest suppression strategy called the Release of Insects carrying a Dominant sterile Drive allele (RIDD) system. Drive conversion can take place in the germline of male heterozygotes, which are released continuously in large number into a population, similar to the RIDL strategy. When drive males are released to the wild population, the drive allele can be transmitted when they mate with wild-type females. Nearly all female progeny will be sterile (regardless of whether they inherit drive alleles or resistance alleles), yet will still contribute to resource competition. The drive male offspring will usually be able to do “homing” by HDR to continuously transmit the drive allele at increased rates. Even when end-joining takes place, they can still pass on a dominant-sterile resistance allele to nearly all progeny that does not receive a drive. (Fig. 4a). Repeatedly releasing drive males into a wild population will result in a steady increase in the drive allele frequency. Eventually, a sufficient number of females will be sterile, and the wild population will be suppressed (Fig. 4b). This system combines the merits of gene drive and RIDL. It is more powerful and efficient than fsRIDL, but still self-limiting, allowing for highly confined population suppression compared to standard homing suppression drive systems.
a Drive inheritance of RIDD. In male drive heterozygotes, germline Cas9 activity converts wild-type alleles to drive alleles by homology-directed repair, while end-joining repair or incomplete homology-directed repair generates nonfunctional resistance (r2) alleles. Females carrying one drive allele are sterile. The most common type of r2 (~ 95%, marked by *) is also dominant sterile in females, though a small fraction are recessive sterile. A small fraction of wild-type alleles (< 1%) in the male germline may also remain uncut. b Concept of RIDD pest control strategy. Drive heterozygous males are continuously released into a wild population. Drive conversion and resistance allele formation take place in the male germline, so nearly all female progeny of drive males are sterile (drive carrier and most r2 carrier females are sterile). Over time, the frequency of drive carriers increases, and with a high enough release level, the population will eventually be suppressed.
Assessment of the RIDD in cages study
To test the Release of Insects carrying a Dominant sterile Drive (RIDD) system, we carried out a cage study. We first conducted a male mating competitiveness assay, so as to test the fitness of drive males compared to wild-type males, a critical performance parameter for systems involving continuous male releases. In 1:1 competitions, drive males successfully mated in 39 of 74 tests, while wild-type males successfully mated in 31 tests. In 4 tests, both males mated with the female, based on the phenotype of the offspring (Supplementary Data Set 2). There was no significant difference between the mating competitiveness of drive males and wild-type males. Then, two replicate cage studies were carried out. Eight weeks were taken to establish an initial population for the cages, which was ~ 2400, and all flies were homozygous for the split nanos-Cas9 allele. Though our construct is a split drive, the cage population dynamics should be close to a complete drive because all individuals in the experiment are homozygous for Cas9. Overlapping-generation cages were maintained by adding fresh food every few days and removing old food. The population size was measured each week (Fig. 5a). To assess the spread of the drive, flies in cages were randomly aspirated for phenotyping (Fig. 5b and Supplementary Fig. 4a) and then put back into the cage. In addition, to monitor the ratio of drive carriers among newly hatched progeny, the old food bottles were often kept for an extra day outside cages, and then newly emerged adults were phenotyped (Supplementary Fig. 4b).
Drive heterozygous males were released each week (usually at two evenly spaced time points per week) into continuously maintained fly populations with overlapping generations. For the first 10 weeks, the release size was ~ 5 % of the total population per week. Starting from week 10 (marked by a vertical dashed line), the release size was increased to ~ 23% of the total population per week. a Population size of cages A and B together with the number of males released each week. After the weekly release size was increased, the population steadily declined. b Frequency of individual phenotypes in the cages based on a small weekly sample of flies. Masculine phenotype females include both sterile drive females and those with a nonfunctional resistance allele. c Female population size in cages. This data was in agreement with stochastic simulations of the cage population (yellow lines). d Frequency of drive individuals in cages A and B together with results from twenty simulations. Error bars show estimated standard error of the mean based on random phenotype samples. Source data are provided in Supplementary Data Sets 3–5.
In the first ten weeks, we released 82 ~ 148 dsx drive males (heterozygous for the drive allele and homozygous for the split nanos-Cas9 allele) into each cage each week, which was about 5% of total population (Fig. 5a). We did not observe a significant decrease in the population size, and the frequency of drive carriers appeared to stabilize at a low level (Fig. 5a). However, the existence of drive females verified that released males mated successfully in the cage population. To increase the suppressive power, starting from week 10, the release size of dsx males was substantially increased to approximately 560 per cage each week, which was about 23% of the total population at first (Fig. 5a). We observed gradually increasing ratios of drive carriers in cages and among newly hatched individuals (Fig. 5b and Supplementary Fig. 4b). In the later period of the study, the drive carrier frequency reached 100% in both cages. We observed the sex ratio biased toward males, and the population size steadily decreased in both cages (Fig. 5a and Supplementary Fig. 4b). No new hatched individuals were produced in both Cage A and B, and the cage collapsed at week 29. At this time, we stopped releasing drive males. At week 32, we collected all the remaining flies for phenotyping and stopped the cages. All flies in cage B were drive males, while all flies in cage A were drive males except two resistance carrier females with strong intersex phenotype.
For comparison, we also maintained a control cage for eleven weeks (Supplementary Fig. 5) with an initial 300 Cas9 flies. The population size gradually increased and achieved an equilibrium at about week 4, which was approximately 1200 flies.
SLiM was used to simulate the population dynamics of our RIDD cage experiments, using estimates for several parameters related to the species-specific ecology of the laboratory cage populations and our observations of their population dynamics (see “methods”). We assumed the same drive conversion rate (69%) as in our experiments and that 92% of remaining wild-type alleles in the germline of male drive heterozygotes were converted to dominant-sterile resistance alleles. We calculated the Root Mean Square Error (RMSE) in order to identify the optimal parameter set for drive fitness, fly lifespan, and low-density growth rate (Supplementary Data Set 6). The best-performing simulation parameters were found to be a fitness of 1.05, a maximum lifespan of 8 weeks, and a low-density growth rate of 6 (Supplementary Data Set 6). The optimal normalized average RMSE value was 4.603, while the lowest was 6.607 (see “methods”). The trajectories of the female population size and drive carrier frequency from twenty simulations using this optimal parameter set are similar to the experimental cage study (Fig. 5c, d). The stochastic fluctuations of cage population dynamics can be probably attributed to small effective population size and limited sampling of phenotypes (even though the total population size was accurately recorded each week), which is considered in simulations of the cage study.
Modeling of the RIDD system
To show the efficiency of RIDD, we model SIT, fsRIDL, RIDD complete drive, and also RIDD split drive in a SLiM program framework. We use panmictic populations averaging 100,000 individuals with a low-density growth rate of 6 and a linear density-dependent growth curve. This creates a highly robust population. High drive conversion was very useful for successful population suppression, proportionally reducing the required release size (Fig. 6). For SIT, the number of released males required for population elimination is over 5.4 times that of the native starting population per generation, while for fsRIDL, the number of released males must be at least 2.9 times as many (Fig. 6). However, for both RIDD complete drive and split drive, when there were over 1.4 males released per generation for each male in the native starting population, the system could be successful even without drive conversion due to the formation of dominant female-sterile r2 alleles (Fig. 6). This highlights RIDD’s high efficiency compared to other systems. RIDD split drive, which released males carrying homozygous split Cas9 allele, requires higher release ratios compared to complete drive when drive conversion is higher than 60% (Fig. 6). This can be attributed to the fact that subsequent drive allele spread at low release ratios relies on Cas9, which tends to separate from the drive at these low release ratios.
A constant number of drive heterozygous males/transgenic males were released each generation at with the number based on the specified ratio of released males to males in the starting population (that is release ratio). The heatmaps show the outcome of stochastic simulations for suppression of a population of 100,000 with overlapping generations and a linear density growth curve. a The blue heat bar shows the average number of weeks to population elimination. b The red heat bar represents the average number of fertile females at equilibrium when population elimination was not successful before 267 weeks. The released RIDD-split drive males are homozygous for the Cas9 allele. Each point in the parameter space had 20 replicates. Note the different vertical axis scale for SIT and fsRIDL. Gray means “not applicable” (NA - where population elimination always occurred for (a), and where it never occurred for (b).
For RIDD and similar technologies such as SIT and fsRIDL, success depends on not just the drive performance and release parameters, but also heavily on the ecology of the species, in particular the density growth curve, which can be highly variable in different scenarios. To allow comparison of suppression system performance independent of ecological parameters, we use constant-population genetic load (see Supplementary Fig. 6 and accompanying text). This shows that RIDD has higher power than even a combination of drive and fsRIDL60.
Despite using gene drive, RIDD is a highly self-limiting system. To demonstrate this, we model a situation where we cease weekly releases after 15 weeks (Fig. 7). Even with high drive performance, the drive frequency immediately declines, and the population can recover shortly after releases stop. The self-limiting property of split drive is even stronger, with rapid elimination of the drive from the population (Fig. 7).
Weekly releases of drive males were conducted into an initial population of 2000 at a ratio of 0.8 (RIDD complete drive) and 1.4 (RIDD split drive) for 15 weeks. The drive conversion rate was 0.9. a Total population of fertile females and (b) the drive carrier frequency in the population is shown. 20 simulations are shown for each drive.
To show the confinement of a RIDD system to a target population, we model two panmictic demes of 100,000 individuals linked by unidirectional migration (Supplementary Fig. 7). Even with high migration when the target population was eliminated, the maximum drive carrier frequency reached in the neighbor deme was less than 18% (Supplementary Fig. 7). Also, the minimum number of fertile females in the neighboring deme always exceeded 42,000, ensuring that there was little effect on this non-target population (Supplementary Fig. 7).
Discussion
In this study, we proposed a new pest control strategy, Release of Insects carrying a Dominant sterile homing gene Drive (RIDD), which would be stronger than SIT, fsRIDL, and fsRIDL with drive because of the key aspect of dominant-sterile resistance alleles, which allow nearly all female progeny of male heterozygotes to be sterile. Also, the RIDD system is potentially simpler to construct, only needing the components of a standard homing suppression drive. This system was tested by targeting doublesex in D. melanogaster. In our drive design, three gRNA were used to target the 5’ intron-exon splicing boundary of the female-specific exon, a highly conserved region. The drive has high inheritance bias in heterozygous drive males, which were healthy and fertile, like a standard homing gene drive. However, all female drive heterozygotes and nearly all female resistance allele heterozygotes (~ 95%) were sterile with a masculinized phenotype. This result is in contrast to recent studies in A. gambiae and D. suzukii that also target doublesex43,49. In the study of A. gambiae, females are sterile only when they are homozygous with drive alleles, though the leftmost target site of our design and the design of Kyrou et al. are both on the splicing boundary43. Our drive had two additional gRNA target sites, which could account for the difference in result. Another possible explanation is that it is fle instead of tra2 that involves altering splicing of dsx in Anopheles, and the recognition sequence of the splicing site is different from Drosophila22,44, so disruptions in splicing may function differently. Our sex-specific transcripts analysis suggests that incorrect splicing in females can account for dominant sterility. Male and female dsx transcripts are co-expressed in drive and r2 females. The regulated splicing enhancer component Tra/Tra2 activates the weak 3’ female-specific splice site by associating with a cis-acting regulatory sequence, the repeat elements in the 3’UTR of the dsx female exon46,47,64,67,68,69. Thus, the correct splicing site and adjacent 3’UTR are two key factors that are required for female-specific splicing. A disrupted splice acceptor site of the female exon and/or a distant disabled 3’UTR by drive insertion or end-joining repair (r2) prevents female-specific splicing components from recognizing the site. However, instead of just stopping and disrupting the female transcript, splicing directly skips to the next splice acceptor at the male-specific exon and, therefore, generates the male version of the dsx transcript. This leads to a sex development disorder in females. This concept can potentially further explain the drive allele and resistance allele performance in the D. suzukii study49. Because the gRNA site in this study was within the female dsx exon, it was distant to the 3’UTR, causing an incorrect dsx splicing result in the dominant sterile drive, but for r2 females, the preserved splicing acceptor site still allowed the transcript of the disrupted allele to splice correctly in spite of end-joining mutations49. Even if one allele is nonfunctional, the other copy of functional dsx is sufficient for female sex development in the absence of the male splice form. Besides, the level of skipping to the male splice form would be a matter of degree, showing higher levels of splicing disruption with increasing sequence disruption throughout the first half or more of the exon. This is suggested by our detailed analysis of nonfunctional resistance alleles.
Three types of non-functional resistance (r2) alleles were detected on the basis of masculine phenotype intensity and fertility: dominant sterile r2 females with stronger masculine phenotype, dominant sterile r2 females with moderate phenotype (the same phenotype as drive alleles in the absence of somatic Cas9 expression), and recessive sterile r2 females with weaker phenotype. We further found that this different degree of masculinization is closely related to the degree of preservation of the AG in the intron-exon boundary (the splice acceptor site). In r2 alleles preserving AG after end-joining, fewer male dsx products will be generated, resulting in a milder phenotype (even more nucleotides being preserved potentially allow for a recessive sterile phenotype, in addition to being visually milder). All types of r2 alleles are soon removed from the population in sterile females, and this process is much faster for the more common dominant-sterile alleles. This result promises a strong population suppression power for homing drives as its frequency reaches equilibrium because most remaining females are sterile. Dominant-sterile r2 alleles will not slow population suppression, which is a problem encountered by recessive-sterile r2 alleles53.
Our overlapping-generation cage study showcased the feasibility of RIDD in population suppression as well as demonstrated a relatively easy model by which cage studies with overlapping generations could be conducted (which is potentially more representative of application scenarios than more commonly used discrete generations). With weekly releases of drive males increasing to ~ 50% of the equilibrium male population size, the population was successfully eliminated in two replicate cages. The performance was somewhat close to the model result. We then further estimated the parameters of cage modeling by RMSE. No drive fitness costs were detected, and ecological parameter estimates appeared realistic. Note that while RMSE is a prevalent method for evaluating the correlation between the model and the experimental results, it may not guarantee high accuracy. A fluctuation early in a cage from the expectation can then result in bad performance of the whole model. For example, released males may have had variable fitness in different time intervals due to slightly different rearing conditions. It is also a little hard to find a parameter set that can both perfectly match the female population and drive carrier frequency. Overall, though, our identified optimal parameter set was reasonable.
The RIDD system is similar to combining fsRIDL with gene drive, which was shown to substantially increase RIDL’s efficiency20,60,61,70. Specifically, homing takes place in drive male germline, biasing the allele’s inheritance, which maintains the RIDL allele in the population for a longer time. Compared to SIT, fsRIDL, and even drive-boosted fsRIDL, RIDD is substantially more efficient and powerful, as indicated by our modeling results (Fig. 6 and Supplementary Fig. 6). Even without homing, RIDD has the advantage of sterilizing females with r2 alleles, not just with drive alleles. It can also maintain its sterile effect in the face of mutations. However, maintaining the drive is more complex than RIDL systems. Though there is no need for an antibiotic repressor, drive males must continually be crossed to wild-type females to keep the drive line. This could be potentially overcome by using a variant of the RIDD system where the drive allele is a modification rescue drive, but also targets dsx from a distant site71. Using a repressible Cas9, the drive can be kept “off” when reared in the laboratory, so homozygotes lines can be stably maintained72. This will also allow RIDD to be more efficient at population suppression because the released males are homozygous.
Nevertheless, though more powerful than similar alternatives, the RIDD system is still self-limiting and confined. If releases are ceased at any time before the population collapses, the population will easily recover because drive females produce no progeny, so the drive frequency will decline and be eliminated in several generations. This property allows for highly confined population suppression compared to standard homing suppression drives.
In addition, apart from the nanos promoter for Cas9, the CG4415 promotor, rcd-1r promotor, and shu 3’UTR were tested for Cas9 constructs in our study. Cas9 guided by rcd-1r has a similar drive conversion as Cas9 regulated by Nanos. Both CG4415 and rcd-1r promotors contribute to higher somatic expression, reflected in a higher proportion of stronger masculine phenotypes among drive carrier offspring (Supplementary Data Set S1 and Supplementary Fig. 4). For RIDD, the property of somatic expression is acceptable because it will not affect males, and drive females are sterile anyway. Indeed, a standard homing suppression drive that does not have any dominant-sterile r2 alleles could still be used for similar suppression if somatic Cas9 expression rendered drive alleles dominant sterile, though such a drive would be less efficient than RIDD due to weaker contribution to population suppression from r2 alleles.
Overall, our study suggests some mechanisms and possible approaches for dominant sterility and drive target site design for dsx. Because the doublesex-based sex determination pathway is highly conserved in dipterans, including mosquitos, moths, and flies, our design utilizing dsx sex-specific splicing with dominant effects has the potential to be applied to a wide variety of pest species control when splicing follows the paradigm in Drosophila rather than Anopheles. Moreover, the RIDD pest control system, verified by our modeling and cage experiments, combines high efficiency with a self-limiting nature, as well as avoiding the negative effects of resistance alleles. It is thus a potentially promising pest control strategy for practical application.
Methods
Ethical statement
This research complies with all relevant ethical regulations and was approved by the Peking University biosafety office.
Plasmid construction
Our plasmid construction was based on TTTgRNAtRNAi, TTTgRNAt, and HSDygU4, which were constructed previously53. dsx-related fragments were amplified from the genome of w1118 flies. We selected gRNA target sites from the website CHOPCHOP. Reagents for restriction digest, PCR, and Gibson assembly, and plasmid miniprep were obtained from New England Biolabs and Vazyme; primers were from Integrated DNA Technologies Company BGI; 5-α competent Escherichia coli from Vazyme; and the ZymoPure Midiprep kit from Zymo Research. Plasmid construction was confirmed by Sanger sequencing. Detailed information about DNA fragments, plasmids, primers, and restriction enzymes used for cloning each construct are listed in the Supplementary material. Plasmid sequences of the final drive insertion plasmid and target gene genomic region with annotation are provided on GitHub in ApE format73 (https://github.com/jchamper/ChamperLab/tree/main/dsx-Suppression-Drive).
Generation of transgenic lines
Embryo injections were conducted by Fungene Transgenic Flies Company. The donor plasmid HSDdsxRed3g (300 ng/ul) was injected into w1118 flies together with TTChsp70c9 (300 ng/ul), providing Cas9 for transformation. Because of an extra DNA addition to the donor plasmid, the boundary between the drive’s gRNA gene and the right homology arm includes a gRNA target site with a mismatch in only the first nucleotide. Fortunately, two of the three final drive allele variants we found still had full homology to the DNA outside the gRNA cut sites. The right arm sequence starting with the last ten nucleotides of the gRNA target sequences were (wild-type: CAACACA | ACG, CAACA-AACG, CA—ACAACG, CAACAC—G, where “-” indicates the missed nucleotide, and “|” indicates the gRNA cut site). Flies were housed in modified Cornell standard cornmeal medium (using 10 g agar instead of 8 g per liter, the addition of 5 g soy flour, and without the phosphoric acid) in a 25 °C incubator on a 14/10-h day/night cycle at 60% humidity.
Phenotypes and morphological analysis
Flies were anesthetized with CO2 and screened for fluorescence using the NIGHTSEA adapter SFA-GR for DsRed and SFA-RB-GO for EGFP. Fluorescent proteins were driven by the 3xP3 promoter for expression and easy visualization in the white eyes of w1118 flies. DsRed was used as a marker to indicate the presence of the split drive allele, and EGFP was used to indicate the presence of the supporting Cas9 allele. Morphological photos were taken by phone using a stereo microscope with 10x/22 magnification. To test drive conversion and somatic expression, our drive line was combined with five split Cas9 lines (BHDaaN: Cas9 with Nanos 5’UTR /nanos 3’UTR; SNc9XsGr: Cas9 with CG4415 promotor/shu 3’UTR; SNc9XnGr: Cas9 with CG4415 promotor/nanos 3’UTR; SNc9DsG: rcd-1r 5’UTR/shu 3’UTR; SNc9DnG: rcd-1r 5’UTR/nanos 3’UTR)66. All lines were tested in drive heterozygotes with one copy of the Cas9 allele. For nanos-Cas9, we did an extra test in flies homozygous for Cas9, which was representative of the situation in the cage experiments.
Male mating competitiveness assay
For preparing drive and non-drive males with similar fitness, their mothers were nanos-Cas9 sisters of similar age, and they were allowed to lay eggs in the same vial. In each vial, we added one nanos-Cas9 female that was previously mated with a nanos-Cas9 male and another that was mated with a drive/nanos-Cas9 male (all these flies were homozygous for Cas9). These were allowed to lay eggs for three days and were then removed. After the offspring hatched, we phenotyped and paired one drive/ nanos-Cas9 male with one nanos-Cas9 male of the same age for a competition test. For each test, we added one w1118 female, one drive/nanos-Cas9 male, and one paired Cas9 male to a vial and allowed mating for one day. Then, we removed the males and allowed the females to lay eggs for several days. We phenotyped the offspring, and if the percentage of individuals with red fluorescent eyes was higher than 64% (the lowest drive inheritance rate we observed from any male drive parent), we scored this as a drive male mating success. If all the offspring were wild type, we scored this situation as a w1118 mating success. Intermediate levels of drive inheritance between these indicated that both males likely mated with the female and were scored as a tie.
Cage study
For the cage study, flies were housed in 25 x 25 x 25 cm enclosures with 7 food bottles at first, with the oldest bottle being replaced every two days (a fourteen-day cycle), and after four weeks of drive, releases was gradually (over two weeks) reduced to 5 bottles with one bottle being replaced every three days (a fifteen-day cycle). Flies that were homozygous for the split nanos-Cas9 allele were initially added to each cage, with an initial group of several hundred varying ages. They mated freely and laid eggs in food bottles for eight weeks in the cage to establish to initial cage population, which was observed to reach an equilibrium density after ~ 4-5 weeks. A control cage was kept on the 15-day cycle without any addition of drive flies. For the drive test cages, drive males were released every week, and the first week was counted as “week zero”. The drive males were heterozygous for dsx drive allele and homozygous for the nanos-Cas9 allele, and they were generated by crossing drive males to females with the nanos-Cas9 line for several generations, selecting males with brighter green fluorescence (which were more likely to be Cas9 homozygotes). To continuously generate drive male flies for release, we set up bottles with crosses between drive/Cas9 males and Cas9 females. Drive male flies were collected and released into the cage each week (usually in two evenly spaced batches) after the initial cage population was established. Detailed release numbers for each week are shown in the Supplementary Data Set S4.
To record the dynamics of the cage population, we took photos with a white background each week of all six sides of the cages to estimate the total population by counting, and we randomly aspirated some flies (about 100 ~ 200) from the cages for phenotyping (Supplementary Data Set S3). These flies were then returned to the cage. Also, to estimate the allele frequency of new hatching flies in the cage, we kept some removed cage bottles for one day and then phenotyped any new adults that emerged during this day (Supplementary Data Set S5).
Phenotype data analysis
Pooled analysis was conducted for calculating drive inheritance, drive conversion and other parameters by combining all data from different individual crosses. However, this pooling approach does not consider potential batch effects (each individual cross is considered as a separate batch with different parameters, which could bias rate and error estimates). To account for such batch effects, we conducted an alternate analysis as in previous studies54,74. In brief, fitting a generalized linear mixed-effects model with a binomial distribution (maximum likelihood, Adaptive Gauss-Hermite Quadrature, nAGQ = 25) enables variance between batches, which then results in marginally different parameter estimates but higher standard error estimates. This analysis was performed with R (3.6.1) and supported by packages lme4 (1.1-21,
https://cran.rproject.org/web/packages/lme4/index.html) and emmeans (1.4.2,
https://cran.rproject.org/web/packages/emmeans/index.html).
The code is available on GitHub
(https://github.com/jchamper/ChamperLab/tree/main/dsx-Suppression-Drive). In our study, the alternate rate estimates and errors were close to the pooled analysis (Supplementary Data Set 1).
Diagnostic PCR
To figure out the transcription of dsx in drive and resistance allele carriers, flies were frozen and homogenized. RNA was extracted by using RNeasy Mini Kit, and reverse transcription was used to obtain cDNA with RevertAid First Strand cDNA Synthesis Kit with oligo(dT) primers. This cDNA was the template for PCR using Q5 DNA Polymerase from New England Biolabs with the manufacturer’s protocol. Primers Exon3_S_F and Exon4_S_R were designed to specifically amplify the female-specific dsx transcript, and Exon3_S_F and Exon5_S_R were designed to amplify the male-specific dsx transcript.
Sanger sequencing
Flies were frozen, and DNA was extracted by grinding single flies in Trizol DNA extraction Reagent. The DNA was used as a template for PCR using Q5 DNA Polymerase from New England Biolabs with the manufacturer’s protocol. To sequence resistance alleles, the region containing the gRNA target sites was amplified using DNA oligo primers DsRed_S_F and dsx_S_R. To determine the sequence of drive allele elements in individuals with faint red fluorescent eyes, the DsRed region was amplified by the primer pairs dsx_S_F/CFD5_S_R. After DNA fragments were isolated by gel electrophoresis, sequences were obtained by Sanger sequencing and analyzed with ApE software.
Population modeling
All modeling files used in this study are available on GitHub (https://github.com/jchamper/ChamperLab/tree/main/dsx-Suppression-Drive). Stochastic simulations were performed in SLiM 4.075 similarly to previous studies70,76,77,78. Our simulations have a single panmictic population of generic diploids with overlapping generations, progressing by time steps. The population size is regulated as a consequence of the balance between individual reproduction and mortality, as in natural populations. Each time step represents one mating and reproduction cycle, labeled as taking place in one week. Age 0 is the larvae stage. Individuals can survive at most six time steps (max age = 6), but their survival rates decline with age to produce a linear decline in the number of individuals in a single age class. The equation for survival is:
One generation is equivalent to 8/3 time steps, which is the time from egg to the point where half the offspring are produced by that individual, on average. In any time step, the population is defined by the number of male and female adults of each genotype. In each time step, each adult female first selects a random adult male in the population to mate with and then produces a number of offspring drawn from a Poisson distribution with an average of max offspring number per female/generation time. Each offspring generated is assigned a random sex, and its genotype is determined by randomly selecting one allele at each genetic locus from each parent, with adjustments for drive activity. Because many insect systems involve larvae competing for resources, we implement density-dependent mortality for age 0 individuals (new larvae):
K is the expected carrying capacity, β is the low-density growth rate, and βmax is the maximum offspring produced by one female. 6/21 is a constant that produces an appropriate survival level to maintain the population at carrying capacity, determined by the age-based death rate.
Implementation of RIDD and other pest control systems
In our model, each individual is specified by its genotype, which can have wild-type alleles, drive alleles, and resistance alleles. Drive alleles and resistance alleles cause dominant sterility in females, but we assume no other fitness costs. For RIDD-complete drive, drive alleles include both Cas9 and gRNA. Drive/wild-type heterozygous males will convert a fraction of wild-type alleles in their germline into drive alleles at the drive conversion rate. The remaining wild-type alleles are all converted to resistance alleles. For the RIDD-split drive, the Cas9 allele is separated at another unlinked locus, so the drive allele only represents gRNAs. Drive conversion and resistance formation can take place only when individuals carry both a Cas9 allele and a drive allele. For fsRIDL, female offspring carrying a dominant lethal allele cannot survive past the larval stage. For SIT, released SIT males will mate with females and thus prevent them from reproducing.
Transgenic males are released at a fixed number in each time step. For the RIDD-complete drive, the released males are drive (gRNAs and Cas9) heterozygotes, while for the RIDD-split drive, the released males are drive (gRNAs) heterozygotes and Cas9 homozygotes. For fsRIDL, released males are homozygous for the female dominant lethal allele. SIT released males are marked with a sterility tag, resulting in the females mated with them producing no progeny. Release ratio refers to the ratio of released males in each time step to the number of native-born males reaching age 1 (reproductive age) in each time step when the population is at natural equilibrium. When studying the self-limiting nature of RIDD, releases only took place during the indicated time steps rather than each time step until the end of the simulation.
Modeling confinement of RIDD
We studied the confinement of RIDD by simulating two panmictic demes connected by unidirectional migration. The migration rate is the probability that individuals in the target deme will move to the neighbor deme in each time step (unidirectional migration avoid the neighboring deme preventing population elimination at the target deme). The capacity of both demes are 100,000, and the drive conversion rate was set to 0.7. To estimate the risk of the neighbor population being suppressed, we recorded the minimum number of fertile females and the maximum drive carrier frequency in the neighbor deme throughout the entire process.
Simulations to support the cage study
A similar SLiM framework was used to simulate the dynamics of RIDD in our Drosophila cage populations. This model is adapted from the general RIDD-split drive model described above, but all flies in the cage carried homozygous Cas9 alleles, thus making it equivalent to a RIDD complete drive in terms of population dynamics. The released male flies were heterozygous for the drive allele and homozygous for the Cas9 allele. Approximating the life cycle of Drosophila melanogaster, age 0 individuals represent eggs and larvae, suffering from competition, while age 1 individuals represent pupae, which cannot participate in reproduction but are not involved in competition. The population capacity was set to 2400, which appeared to be close to the equilibrium population during the cage experiment. The number of released drive males each week was the same as the cage experiment. Female survival was adjusted to be lower than male survival to account for higher mortality during egg laying (where overcrowding was observed to produce high death rates, resulting in a male-skewed sex ratio, though males also experienced some death at the bottom of bottles), thus producing the sex ratio observed in cage adults.
To better understand cage dynamics, we varied the adult lifespan (affecting age-based survival rate, varying from 4 to 8 weeks with steps of 0.5), low-density growth rate (varying from 6 to 10 with steps of 0.5), and male drive mating fitness (varying from 0.7 to 1.1 with steps of 0.05) to identify the optimal parameter sets that best matched the experimental data. This was done by calculating the Root Mean Square Error (RMSE) of 100 simulations compared to cage data (separately compared to both cages, for both drive carrier frequency and female population size). Because RMSE of population size and RMSE of drive carrier frequency are not on the same scale, we normalized them by dividing each by their respective maximum values. We then averaged the four normalized RMSEs to find the optimal parameter set.
Because the effective population size in our cages was likely lower than the censuses sizes, the actual simulations have all population sizes divided by twenty to produce stochastic variation based on typical effective population sizes79,80,81. Also, to simulate the situation of using an aspirator to phenotype only a portion of cage flies, the female fraction and drive carrier frequency are also estimated from sampling a similar number (~ 150) of individuals during the course of the model at each time step.
Statistics and reproducibility
For Fig. 2c, we used several different primer pairs to detect and got similar result. The presented result was from the most specific primer pair, showing clear and representative bands. For Fig. 2b and Supplementary Fig. 1, the flies we chose had representative features on behalf of their categories. More than 100 individuals for each category had similar phenotype with the presented individual.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All raw data is available in the Source Data file, provided with this paper. Plasmid sequence is available in https://github.com/jchamper/ChamperLab/tree/main/dsx-Suppression-Drive. A doi link is https://doi.org/10.5281/zenodo.13683350.
Code availability
All code is available in https://github.com/jchamper/ChamperLab/tree/main/dsx-Suppression-Drive. A doi link is https://doi.org/10.5281/zenodo.13683350.
References
Ferguson, N. M. Challenges and opportunities in controlling mosquito-borne infections. Nature 559, 490–497 (2018).
Maurya, R. P. et al. Biological control: A global perspective. Int. J. Trop. Insect Sci. 42, 3203–3220 (2022).
Gould, F., Brown, Z. S. & Kuzma, J. Wicked evolution: Can we address the sociobiological dilemma of pesticide resistance? Science 360, 728–732 (2018).
Walsh, T. K. et al. Determinants of insecticide resistance evolution: Comparative analysis among heliothines. Annu. Rev. Entomol. 67, 387–406 (2022).
Tudi, M. et al. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public. Health 18, 1112 (2021).
Bourtzis, K. & Vreysen, M. J. B. Sterile insect technique (SIT) and its applications. Insects 12, 638 (2021).
Burt, A. & Crisanti, A. Gene drive: Evolved and synthetic. ACS Chem. Biol. 13, 343–346 (2018).
Bier, E. Gene drives gaining speed. Nat. Rev. Genet. 1–18 https://doi.org/10.1038/s41576-021-00386-0 (2021).
Quinn, C. M. & Nolan, T. Nuclease-based gene drives, an innovative tool for insect vector control: advantages and challenges of the technology. Curr. Opin. Insect Sci. 39, 77–83 (2020).
Benedict, M. Q. Sterile insect technique: Lessons from the past. J. Med. Entomol. 58, 1974–1979 (2021).
Grilli, S., Galizi, R. & Taxiarchi, C. Genetic technologies for sustainable management of insect pests and disease vectors. Sustainability 13, 5653 (2021).
Wang, G.-H. et al. Symbionts and gene drive: Two strategies to combat vector-borne disease. Trends Genet. 38, 708–723 (2022).
Maselko, M. et al. Engineering multiple species-like genetic incompatibilities in insects. Nat. Commun. 11, 4468 (2020).
Li, M. et al. Suppressing mosquito populations with precision guided sterile males. Nat. Commun. 12, 5374 (2021).
Hay, B. A., Oberhofer, G. & Guo, M. Engineering the composition and fate of wild populations with gene drive. Annu. Rev. Entomol. 66, 407–434 (2021).
Knipling, E. F. Sterile-male method of population control. Science 130, 902–904 (1959).
Scott, M. J., Concha, C., Welch, J. B., Phillips, P. L. & Skoda, S. R. Review of research advances in the screwworm eradication program over the past 25 years. Entomol. Exp. Appl. 164, 226–236 (2017).
Hendrichs, J., Franz, G. & Rendon, P. Increased effectiveness and applicability of the sterile insect technique through male-only releases for control of mediterranean fruit flies during fruiting seasons. J. Appl. Entomol. 119, 371–377 (1995).
Harris, A. F. et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 30, 828–830 (2012).
Thomas, D. D., Donnelly, C. A., Wood, R. J. & Alphey, L. S. Insect Population Control Using a Dominant, Repressible, Lethal Genetic System. Science 287, 2474–2476 (2000).
Black, W. C., Alphey, L. & James, A. A. Why RIDL is not SIT. Trends Parasitol. 27, 362–370 (2011).
Siddall, A., Harvey-Samuel, T., Chapman, T. & Leftwich, P. T. Manipulating insect sex determination pathways for genetic pest management: Opportunities and challenges. Front. Bioeng. Biotechnol. 10, 867851 (2022).
Vásquez, V. N.; Reddy, M. R.; Marshall, J. M. Environmentally appropriate vector control is facilitated by standard metrics for simulation-based evaluation. Front. Trop. Dis. 3, https://doi.org/10.3389/fitd.2022.953212 (2022).
Leftwich, P. T. et al. Genetic elimination of field-cage populations of mediterranean fruit flies. Proc. R. Soc. B Biol. Sci. 281, https://doi.org/10.1098/rspb.2014.1372 (2014).
Labbé, G. M. C., Scaife, S., Morgan, S. A., Curtis, Z. H. & Alphey, L. Female-specific flightless (fsRIDL) phenotype for control of Aedes Albopictus. PLoS Negl. Trop. Dis. 6, e1724 (2012).
Waltz, E. First genetically modified mosquitoes released in the United States. Nature 593, 175–176 (2021).
Harris, A. F. et al. Field performance of engineered male mosquitoes. Nat. Biotechnol. 29, 1034–1037 (2011).
Carvalho, D. O. et al. Suppression of a field population of Aedes Aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis. 9, e0003864 (2015).
Harvey-Samuel, T., Ant, T., Gong, H., Morrison, N. I. & Alphey, L. Population-level effects of fitness Costs associated with repressible female-lethal transgene insertions in two pest insects. Evol. Appl. 7, 597–606 (2014).
Jin, L. et al. Engineered female-specific lethality for control of pest Lepidoptera. ACS Synth. Biol. 2, 160–166 (2013).
Bax, N. J. & Thresher, R. E. Ecological, behavioral, and genetic factors influencing the recombinant control of invasive pests. Ecol. Appl. Publ. Ecol. Soc. Am. 19, 873–888 (2009).
Teem, J. L., Gutierrez, J. B. & Parshad, R. D. A comparison of the Trojan Y chromosome and daughterless carp eradication strategies. Biol. Invasions 16, 1217–1230 (2014).
Thresher, R. E. Autocidal technology for the control of invasive fish Fisheries 33, 114–121 (2008).
Lutrat, C. et al. Combining two genetic sexing strains allows sorting of non-transgenic males for Aedes genetic control. Commun Biol 6, 646 (2023).
Kojin, B. B., Compton, A., Adelman, Z. N. & Tu, Z. Selective targeting of biting females to control mosquito-borne infectious diseases. Trends Parasitol. 38, 791–804 (2022).
Wilke, A. B. B. & Marrelli, M. T. Genetic control of mosquitoes: Population suppression strategies. Rev. Inst. Med. Trop. Sao Paulo 54, 287–292 (2012).
Qsim, M. et al. Genetically modified Aedes Aegypti to control dengue: A review. Crit. Rev. Eukaryot. Gene Expr. 27, 331–340 (2017).
Facchinelli, L. et al. Field cage studies and progressive evaluation of genetically-engineered mosquitoes. PLoS Negl. Trop. Dis. 7, e2001 (2013).
Verkuijl, S. A. N., Ang, J. X. D., Alphey, L., Bonsall, M. B. & Anderson, M. A. E. The challenges in developing efficient and robust synthetic homing endonuclease gene drives. Front. Bioeng. Biotechnol. 0, 426 (2022).
Burt, A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc. R. Soc. B Biol. Sci. 270, 921–928 (2003).
Hammond, A. et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the Malaria Mosquito vector Anopheles gambiae. Nat. Biotechnol. 34, 78–83 (2016).
Verhulst, E. C. & van de Zande, L. Double nexus–doublesex is the connecting element in sex determination. Brief. Funct. Genomics 14, 396–406 (2015).
Kyrou, K. et al. A CRISPR–Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae Mosquitoes. Nat. Biotechnol. 36, 1062–1066 (2018).
Krzywinska, E. et al. Femaleless controls sex determination and dosage compensation pathways in females of Anopheles Mosquitoes. Curr. Biol. 31, 1084–1091.e4 (2021).
Koukidou, M. & Alphey, L. Practical applications of insects’ sexual development for pestcontrol. Sex. Dev. 8, 127–136 (2014).
Nagoshi, R. N., McKeown, M., Burtis, K. C., Belote, J. M. & Baker, B. S. The control of alternative splicing at genes regulating sexual differentiation in D. Melanogaster. Cell 53, 229–236 (1988).
Nagoshi, R. N. & Baker, B. S. Regulation of sex-specific RNA splicing at the Drosophila doublesex gene: cis-acting mutations in exon sequences alter sex-specific RNA splicing patterns. Genes Dev. 4, 89–97 (1990).
Ranian, K., Kashif Zahoor, M., Zulhussnain, M. & Ahmad, A. CRISPR/Cas9 Mediated sex-ratio distortion by sex specific gene editing in Aedes Aegypti. Saudi J. Biol. Sci. 29, 3015–3022 (2022).
Yadav, A. K. et al. CRISPR/Cas9-based split homing gene drive targeting doublesex for population suppression of the global fruit pest Drosophila suzukii. Proc. Natl. Acad. Sci. USA 120, e2301525120 (2023).
Champer, J. et al. Reducing resistance allele formation in CRISPR gene drive. Proc. Natl. Acad. Sci. USA 115, 5522–5527 (2018).
Unckless, R. L., Clark, A. G. & Messer, P. W. Evolution of resistance against CRISPR/Cas9 gene drive. Genetics 205, 827–841 (2017).
Beaghton, A. K., Hammond, A., Nolan, T., Crisanti, A. & Burt, A. Gene drive for population genetic control: non-functional resistance and parental effects. Proc. R. Soc. B Biol. Sci. 286, 20191586 (2019).
Yang, E. et al. A homing suppression gene drive with multiplexed gRNAs maintains high drive conversion efficiency and avoids functional resistance alleles. GenesGenomesGenetics. https://doi.org/10.1093/g3journal/jkac081 (2022).
Champer, S. E. et al. Computational and experimental performance of CRISPR homing gene drive strategies with multiplexed gRNAs. Sci. Adv. 6, eaaz0525 (2020).
Marshall, J. M. & Hay, B. A. Confinement of gene drive systems to local populations: A comparative analysis. J. Theor. Biol. 294, 153–171 (2012).
Adolfi, A. et al. Efficient population modification gene-drive rescue system in the Malaria Mosquito Anopheles Stephensi. Nat. Commun. 11, 1–13 (2020).
Champer, J. et al. Molecular safeguarding of CRISPR gene drive experiments. ELife 8, e41439 (2019).
Terradas, G. et al. Inherently confinable split-drive systems in Drosophila. Nat. Commun. 12, 1–12 (2021).
Akbari, O. S. et al. Safeguarding gene drive experiments in the laboratory: Multiple stringent confinement strategies should be used whenever possible. Science 349, 927–929 (2015).
Zhu, J., Chen, J., Liu, Y., Xu, X. & Champer, J. Population suppression with dominant female-lethal alleles is boosted by homing gene drive. BMC Biol 22, 201 (2024).
Burt, A. & Deredec, A. Self-limiting population genetic control with sex-linked genome editors. Proc. R. Soc. B Biol. Sci. 285, https://doi.org/10.1098/rspb.2018.0776 (2018).
Epper, F. Morphological analysis and fate map of the intersexual genital disc of the mutant doublesex-dominant in Drosophila Melanogaster. Dev. Biol. 88, 104–114 (1981).
Waterbury, J. A., Jackson, L. L. & Schedl, P. Analysis of the doublesex female protein in drosophila melanogaster: Role on sexual differentiation and behavior and dependence on intersex. Genetics 152, 1653–1667 (1999).
Burtis, K. C. & Baker, B. S. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell 56, 997–1010 (1989).
Su, M. P. et al. Assessing the acoustic behaviour of anopheles gambiae (s.l.) dsxF mutants: Implications for vector control. Parasit. Vectors 13, 507 (2020).
Du, J. et al. Germline Cas9 promoters with improved performance for homing gene drive. Nat Commun 15, 4560 (2024).
Lynch, K. W. & Maniatis, T. Assembly of specific SR protein complexes on distinct regulatory elements of the Drosophila doublesex splicing enhancer. Genes Dev. 10, 2089–2101 (1996).
Hedley, M. L. & Maniatis, T. Sex-specific splicing and polyadenylation of Dsx pre-mRNA requires a sequence that binds specifically to tra-2 protein in vitro. Cell 65, 579–586 (1991).
Ryner, L. C. & Baker, B. S. Regulation of doublesex Pre-mRNA processing occurs by 3’-splice site activation. Genes Dev. 5, 2071–2085 (1991).
Zhu, Y. & Champer, J. Simulations reveal high efficiency and confinement of a population suppression CRISPR toxin-antidote gene drive. ACS Synth. Biol. 12, 809–819 (2023).
Faber, N. R. et al. Improving the suppressive power of homing gene drive by co-targeting a distant-site female fertility gene. Nat Commun (in press), (2024).
López Del Amo, V. et al. Small-molecule control of super-mendelian inheritance in gene drives. Cell Rep. 31, 107841 (2020).
Davis, M. W. & Jorgensen, E. M. ApE, a Plasmid editor: A freely available DNA manipulation and visualization program. Front. Bioinforma. 0, 5 (2022).
Champer, J. et al. A CRISPR homing gene drive targeting a haplolethal gene removes resistancealleles and successfully spreads through a cage population. Proc. Natl. Acad. Sci. USA 117, 24377–24383 (2020).
Haller, B. C. & Messer, P. W. SLiM 4: Multispecies eco-evolutionary modeling. Am. Nat. 201, E127–E139 (2023).
Liu, Y., Teo, W., Yang, H. & Champer, J. Adversarial interspecies relationships facilitate population suppression by gene drive in spatially explicit models. Ecol. Lett. 26, 1174–1185 (2023).
Champer, S. E., Kim, I. K., Clark, A. G., Messer, P. W. & Champer, J. Anopheles homing suppression drive candidates exhibit unexpected performance differences in simulations with spatial structure. ELife, 11, https://doi.org/10.7554/ELIFE.79121 (2022).
Liu, Y.; Champer, J. Modelling homing suppression gene drive in haplodiploid organisms. Proc. R. Soc. B, 289, https://doi.org/10.1098/RSPB.2022.0320 (2022).
Liu, J. et al. Maximum likelihood estimation of fitness components in experimental evolution. Genetics 211, 1005–1017 (2019).
Sjödin, P., Kaj, I., Krone, S., Lascoux, M. & Nordborg, M. On the meaning and existence of an effective population size. Genetics 169, 1061–1070 (2005).
Nürnberger, B. Ecological genetics. In Encyclopedia of Biodiversity; Levin, S. A., Ed.; Elsevier: New York; pp 245–258 (2001).
Acknowledgements
Thanks to Jie Du, Jingheng Chen, Xuqing Feng, and Weiwen Yang for assistance with cage experiments. Thanks to Xinyue Zhang for assistance with modeling. This study was supported by laboratory startup funds from Peking University and the Center for Life Sciences, as well as the National Science Foundation of China (grant 32270672).
Author information
Authors and Affiliations
Contributions
Weizhe Chen and Jackson Champer designed the research. Weizhe Chen, Jialiang Guo, and Yiran Liu performed the research. Weizhe Chen analyzed the data. Weizhe Chen and Jackson Champer contributed to writing and editing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors have declared no competing interest.
Peer review
Peer review information
Nature Communications thanks Jared Bennett, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, W., Guo, J., Liu, Y. et al. Population suppression by release of insects carrying a dominant sterile homing gene drive targeting doublesex in Drosophila. Nat Commun 15, 8053 (2024). https://doi.org/10.1038/s41467-024-52473-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52473-5
- Springer Nature Limited