Abstract
Compensation and intracellular storage of PD-L1 may compromise the efficacy of antibody drugs targeting the conformational blockade of PD1/PD-L1 on the cell surface. Alternative therapies aiming to reduce the overall cellular abundance of PD-L1 thus might overcome resistance to conventional immune checkpoint blockade. Here we show by bioinformatics analysis that colon adenocarcinoma (COAD) with high microsatellite instability (MSI-H) presents the most promising potential for this therapeutic intervention, and that overall PD-L1 abundance could be controlled via HSC70-mediated lysosomal degradation. Proteomic and metabolomic analyses of mice COAD with MSI-H in situ unveil a prominent acidic tumor microenvironment. To harness these properties, an artificial protein, IgP β, is engineered using pH-responsive peptidic foldamers. This features customized peptide patterns and designed molecular function to facilitate interaction between neoplastic PD-L1 and HSC70. IgP β effectively reduces neoplastic PD-L1 levels via HSC70-mediated lysosomal degradation, thereby persistently revitalizing the action of tumor-infiltrating CD8 + T cells. Notably, the anti-tumor effect of lysosomal-degradation-based therapy surpasses that of antibody-based immune checkpoint blockade for MSI-H COAD in multiple mouse models. The presented strategy expands the use of peptidic foldamers in discovering artificial protein drugs for targeted cancer immunotherapy.
Similar content being viewed by others
Introduction
The advent of immune checkpoint blockade (ICB) therapies, particularly those targeting programmed cell death 1 (PD-1) and its ligand PD-L1, has revolutionized the treatment paradigm for tumors and propelled tumor immunotherapy to the forefront of cancer care1,2. There is compelling evidence to suggest that the current monoclonal antibodies targeting PD-L1 and PD-1 offer a promising avenue for improving survival rates in various advanced malignancies3,4. Nevertheless, the clinical benefit is achieved by less than one-third of the patients, with only a small fraction of them experiencing enduring remission5. A growing body of research indicates that two broad mechanisms contribute to this phenomenon: 1) cell drug resistance mechanism involving antibody endocytosis degradation by tumor and/or tumor-associated cells6,7, as well as repopulation of recycling endosome-residing PD-L17; and 2) immune evasion mechanisms encompassing compensatory upregulation of PD-L1 and its isoproteins8, along with neutralization of Anti-PD-L1 by tumor exosomes expressing PD-L19.To address this challenge, a dependable and long-lasting approach is to decrease the level of PD-L1 in tumor cells, including those found in the cytoplasm, membrane, and exosomes6. Recently, various techniques have been employed to degrade ICB-related proteins, particularly PD-L1. These techniques include but are not limited to proteolysis-targeting chimeras (PROTACs), lysosome-targeting chimeras (LYTACs), and molecular glues10,11,12,13,14,15,16,17. Although some of them have successfully achieved overcoming the PD-L1-mediated immune escape and the congenital/acquired drug resistance in antibody-based ICB therapy, significant challenges remain in identifying potential beneficiaries for neoplastic PD-L1 degradation therapy and precisely reducing the abundance of intratumoral PD-L1.
A series of recent breakthroughs in anti-cancer medication indicates that precision medicine has evolved from a mere aspiration and exaggeration to a practical reality18,19. By this way, the comprehensive use of multivariate data analysis, including but not limited to genomic, proteomic, and metabolomic, will facilitate evidence-based decision-making in drug design, drug delivery and beneficiary prediction, thus maximizing healthcare quality through the innovative therapy which aim to reduce PD-L1 cellular abundance in tumors. In this particular context, bioinformatics data from 30 common tumors were analyzed herein, and colon adenocarcinoma (COAD) with high microsatellite instability (MSI-H) presents the most promising potential for therapeutic intervention. Furthermore, the bioinformatic analysis of clinical samples from COAD patients with MSI-H, combined with single-cell mRNA sequencing from MSI-H COAD mice overexpressing PD-L1, suggested that HSC70-mediated lysosomal degradation may alleviate the burden of PD-L1. Therefore, a mesomeric peptidic foldamer MPHP is designed here with specific levorotatory (L) and dextrorotatory (D) peptide patterns to facilitate the affinity between PD-L1 and HSC70. For MPHP targeted delivery, proteomic and metabolomic analyses were conducted on in situ mice COAD with MSI-H, revealing the presence of a prominent tumor acid microenvironment (TAME).
As a promising avenue for drug discovery, protein drugs possess the ability to perform intricate tasks such as catalyzing biochemical reactions and regulating signaling pathways20,21, providing an opportunity to implement TAME-responsive PD-L1 degradation. While rapid advances in genome sequencing and structural biology have revealed an increasing number of protein blueprints that are nature’s masterpieces for multifarious functions22, customizing them with specific functions for drug discovery remains a significant challenge today. Foldamers are oligomers with regular secondary structures that aim to replicate the structural and functional features of natural biomacromolecules using non-natural building blocks23,24. This approach enables a bottom-up strategy for chemically or genetically introducing non-natural functions into proteins25,26.
Within this context, MPHP is modified to exhibit pH-responsive behavior through the conjugation of a charge-reversible motif HEHE at its N-terminus and a positively charged motif DRDR at its C-terminus. Subsequently, this pH-responsive MPHP (pH-MPHP) is assembled into an immune-regulatory globulin-like particle (IgP β). As anticipated, IgP β exhibits preferential accumulation at COAD tumor sites and effectively triggeres PD-L1 lysosomal degradation, thereby reactivating cytotoxic T lymphocytes (CTL) and facilitating immune clearance. Not only is this Anti-PD1/PDL1 alternative therapeutic approach reported here effective in degrading neoplastic PD-L1 and achieving long-term remission in MSI-H COAD, but provides a splendid exemplification for the advancement of precision medicine in guiding drug discovery and delivery.
Results
COAD with MSI-H presents the most promising potential for alleviating therapeutic burden of PD-L1
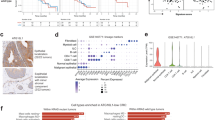
In hopes of finding the most promising beneficiaries of PD-L1 degradation therapy, expressions of immunoinhibitory factors were evaluated in 30 common tumors in relationship with the expression of neoplastic PD-L1 using the genetic and immune information of 9993 PD-L1-positive tumor samples from The Cancer Genome Atlas (TCGA) database. The colon adenocarcinoma (COAD) exhibited the highest degree of relevance, as PD-L1 demonstrated significant positive correlations with all 20 detectable immunoinhibitory factors (Fig. 1A), indicative of a potential immune escape mechanism stemming from PD-L1. Next, the abundance of PD-L1 was investigated in the three molecular subtypes of COAD including high microsatellite instability (MSI-H), low microsatellite instability (MSI-L) and microsatellite stability (MSS), and it was found that the highest expression of PD-L1 occurred in COAD with MSI-H (Fig. 1B). Furthermore, a statistically significant decrease in overall survival was observed in patients with high PD-L1 expression (Fig. 1C). The findings of this study suggest that PD-L1 can serve as a valuable prognostic risk factor in patients with MSI-H COAD. To investigate potential mechanisms, we utilized single-sample gene set enrichment analysis (ssGSEA) to evaluate the abundance of tumor-infiltrating immune cells in 78 clinical samples of COAD with MSI-H, and examined their relationship with neoplastic PD-L1 expression. As shown in Fig. 1D, T lymphocytes exhibit the strongest correlation with PD-L1 expression. Furthermore, a repeated correlation analysis was conducted between the abundance of T cell subsets and PD-L1 expression, and cytotoxic T lymphocytes exhibited the strongest correlation (Fig. 1E). Moreover, compared to the other two molecular typing subsets for COAD (MSI-L and MSS), MSI-H tumors exhibited a statistically significant increase in T cell and CTL abundance (Fig. 1F). Besides, in these MSI-H COAD cases, all CTL immunoinhibitory factors exhibited a statistically significant correlation with PD-L1 expression (Fig. 1G). Moreover, there is an inverse association between PD-L1 expression and the type II IFN response, while a positive correlation exists between PD-L1 expression and T cell co-inhibition pathway as determined by ssGSEA analysis in these cases (Fig. 2A). The results presented in Figs1. D-H collectively suggest that the underlying primary mechanism of immune evasion in COAD with MSI-H is the inhibition of CTL function, which is hindered by a high burden of PD-L1.
A The correlation between the expression of immunoinhibitory factors and neoplastic PD-L1 in 30 common tumors using genetic and immune information from 9993 PD-L1-positive tumor samples in The Cancer Genome Atlas (TCGA) database. B The abundance of PD-L1 in the three molecular subtypes of COAD. MSI-H, high microsatellite instability; MSI-L, low microsatellite instability; MSS, microsatellite stability. p value calculated by two-tail t-test, p (MSI-H to MSI-L) = 1.0e-6, p (MSI-H to MSS) = 1.0e-6, p (MSI-L to MSS) = 0.026. C Correlation of expression of neoplastic PD-L1 with overall survival. PD-L1 expression > median (red line); PD-L1 expression ≤ median (blue line). D The correlation between neoplastic PD-L1 and the abundance of tumor-infiltrating immune cells using single-sample gene set enrichment analysis (ssGSEA) in 78 clinical samples of COAD with MSI-H. E The correlation between neoplastic PD-L1 and the abundance of T cell subsets. F The abundance of T and CTL in the three molecular subtypes of COAD. p value calculated by two-tail t-test. As for T cell abundance, p (MSI-H to MSI-L) = 0.005, p (MSI-H to MSS) = 1.0e-5. As for CTL abundance, p (MSI-H to MSI-L) = 1.0e-5, p (MSI-H to MSS) = 1.0e-5. G The correlation between neoplastic PD-L1 and CTL immunoinhibitory factors in clinical samples of COAD with MSI-H. All box-whisker plots B, F center on the median; the bounds of the boxes mark the upper and lower quartile; the whiskers extend to the upper and lower extremes (1.5x interquartile range from the upper and lower quartiles). (* p < 0.05, ** p < 0.01, ***p < 0.001).
A The correlation between neoplastic PD-L1 and type II IFN response/T cell co-inhibition pathway as measured by ssGSEA in clinical samples with MSI-H COAD. B t-Distributed Stochastic Neighbor Embedding (t-SNE) plots of broad cell types in mouse model of COAD with MSI-H establishing by subcutaneously transplanting either PD-L1 overexpressed (high PD-L1) or normal-expressed (WT) MC38 cell lines (n = 3/group). C UMAP plots showing the expression of selected marker genes in cancer cells. D GSEA analysis of reactome cell cycle in scRNA-seq data from the cancer cell cluster. E UMAP plots showing the expression of selected marker genes in CD8 + T cells. F, multichannel flow cytometry analysis of Treg cells in the two distinct MC38 tumor types. The bar charts depict the proportions of Treg (n = 3 biological replicates in each group). p value calculated by two-tail t-test. p = 0.002. The gating strategy for Treg involves two sequential steps: (1) identification of cells expressing CD45+ and CD4 + , and (2) characterization of Treg as cells positive for both CD25+ and FOXP3+ within the CD45+ and CD4+ population. G Multichannel flow cytometry analysis of CTLs in the two distinct MC38 tumor types. The bar charts illustrate the proportions of CTLs cells that are positive for GzmB or IFN γ (n = 3 biological replicates in each group). p value calculated by two-tail t-test. p = 0.024 and 0.002. The gating strategy for CTL involves two sequential steps: (1) identification of CD45+ expressing cells, and (2) characterization of CTL as cells positive for both CD8+ and CD3+ within the CD45+ population. Subsequently, GzmB+ or IFN γ + CTL were identified in this population.
To further validate this hypothesis, a mouse model of colorectal adenocarcinoma (COAD) with microsatellite instability-high (MSI-H) was established by subcutaneously transplanting either PD-L1 overexpressed or normal-expressed MC38 cell lines with MSI-H. Following the assessment of PD-L1 expression in a series of mouse colon cancer cell lines (Supplementary Fig. 1A), it was observed that MC38 exhibited the most significant upregulation of PD-L1, thus rendering it an optimal choice for modeling purposes. The following week, our group performed single-cell transcriptome sequencing (scRNA-seq) on two distinct MC38 tumor types in an effort to delineate both the cellular and molecular characteristics of immune cells as well as cancer cells. As shown in the single-cell expression atlas in Fig. 2B, there is no significant difference in T cell or cancer cell percentage between overexpressed (high PD-L1) and normal-expressed (WT) MC38 tumors. Furthermore, molecular characteristics were investigated within the cancer cell cluster, revealing a strikingly apparent up-regulation of PD-L1 in high PD-L1 tumors, thus confirming successful model construction (Fig. 2C). Additionally, the proportion of Ki67/PCNA/β-catenin positive cells was significantly increased in high PD-L1 tumors, indicating an accelerated cellular proliferation (Fig. 2C). This finding was further validated through GSEA analysis of scRNA-seq data from the cancer cell cluster, revealing a significant upregulation of gene sets related to the cell cycle in high PD-L1 tumors compared to WT tumors (Fig. 2D; Supplementary Fig. 1B). Besides, the molecular characteristics of T cells indicate that both CD25/FOXP3-positive regulatory T cells (Treg) and CD8-positive T lymphocytes (CD8+ T) are elevated in high PD-L1 tumors compared to WT tumors (Fig. 2E). Furthermore, high PD-L1 tumors exhibit reduced expression of granzyme B (GamB) and interferon γ (IFN γ) (Fig. 2E). Additionally, GSEA analysis revealed significant downregulation of gene sets related to T cell-mediated cytotoxicity, T cell cytokine production, and cancer immunotherapy via PD-1 blockade in high PD-L1 tumors (Supplementary Figs. 1C-E), indicating a higher level of exhaustion among CD8-positive T cells in high PD-L1 COAD with MSI-H. Consistent with these findings, the proportion of Treg cells increased by 48.4% in high PD-L1 tumors (Fig. 2F), while the percentages of GamB-positive and IFN γ-positive cells decreased by 12.0% and 37.5%, respectively (Fig. 2G). As a result of the collective findings, high levels of expression of PD-L1 impede the function of CTLs and facilitate the evasion of the immune system in MSI-H COADs. Furthermore, a PD-L1 knockout MC38 cell line was established using CRISPR-Cas9 technology, and the successful deletion of PD-L1 was confirmed through Western blot analysis (Supplementary Fig. 1F). Importantly, compared to the wild-type MC38 tumors expressing PD-L1, the PD-L1 knockout tumors exhibited significantly suppressed growth (Supplementary Fig. 1G, H), accompanied by enhanced anti-tumor immune response (Supplementary Figs. 1I,J). The aforementioned findings illustrate that the deletion of PD-L1 may enhance CTL function and impede immune evasion in MSI-H COAD.
A mesomeric peptidic foldamer, MPHP, has been designed to mitigate the burden of PD-L1 by inducing HSC70-dependent lysosomal degradation
PD-L1 can be regulated through HIP1R-mediated lysosomal degradation8, which prompted us to explore an appropriate strategy for alleviating the burden of PD-L1 by directing it towards lysosomes. Towards this objective, the correlation between 19 lysosome translocators and PD-L1 was investigated in COAD with MSI-H. As depicted in Fig. 3A, HIP1R exhibited a negative correlation with PD-L1, while ANNA5, ANXA10, and HSC70 displayed the highest degree of positive correlation. To comprehensively evaluate these candidates further, their expression differences between COAD tumor and normal tissue were analyzed in Supplementary Fig. 3A. Only HSC70 exhibited a statistically significant increase in expression levels in tumors compared to normal tissues (Supplementary Fig. 2A), indicating its potential suitability as a degradative pathway for PD-L1. Moreover, the molecular characteristics of HSC70 in PD-L1-overexpressing COAD with MSI were investigated through scRNA-seq analysis of the MC38 mice model mentioned above (Fig. 3B). The results demonstrated a significant upregulation of HSC70 expression in cancer cells compared to other cell types, further validating its specific association with tumor cells in COAD with MSI. Besides, the process of HSC70-mediated lysosomal degradation requires the cooperation of lysosome associated membrane protein 2 (LAMP2), which facilitates the transportation of both HSC70 and its cargo into this organelle (Fig. 3C)27. Moreover, the expression of LAMP2 in tumor samples from COAD patients was demonstrated to be positively correlated with the expression of PD-L1 (Fig. 3D) and significantly higher than the expression in normal tissue (Fig. 3E). These findings indicate that the HSC70/LAMP2-mediated lysosomal degradation pathway represents a promising method to alleviate the burden of PD-L1 in MSI-H COAD cancer cells.
A bubble diagram and heatmap of correlation between expression of PD-L1 and multiple molecular chaperones related to protein degradation in COAD. B HSC70 expression in cell clusters from the sc-RNA seq of mouse model of COAD with MSI-H establishing by subcutaneously transplanting either PD-L1 overexpressed MC38 cell lines (n = 3 biological replicates in group). C schematic illustration for the potential PD-L1 degradation pathway in a HSC70 dependent manner. D The correlation between neoplastic PD-L1 and LAMP2 in clinical samples with MSI-H COAD. E The expression of LAMP2 in COAD tumor or normal tissue (n (Tumor)=275, n (Normal) = 349, and all date obtained from independent sample). p value calculated by two-tail t-test. F Schematic diagram of the MPHP. G The spatial structure of the MPHP/PD-L1 complex obtained through molecular docking. H The spatial structure of the heterotrimer of PD-L1/MPHP/HSC70 obtained through molecular docking. I The HPLC and MASS chromatogram of MPHP. J, K Immunofluorescence showed the colocalization between PD-L1 and HSC70 (J) or LAMP2 (K) after treated with Arg6-MPHP for 10 h. The fluorescence intensity profiles curves of PD-L1 and HSC70 or LAMP2 along the white line were plotted by Image J with colocalization factor (Pearson’s R value). L PD-L1 expression in HCT116 cells was assessed by western blot analysis and quantified using Image J software following a 48-hour incubation with isodiluent Arg6-MPHP. M The immunofluorescence assay revealed the degradation effects of Arg6-MPHP on PD-L1 in HCT116 cells. Simultaneous degradation of PD-L1 in cell membrane and cytoplasm were showed by co-staining with phalloidin (staining cytoskeleton). Scale bars indicate 100 μm; local magnification, 25 μm. N, P The western blot assay was conducted to investigate the impact of Arg6-MPHP on PD-L1 expression in the presence and absence of proteasome inhibitors (MG132, 0.06 μM) N, autophagy inhibitors (3-MA, 40 μM) O, and lysosomal inhibitors (NH4Cl, 250 μM) P for a duration of 48 h, and the quantification is shown below (n = 3, means ± SD). All box-whisker plots (B, E) center on the median; the bounds of the boxes mark the upper and lower quartile; the whiskers extend to the upper and lower extremes (1.5× interquartile range from the upper and lower quartiles).
Therefore, we aim to design a bifunctional mesomeric peptidic foldamer capable of simultaneously binding HSC70 and PD-L1 (MPHP), with the ultimate goal of facilitating lysosomal degradation of PD-L1 via the HSC70/LAMP2 pathway. In details, MPHP is composed of three functional motifs spliced together from N-terminal to C-terminal: a PD-L1 dodecamerous peptide ligand consisting of D-enantiomeric amino acid residues (sequence: nyskptdrqyhf)13,15,16, a flexible trimer PEG linker28,29, and a hexamerous L-enantiomeric peptide motif (sequence: CKEFRQ) recognized by Hsc70 (Fig. 3F)27. To elucidate the interaction between MPHP and PD-L1, molecular docking was conducted using Discovery Studio (DS)30,31. As illustrated in Fig. 3G, MPHP demonstrated a robust binding Gibbs free energy (∆G) of -6.5 kcal/mol to the lgV region of PD-L1 ectodomain. Subsequently, the MPHP/PD-L1 complex was subjected to molecular docking with HSC70, revealing a strong interaction between MPHP and HSC70 with an interface area of 378.3 Å2 and ∆G value of -5.2 kcal/mol (Fig. 3H).
To confirm its bioactivity, a solid phase peptide synthesis (SPPS) of MPHP was carried out using L- or D-amino acids protected by Fluorene methoxyl (Fmoc) in HBTU/HOBT-mediated condensation reactions32,33,34,35. Liquid chromatography-mass spectrometry was used to determine the purity and molecular weight of the peptides (Fig. 3I). The biofunctional properties of MPHP in binding with HSC70 and PD-L1 were initially investigated through fluorescence polarization analysis, wherein FITC-labeled MPHP was incubated with isodilute solutions of HSC70 or PD-L1 at pH 7.4. As demonstrated in Supplementary Fig. 2B, MPHP exhibits a high binding affinity to both HSC70 and PD-L1, with values of 55.1 nM and 295.1 nM, respectively. Additionally, a random sequence of MPHP (MPHPCtrl) was synthesized as a control. It is noteworthy that to enhance cell membrane penetration, a cationic transmembrane peptide hexamer Arg (Arg6) was attached to the N-terminal of MPHP and MPHPCtrl for subsequent in vitro bioactivity testing. To confirm the ability of MPHP to facilitate the PD-L1/HSC70 affinity, a co-localization analysis was conducted using Laser Scanning Confocal Microscopy (LSCM) imaging in HCT116 colon cancer cells. As depicted in Fig. 3J, a conspicuous colocalization was observed between red-dye-labeled PD-L1 and green-dye-labeled HSC70 after incubation with Arg6-MPHP for 10 h, whereas negligible colocalization was detected in the Arg6-MPHPCtrl-treated group. Also, notable colocalization of PD-L1 with LAMP2 (Fig. 3K) suggests that Arg6-MPHP triggers PD-L1 translocation to lysosomes36,37. Furthermore, the lysosomal total proteins were extracted, and the level of PD-L1 was quantified using Western blotting (WB) and enzyme-linked immunosorbent assay (ELISA). As anticipated, treatment with Arg6-MPHP significantly increased the accumulation of PD-L1 in lysosomes (Supplementary Fig. 2C), indicating enhanced PD-L1 levels upon MPHP treatment. Accordingly, Arg6-MPHP leaded to the degradation of total PD-L1 in HCT116 cells in a dose-dependent manner with an inhibitory concentration (IC50) of 1.62 ± 0.20 μM (Fig. 3L) Furthermore, immunofluorescent staining (Fig. 3M) and western blot results (Supplementary Figs. 2 D&E) revealed that Arg6-MPHP down-regulated PD-L1 not only in the cytoplasm but also on the cell membrane. Additionally, Arg6-MPHP significantly decreased the amount of PD-L1 in exosomes as measured by western blot (Supplementary Fig. 2F) and a protein-targeted quantification technology executed by LC-MS termed parallel reaction monitoring (Supplementary Fig. 2G). For further validation of the PD-L1 degradation mechanism, we employed proteasome inhibitor MG132, autophagosome inhibitor 3-MA, lysosome inhibitor NH4Cl and chloroquine to suppress PD-L1 degradation induced by Arg6-MPHP (Fig. 3N-3P and Supplementary Fig. 2H). As anticipated, MG132 and 3-MA exhibited negligible impact on Arg6-MPHP-derived PD-L1 degradation while NH4Cl and chloroquine impeded the function of Arg6-MPHP in degrading PD-L1 (Fig. 3N-3P and Supplementary Fig. 2H). To further confirm the dependence of PD-L1 degradation on LAMP2 and HSC70, we employed Small interfering RNA (siRNA) targeting LAMP2 and a specific inhibitor (VER-155008) of HSC70 to challenge the biofunction of Arg6-MPHP. As anticipated and demonstrated in Supplementary Fig. 2I, both LAMP2 siRNA and VER-155008 effectively impede the function of Arg6-MPHP in promoting PD-L1 degradation. Besides, the impact of Arg6-MPHP on other PD-L1 family proteins, such as PD-L2, B7H3 (CD276), B7H4, or Vista, was found to be negligible (Supplementary Fig. 2J). Collectively, MPHP specifically triggers the degradation of PD-L1 via the lysosome-dependent protein degradation pathway. The potency of Arg6-MPHP extends to other colon cancer cell lines, such as MC38 and CMT93 (Supplementary Fig. 2K), highlighting its broad-spectrum potential in colon cancer therapy.
Construction of TAME-responsive artificial protein IgP β that targets PD-L1-overexpressing MSI-H COAD
In order to determine effective strategies for targeted delivery of MPHP to COAD with MSI-H, we established an in-situ mouse model of COAD with MSI-H by transplanting PD-L1-overexpressed MC38 cells into the submesentery at colon, and then conducted comprehensive proteomic analyses to identify its molecular characteristics. Compared to the normal colon near the nidus, 1191 proteins were down-regulated and 1187 were up-regulated in MSI-H COAD tumors (Fig. 4A). Through Gene Set Enrichment Analysis (GSEA), it was observed that among the top 20 up-regulated pathways, two gene sets related to anaerobic metabolism were identified (Fig. 4B), while eight gene sets associated with aerobic metabolism were found to be the top eight down-regulated pathways (Fig. 4C). The observed differences indicate an acidic tumor microenvironment resulting from anaerobic respiratory metabolites. To further validate this, metabolomic analyses were performed on the model, revealing 27 down-regulated and 36 up-regulated metabolites in tumor compared to normal colon (Fig. 4D). Clustering analysis of these differential metabolites revealed that most down-regulated metabolites were enriched in anaerobic metabolic pathways, while more than half of the up-regulated metabolites were associated with anaerobic metabolism (Fig. 4E). Moreover, the majority of significantly up-regulated metabolites were found to be acid metabolites (Supplementary Fig. 3A), indicating a prominent tumor acid microenvironment (TAME) in MSI-H COAD. This discovery was further confirmed by the correlation analysis of acid-related proteins and PD-L1 expression in MSI-H COAD using TCGA data, which revealed significant positive correlations between PD-L1 and key glycolysis enzymes (ADPGK, BPGM, ENO1, and PKM) as well as acid-responsive proteins (LAMP5 and GPR68) (Fig. 4F). Taken together, TAME is a prominent characteristic of COAD with MSI-H overexpressing PD-L1, and targeting TAME may represent a viable strategy for MPHP delivery.
A The proteome heatmap of differential proteins at tumor sites and normal colon near the nidus in an in-situ mouse model of COAD with MSI-H by transplanting PD-L1-overexpressed MC38 cells into the submesentery at colon. B, C The top 20 up-regulated B and down-regulated C pathways at tumor sites in comparison with normal colon near the nidus. D Volcano Plot of differential metabolites at tumor sites in comparison with normal colon near the nidus. E Clustering analysis of differential metabolites in metabolic pathways. F The correlation between neoplastic PD-L1 and key glycolytic enzymes or acid responsive proteins in clinical samples with MSI-H COAD.
Within this context, MPHP was modified to exhibit pH-responsive behavior through the conjugation of a charge-reversible motif HEHE at its N-terminus and a positively charged motif DRDR at its C-terminus. As illustrated in Fig. 5A, the PD-L1 recognition capacity of MPHP can be modulated by charge attraction between its head and tail at neutral pH, and restored under acidic conditions due to the charge reversal of HEHE motif. The modification of MPHP to pH-MPHP did not alter its biofunction in terms of binding with HSC70 and PD-L1, and the change in pH had minimal impact on this binding affinity (Supplementary Fig. 3B, C). To optimize their pharmacokinetic properties, both MPHP and pH-responsive MPHP (pH-MPHP) were incorporated into immune-regulatory globulin-like particles, referred to as IgP α and IgP β respectively (Fig. 5A). It has been demonstrated previously that thiol-N-terminal modified peptides are capable of forming comonomer precursors through infinite Auric-sulfhydryl coordination and then self-assemble into sphere-shaped nanostructures driven by aurophilia13,15,33,38,39. Using this self-assembly approach, both MPHP and pH-MPHP modified with N-terminal Cys can form spherical globulin-like particles, as demonstrated by individual nanoparticles observed through transmission electron microscopy (TEM) and the uniform granulometric distribution characterized by dynamic light scattering (DLS) (Fig. 5B–E). Moreover, to confirm the self-assembly of IgP β from pH-MPHP-Au(I) precursors, we conducted X-ray photoelectron spectroscopy (XPS) and Fourier-transform infrared (FT-IR) analysis to characterize the Auric(I)-sulfhydryl bond. The XPS analysis of Au(4 f) and S(2p), as depicted in Supplementary Fig. 3D, is consistent with the characteristic energy band spectrum of the bond between Au(I) ions and alkanethiols15,16,33. This result was further confirmed by the FT-IR spectra of IgP β presented in Supplementary Fig. 3E, where the distinctive absorption peak of gold(I)-thiolate is observed at 2950 cm-1. Furthermore, the composition of IgP β was characterized using High Angle Annular Dark Field (HAADF) Scanning Transmission Electron Microscopy (STEM) combined with Energy Dispersive X-rayspectroscopy (EDX). The overlay chart of elements and HAADF image of IgP β exhibit a uniform distribution of Nitrogen (N), Sulphur (S), Oxygen (O) and Gold (Au) as depicted in Fig. 5F. These results suggested that IgP β was formed through the assembly of pH-MPHP-Au(I) precursors rather than surface modification of pH-MPHP on gold nanoparticles. As a result, a spherical globulin-like particle named IgP β was successfully constructed with the added capability of charge reversal in response to an acidic environment (Fig. 5G), which endowed it with the potential to respond to TAME.
A Schematic representation of the design and synthesis of artificial immunoglobulin derived from MPHP (IgP α) or pH-MPHP (IgP β). B, C TEM images B and hydrodynamic diameter C of IgP α. D, E TEM images D and hydrodynamic diameter E of IgP β. F IgP β was characterized using High Angle Annular Dark Field (HAADF) Scanning Transmission Electron Microscopy (STEM) combined with Energy Dispersive X-rayspectroscopy (EDX). G, ζ potentials of IgP α and IgP β in response to various pH levels (n = 3 independent replicates). The experiments in H-M were independently replicated three times, yielding consistent results. H, I Accumulation of IgP α and IgP β in organs and tissues were reflected by the concentration of Au detected by ICP-MS in tumor-bearing C57BL/6 mice. Serial euthanasia was carried out at different time points (4 h, 10 h, 24 h, 48 h, 72 h) after systemic injection. Dynamic distribution H of IgP α and IgP β in several organs/tissues, including heart, liver, spleen, lung, kidney, stomach, intestine and tumor. Tumor-to-organ ratios I of IgP α and IgP β at 10 h after administration (n (biological) =3/group). p value calculated by two-tail t-test. All p value = 1.0e-6. Tu, tumor; He, heart; Li, liver; Sp, spleen; Lu, lung; Ki, kidneys; St, stomach; In, intestine. J Cellular uptakes of FITC-labeled IgP β measured by flow cytometry. K The colloidal stability of IgP β measured at pH 7.4 by DLS. L MPHP release from IgP β in response to GSH measured by HPLC. M Changes of body weight of C57BL/6 mice were continuously detected for 10 days after dosage of 100 mg/Kg IgP β.
IgP β exhibited favorable pharmaceutical properties
As per our design, the TAME responsiveness is expected to enhance tumor accumulation of IgP β. To verify this hypothesis, we comparatively investigated the dynamic organ and tumor distribution of acid-insensitive IgP α and acid-responsive IgP β in an MC38 in-situ mice model using inductively coupled plasma mass spectrometry (ICP-MS). As shown in Fig. 5H, IgP β exhibited a 3.1-fold increase in tumor accumulation compared to IgP α, indicative of enhanced tumor targeting through TAME-responsiveness. This is further supported by the more pronounced tendency for tumor accumulation of IgP β over IgP α (Fig. 5I). Furthermore, the acid responsiveness in IgP β resulted in reduced drug accumulation in the reticuloendothelial system (liver and spleen) and digestive tract following intravenous injection (stomach and intestine), indicating an optimized pharmacokinetic profile (Fig. 5H). Additionally, the acid responsiveness of IgP β confers upon it the ability to selectively internalize into cells in response to extracellular pH microenvironments40. As expected, at pH 6.5 which mimics the acidic tumor microenvironment, IgP β exhibited robust cellular internalization in two COAD cell lines HCT116 and MC38, while its internalization was weakened at pH 7.4 (Fig. 5J). In summary, TAME-responsiveness enhances the tumor accumulation and cellular internalization of IgP β, which is a desirable characteristic for increased potency and reduced toxicity.
Furthermore, in order to investigate the colloidal stability of IgP β, it was diluted into a standard PBS solution containing 20% fetal bovine serum (FBS) at pH 7.4 and its hydrodynamic size was monitored using DLS. Over a period of 72 h incubation, there was less than a 10% change in hydrodynamic size observed, indicating excellent monodispersion and satisfactory colloidal stability (Fig. 5K). Under these conditions, it is noteworthy that a 24-hour incubation did not result in significant MPHP release as measured by High Performance Liquid Chromatography (HPLC) (Fig. 5L). However, the presence of 10 mM glutathione (GSH), which is commonly found in the intracellular environment of cancer cells, led to a marked release of MPHP with a half-release time of 2.2 h (Fig. 5L). To assess the acute toxicity of IgP β, ten healthy C57BL/6 mice were intravenously administered a high dose of 100 mg/kg of IgP β, while another ten C57BL/6 mice received isopycnic normal saline as a control group. After the injection, the body weights of five randomly selected mice in per group were continuously monitored for a period of 10 days, and no statistically significant differences were observed during this time (Fig. 5M).
The remaining five mice in each group were euthanized 24 h post-injection, and their blood and major organs were collected for further analyses of acute toxicity. In the course of routine blood analysis after administration, hemolysis, myelosuppression, anemia, leukopenia, and thrombocytopenia were not observed (Fig. 6A). To investigate the anaphylactic potential of IgP β, we assessed a range of serum immunotoxic factors, including IFNγ, IL1β, IL2, IL4, IL5, IL6, IL10, IL12 and TNFα. The results of our study reveal a complete absence of any immunological risk (Fig. 6B). Additionally, the absence of hepatotoxicity in IgP β is supported by the healthy pathological sections of the liver and hepatic biochemical indexes (Fig. 6C). Meanwhile, there was no evidence of nephrotoxicity in the pathological sections or renal function indices, including blood urea nitrogen [BUN], creatinine [CREA], and albumin [ALB] (Fig. 6D). In comparison with Normal saline administration, the injection of IgP β exhibited negligible impact on lung morphology and IFNγ, TNFα, IL2, IL6 levels in pulmonary lavage fluid (Fig. 6E). Furthermore, the spleen and heart showed no pathological changes (Fig. 6F, G). Collectively, these findings demonstrate that IgP β exhibits enough avirulence for clinical translation.
A Heat map showing the changes of blood corpuscle after indicated treatment. B Serum biochemical markers related to immunotoxicity measured in IgP β- or mock-treated mice. C Hepatotoxicity of IgP β was detected by H&E staining of liver pathological sections, alanine aminotransferase (ALT), aspartate transaminase (AST) and total bilirubin (TBIL). D Nephrotoxicity of IgP β was showed by H&E staining of renal pathological sections, creatinine (CRE), blood urea nitrogen (BUN) and serum albumin (ALB). E Pulmonary toxicity of IgP β was reflected by H&E staining of lung pathological sections and the changes of inflammatory factors (IFN- γ, TNF- α, IL-2 and IL-6) in pulmonary lavage fluid. F, G H&E staining of pathological sections of spleen F and heart G. The data were presented as mean ± SD. H, body weights of mice in cumulative toxicity test of IgP β. I Heat map showing the changes of blood corpuscle and blood biochemical indexes after indicated treatment. (B–G, n = 3 biological replicates; H, I n = 5 biological replicates. Data are presented as mean values +/-SD, Statistical analysis was performed using two-sided t-test, ***p < 0.001. Scale bar, 50 µm). The experiments depicted in C-F were independently replicated three times, resulting in consistent findings.
To assess the cumulative toxicity of IgP β, a three-week administration study was conducted on 20 mice randomly divided into four groups and given dosages of 0 (Control), 10, 20, and 40 mg/Kg every other day. After the 21-day treatment period, there was an increase in body weights across all groups (Fig. 6H), indicating a favorable safety profile of IgP β. Moreover, the injection of IgP β at three different concentrations did not yield statistically significant alterations in blood corpuscle counting, nephrotoxicity indices, and hepatotoxicity indices when compared to the control group (Fig. 6I). Aside from that, no pathological changes were observed in the hearts, livers, spleens, lungs, or kidneys of mice given IgP β (Supplementary Fig. 4). In conclusion, the above results have demonstrated that IgP β possesses favorable pharmaceutical properties, including tumor-specific accumulation, efficient cellular internalization, colloidal stability, GSH-triggered release and safety profiles.
IgP β effectively induced lysosomal degradation of PD-L1
These advantageous pharmaceutical characteristics of IgP β prompted us to investigate its effectiveness. the bioactivity of IgP β was evaluated using HCT116, a COAD cell line with high expression levels of PD-L1. As demonstrated by the CLSM measurements in Fig. 7A, B, IgP β significantly reduced the abundance of PD-L1 after a 10-hour incubation period with greater efficacy than IgP α. To further investigate its pharmacodynamics, the degradation kinetics curves of PD-L1 in response to IgP α and IgP β were measured via Western Blot. It was observed that both IgP α and IgP β exhibited dose-dependent degradation (Fig. 7C), and IgP β induced a median inhibitory concentration (IC50) decrease approximately four times greater than that of IgP α (Fig. 7D), indicating significantly enhanced bioactivity. Furthermore, neithnor proteasome inhibitor MG132 nor autophagosome inhibitor 3-MA suppress the IgP β-derived PD-L1 degradation (Fig. 7E). However, lysosome inhibitors NH4Cl and chloroquine were effective in inhibiting this process (Fig. 7E), indicating a protein degradation pathway that is dependent on lysosomes. Moreover, IgP β also induced colocalization of PD-L1 with both HSC70 and LAMP2 (Supplementary Fig. 5A), suggesting a lysosomal degradation pathway that is dependent on HSC70. Furthermore, IgP β potently triggers PD-L1 degradation in vitro, resulting in decreased levels of PD-L1 throughout the entire tumor cell, including in cytoplasmic, membrane-bound and exosomal compartments (Fig. 7F and Supplementary Fig. 5B). The collective findings strongly support the effectiveness of IgP β in reducing intratumoral PD-L1 burden. Furthermore, to validate the reliance of PD-L1 degradation on LAMP2 and HSC70, we also utilized LAMP2 siRNA and VER-155008 to challenge the biofunction of IgP β, both of which effectively hindered the function of IgP β in promoting PD-L1 degradation (Supplementary Fig. 5C). Additionally, negligible impact was observed on other PD-L1 family proteins such as PD-L2, B7H3 (CD276), B7H4, or Vista by IgP β (Supplementary Fig. 5D). Moreover, the duration of IgP β‘s involvement in PD-L1 degradation extends up to at least 72 h, surpassing that of IgP α (Supplementary Fig. 5E). Furthermore, the efficacy of IgP β extends to other colon cancer cell lines like MC38 and CMT93 (Supplementary Fig. 5F), underscoring its broad-spectrum potential in colon cancer therapy. The findings from this study demonstrate that IgP β specifically initiates the lysosome-dependent protein degradation pathway for PD-L1.
A The immunofluorescence assay revealed the degradation effects of IgP α and IgP β on PD-L1 in HCT116 cells. Scale bars indicate 100 μm. B Fluorescent quantitation of PD-L1 from immunofluorescence images in (A). p value calculated by two-tail t-test. p (Ctrl to IgPα)= 1.0e-6, p (IgPα to IgPβ)= 1.0e-6. C PD-L1 expression in HCT116 cells was assessed by western blot analysis and quantified using Image J software following a 48-hour incubation with isodiluent IgP α or IgP β. D A median inhibitory concentration (IC50) of PD-L1 level. p value calculated by two-tail t-test. p (Arg6-MPHP to IgPβ)= 0.045, p (IgPα to IgPβ)= 1.0e-6. E The western blot assay was conducted to investigate the impact of IgP β on PD-L1 expression in the presence and absence of proteasome inhibitors (MG132, 0.06 μM), autophagy inhibitors (3-MA, 40 μM), and lysosomal inhibitors (NH4Cl, 250 μM; chloroquine, 20 μM) for a duration of 48 h. The quantification is shown below (n = 3, the data were obtained from three independent biological replicates, and the results are presented as means ± standard deviation (SD)). F PD-L1 in HCT116 cell membrane, cytoplasm and exosome incubating with different concentrations of IgP β for 48 h. p value calculated by two-tail t-test. G immunohistochemical staining of the tumor for PD-L1 in C57BL/6 mice bearing subcutaneous homologous PD-L1-overexpressed MC38 COAD. (Scale bar: 50 μm) H IHC score of the PD-L1 at tumor sites. I Tumor growth curve during the administration. p value calculated by two-tail t-test. J Photos of tumor isolated from mice after administration. K Weight of tumors in J, p value calculated by two-tail t-test, p (Ctrl to Anti-PD-L1 15 mg/kg)= 0.001, p (Ctrl to IgPβ 15 mg/kg)= 1.0e-6, p (Ctrl to Anti-PD-L1 30 mg/kg)= 0.001, p (Ctrl to IgPβ 30 mg/kg)= 1.0e-6, p (Anti-PD-L1 15 mg/kg to IgPβ 15 mg/kg)=0.003, p (Anti-PD-L1 30 mg/kg to IgPβ 30 mg/kg)=0.002. (A-F, n = 3 biological replicates. G–K n = 5 biological replicates; data are presented as means ± SD, * p < 0.05, ** p < 0.01, ***p < 0.001).
Next, C57BL/6 mice bearing homologous PD-L1-overexpressed MC38 COAD subcutaneously were used to investigate the function of IgP β in vivo. When the tumor volume reached 100 ± 20 mm3, a total of 25 mice were randomly divided into five groups: normal saline (Control), IgP β at doses of 15 mg/Kg and 30 mg/Kg, Anti-PD-L1 at doses of 15 mg/Kg and 30 mg/Kg. On day1 and day6, all mice received intravenous injections as scheduled. After a 10-day administration period, immunohistochemical staining of the tumor for PD-L1 revealed that IgP β significantly reduced the burden of neoplastic PD-L1, while Anti-PD-L1 exhibited minimal impact on the level of PD-L1 (Fig. 7G, H). Furthermore, compared to isodosing Anti-PD-L1, IgP β significantly upregulated immune activity factors including Granzyme A, Granzyme B, Perforin-1 and CD80 at tumor sites (Supplementary Fig. 6A). As a desired result, the isodosing IgP β exhibited superior tumor suppression rates compared to Anti-PD-L1 (Fig. 7I). As with the volumetric measurements of tumors, the photographs of tumors (Fig. 7J) and direct weight measurements (Fig. 7K) obtained at the end of the experiment provided similar conclusions. Moreover, a significant increase in tumor cell apoptosis was observed in IgP β-treated samples compared to other groups, as evidenced by H&E staining and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay (Supplementary Fig. 6B). Collectively, the aforementioned data demonstrate the effective in vivo degradation of neoplastic PD-L1 by IgP β.
IgP β rejuvenated CTL action in vivo through the PD-L1 degradation
To investigate the underlying mechanism of IgP β-based immunotherapy, single-cell RNA sequencing was performed on mice bearing subcutaneous MC38 tumors overexpressing PD-L1. Figure 8 A&B presented a distinct single-cell expression atlas following a single injection of either 30 mg/kg IgP β or isometric normal saline (Control), wherein the proportion of cancer cells significantly decreased in response to IgP β treatment. Furthermore, the percentage of Ki67/PCNA positive cells was significantly reduced in tumors treated with IgP β, indicating a suppressed cellular proliferation (Fig. 8C). Additionally, GSEA analysis of scRNA-seq data obtained from cancer cell clusters revealed a significant upregulation of apoptosis and downregulation of cell cycle following IgP β treatment (Fig. 8D and Supplementary Fig. 7A), indicating potential tumor inhibition. Besides, the molecular characteristics of T cells suggest a reduction in CD25/FOXP3-positive Treg cells and an increase in CD8-positive T lymphocytes (CD8+ T) following treatment with IgP β (Fig. 8E). Further analysis reveals a significant upregulation of granzyme A (GamA), granzyme B (GamB) and interferon γ (IFN γ) in CD8 + T cells following IgP β treatment (Fig. 8E), indicating a reversal of CTL exhaustion. GSEA analysis of the T cell cluster provides further evidence for IgP β-mediated restoration of CTL function, as demonstrated in Fig. 8F, where the top 10 up-regulated gene sets are all related to immune activation. These significant upregulation of gene sets related to CD8 TCR, T cell activation involved in immune response, CD8+ T cell activation and T cell cytokine and downregulation of caspase activation in response to IgP β treatment indicated an anti-tumor immune activation of CTL (Fig. 8G and Supplementary Fig. 7B). Consistent with these findings, a comparative study utilizing multichannel flow cytometry was conducted to examine the effects of IgP β treatment on MC38 tumor types. The results showed a significant increase in the percentage of GamB-positive and IFN γ-positive CTL by 10.6-fold and 13.1-fold, respectively (Fig. 8H), while Treg cells decreased by 47.4% in response to IgP β treatment (Fig. 8I). The collective findings demonstrate that IgP β is capable of effectively inducing lysosomal degradation of PD-L1, thereby rejuvenating in vivo CTL activity in COAD with MSI-H.
A&B, t-SNE plots of broad cell types in mouse model of COAD with MSI-H establishing by subcutaneously transplanting PD-L1 overexpressed MC38 cell lines after IgP β or mock treatment (n = 3/group). C, t-SNE plots showing the expression of selected marker genes in cancer cells. D, GSEA analysis of apoptosis and cell cycle in scRNA-seq data from the cancer cell cluster. E, UMAP plots showing the expression of selected marker genes in CD8 + T cells. F, Top 10 up-regulated gene sets of GSEA analysis of scRNA-seq data from the CD8 + T cell cluster. G, GSEA analysis of CD8 TCR, T cell activation involved in immune response, CD8 + T cell activation and caspase activation in scRNA-seq data from the CD8 + T cell cluster. H, multichannel flow cytometry analysis of CTLs in the two distinct MC38 tumor types. The bar charts depict the proportions of Treg (n = 3 biological replicates in each group). p value calculated by two-tail t-test. p = 0.002. The gating strategy for Treg involves two sequential steps: 1) identification of cells expressing CD45+ and CD4 + , and 2) characterization of Treg as cells positive for both CD25+ and FOXP3+ within the CD45+ and CD4+ population. I, multichannel flow cytometry analysis of Treg cells in the two distinct MC38 tumor types. The bar charts illustrate the proportions of CTLs cells that are positive for GzmB or IFN γ (n = 3 biological replicates in each group). p value calculated by two-tail t-test. p = 0.024 and 0.002. The gating strategy for CTL involves two sequential steps: 1) identification of CD45+ expressing cells, and 2) characterization of CTL as cells positive for both CD8+ and CD3+ within the CD45+ population. Subsequently, GzmB+ or IFN γ + CTL were identified in this population. (H-I, Data are presented as means ± SD; p value in H and I calculated by two-tail t-test, and all p = 1.0e-6).
Furthermore, to assess the impact of IgP β in a more natural context, we established an anti-PD-1-resistant COAD mouse model by continuously stimulating MC38 mice with PD-1 neutralizing antibodies (5 mg/kg every 5 days) across three generations. In this model, the level of PD-L1 in anti-PD-1-resistant MC38 tumors is higher compared to the baseline (Supplementary Fig. S7A). Subsequently, we evaluated the anti-tumor efficacy of IgP β and Anti-PD-1 in this endogenous PD-L1 high model and observed that IgP β significantly inhibited tumor growth, while Anti-PD-1 had minimal effect (Supplementary Fig. S7A). Moreover, multichannel flow cytometry results demonstrated a significant increase in the percentage of GamB-positive and IFN γ-positive CTLs, along with a significant decrease in Treg cells following treatment with IgP β (Supplementary Fig. S7B). These collective findings demonstrate that IgP β is also capable of rejuvenating CTL activity in vivo within an Anti-PD-1 resistant COAD with MSI-H.
IgP β potently suppressed tumor progression in humanized PDX and PDOX mice model of COAD with MSI-H
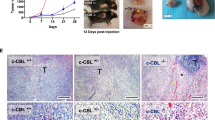
An NSG mouse model of MSI-H COAD was created in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice such that the humanized patient-derived xenograft (Hu-PDX) could be further validated as a clinical translational model. The tumor was obtained from a patient with COAD who had relapsed and become refractory. This tumor carried three malignant gene mutations of Kras, PIK3CA, and ARID1A (Supplementary Fig. 9A). Notably, the tumor also exhibited mismatch repair-deficient mutations at MLH1, MSH2, MSH6, and PMS2, indicating microsatellite instability-high (MSI-H) (Supplementary Fig. 8A)41. A patient-derived xenograft (PDX) tumor implanted subcutaneously and allowed to reach a volume of 100-150 mm3 was irradiated with sub-lethal radiation (0.5 Gy). To modulate the immune response, 5 × 106 human peripheral blood mononuclear cells (PBMCs) were transplanted from the same patient. As soon as the mice had >1% engraftment, they were randomly divided into three groups and given 30 mg/kg doses of PBS (control), IgP β, or Anti-PD1 intravenously every four days. In contrast to the mock and Anti-PD1 treatments, IgP β significantly reduced tumor PD-L1 abundance in both tumor tissue (Fig. 9A) and exosomes from tumor and peripheral blood after four treatment cycles (Supplementary Fig. 8B). Compared to Anti-PD1, IgP β demonstrated superior efficacy in reducing intratumoral CD4+/CD25+ cells (Fig. 9B and Supplementary Fig. 8C) and increasing intratumoral CD3+/CD8+ cells (Fig. 9C and Supplementary Fig. 8D), while also inducing upregulation of immunoactivators (Fig. 9D–G and Supplementary Fig. 8E). The findings suggest that IgP β elicits a greater degree of immune activation in CTLs compared to Anti-PD1. Consequently, a higher proportion of apoptotic cells was observed in IgP β-treated tumors compared to those treated with Anti-PD1 (Fig. 9H and Supplementary Fig. 8F). At the end of IgP β treatment, 57.03% of PDX tumors exhibited significant inhibition, which was statistically superior to the 30% inhibition observed with Anti-PD1 treatment (Fig. 9I, J). This finding was further supported by tumor weight measurements obtained at the conclusion of experiments (Fig. 9K). The administration of IgP β for 17 days, remarkably, did not lead to any significant loss in body weight (Fig. 9L) or alterations in H&E staining of organs (Supplementary Fig. 8G), thereby highlighting the impeccable safety profile of IgP β.
A The expression of PD-L1 involved in tumor tissue tested by IHC. B, C The percentage of double positive cells counted from immunofluorescence images of tumor sections staining with CD4 /CD25 B and CD3/CD8 C after indicated treatments. D–G IHC score of tumor sections staining with Granzyme-A D, Granzyme-B E, Perforin-1 F, CD80 G after indicated treatments. H TUNEL staining of tumor tissue sections with the different treatments. (Scale bar: 50 μm). I Photographic images of NSG mice bearing colon-carcinoma-PDX tumors during treatments. J Tumor growth curves of mice during the administration (mean ± SEM, n = 6 per group). K Weights of tumor isolated from mice at the end of experiment. L Body weights of mice during the administration. (A–L the PDX model n = 6 biological replicates; A, D–H, J–L, data are presented as means ± SD, statistical analysis was performed using two-sided t-test, * p < 0.05, ** p < 0.01, ***p < 0.001). The experiments depicted in this figure were independently replicated a minimum of three times, resulting in consistent findings.
To further evaluate the efficacy of IgP β, a patient-derived orthotopic xenograft (PDOX) model of COAD was established by implanting carcinoma cells derived from surgically resected tumors into the inferior mesenteric tissue at the colon of NSG mice. Two weeks after tumor transplantation, the same protocol was used to construct a Hu-PDOX model as described above for the Hu-PDX model. Subsequently, 24 mice were randomly and averagely allocated into three groups (n = 8/group): the control group (NS, 100 μL), the study group (IgP β, 15 mg/Kg), and the positive control group (Anti-PD1, 15 mg/Kg). They received a two-week treatment consisting of intravenous injections of medication twice. Following administration, Anti-PD1 demonstrated a tumor inhibitory rate (TIR) of less than 40% on the PDOX tumor, as evidenced by both tumor photos (Fig. 10A, B and Supplementary Fig. 9A) and weights (Fig. 10C). In contrast, IgP β exhibited robust efficacy with a TIR exceeding 83.7% (Fig. 10B&C and Supplementary Fig. 9A). Consistent with this discovery, IgP β effectively degraded PD-L1 in both tumor tissue (Fig. 10D) and tumor exosomes (Supplementary Fig. 9B), providing further evidence of its stable and potent bioactivity. Furthermore, the presence of immunosuppressive CD4 + /CD25+ cells (Fig. 10E and Supplementary Fig. 9C), coupled with the absence of infiltrated CD3 + /CD8+ cells (Fig. 10F and Supplementary Fig. 9D) and a deficiency in CTL-related immunoactivators such as Granzyme A, Granzyme B, Perforin-1 and CD80 (Fig. 10G–J and Supplementary Fig. 9E–H) within mock-treated tumors collectively demonstrate their characteristic immune evasion. Fortunately, IgP β effectively activated the T-cell immune response in this colorectal cancer with greater efficacy than Anti-PD1 (Fig. 10E–J and Supplementary Figs. 9E–H). Moreover, the results were reinforced by TUNEL (Fig. 10K) and H&E (Fig. 10L) staining of the tumor tissue. In conclusion, these findings suggest that IgP β can significantly reduce PD-L1 expression in Hu-PDX/PDOX colon cancers and reactivate antitumor immunity by expanding CTLs.
A, B Photos of tumors excised at the end of experiment. C Weights of tumor isolated from PDOX mice at the end of experiment. D The change of PD-L1 expression involved in tumor tissue of PDOX model tested by IHC. E, F The percentage of double positive cells counted from immunofluorescence images of tumor sections staining with CD4 /CD25 E and CD3/CD8 F after indicated treatments (Scale bar: 100 μm). G–J IHC score of tumor sections staining with Granzyme-A G, Granzyme-B H, Perforin−1 I, CD80 J after indicated treatments. K, L TUNEL staining images K and H&E staining images L of the tumors after indicated treatment. (C, the PDOX model, n = 8 biological replicates; D, K The PDOX model, n = 6 biological replicates. C, K Data are presented as means ± SD, statistical analysis was performed using two-sided t-test, *p < 0.05, **p < 0.01, ***p < 0.001). All box-whisker plots C, D, G, H, I, J, K center on the median; the bounds of the boxes mark the upper and lower quartile. The experiments depicted in this figure were independently replicated a minimum of three times, resulting in consistent findings.
Discussion
In this study, we analyzed a TCGA data package comprising 9993 cases of 30 common tumors, which included tumor immunosuppressive factors, immunocytotomic data and PD-L1 expression data to identify potential candidates for PD-L1 degradation therapy in neoplastic tumors. Our findings suggest that colon adenocarcinoma (COAD) with microsatellite instability-high (MSI-H) may hold the greatest therapeutic promise. It is worth noting that COAD represents the largest subset of colorectal cancer (CRC). MSI-H CRCs constitute approximately 15% of all CRC cases and are consistently associated with higher neoantigen loads and immune cell infiltration, rendering them a more promising candidate for first-line treatment of metastasis as well as neoadjuvant therapy in non-metastatic cases using PD-1/PD-L1-related ICB therapies42,43. Nevertheless, since up to 50% of MSI-H CRC patients resist immunotherapy and progress as a result41,44,45, there is still a challenge associated with the use of ICI treatment, which must be addressed in order to enhance the efficacy of current therapies. To achieve this objective, we performed bioinformatic analyses on clinical samples of COAD with MSI-H and conducted single-cell mRNA sequencing (scRNA-seq) analysis on over-expressed PD-L1 mouse models with MSI-H COAD, in order to elucidate the underlying mechanism of PD-L1-mediated immune evasion. Based on our findings, it has been determined that (1) PD-L1 disrupts immune clearance and promotes immune evasion in MSI-H COAD by inhibiting the function of CTL, and (2) HSC70-mediated lysosomal degradation represents a viable strategy for reducing PD-L1 levels in COAD. Furthermore, a peptidic foldamer based on MPHP was developed along this line to stimulate the degradation of PD-L1 by the lysosomal system in a HSC70-dependent manner.
As well, comparisons of proteomic and metabolomic analyses among PD-L1-over-expressed MC38 (MSI-H) orthotopic CRCs in C57BL/6 mice revealed the presence of a prominent tumor acid microenvironment (TAME). There is therefore a need for multifunctional drugs capable of performing this intricate process, which involves the response of TAME outside tumor cells and the degradation of PD-L1 within the tumor cells. In terms of possible therapeutic solutions, protein drugs represent a class of multifunctional therapeutics capable of performing complex functions simultaneously, such as catalyzing biochemical reactions and regulating signaling pathways20,21. In the past decade, a long-range objective in chemical engineering and synthetic biology is to elaborate protein with enhanced or extensional functionality46. Through the incorporation of natural and/or non-natural functionality into template proteins, considerable efforts have been made to re-engineer existing natural proteins using epitope grafting, sequence randomization and directed screening47,48,49. Though promising, this strategy is first hindered by the limited number of template proteins22. Nor, even if this obstacle is overcome, can the strategy be quite the panacea that take full advantage of topological structure in template proteins and achieve global shape complementarity with targets50. There is therefore a need for a general and feasible approach to creating natural or non-natural proteins as drug candidates with well-defined structures and functions, and this work may fill the void.
Upon the basic building principles of peptidic foldamers and peptidic backbone engineering, in this work, the mesomeric peptidic foldamer pH-MPHP were designed by backbone conjugation of functional levorotatory and dextrorotatory peptide motifs. Through Au-peptide precursor-mediated aurophilic interaction, pH-MPHP can undergo self-assembly to form an immune-regulatory globulin-like proteinoid nanospheres (IgP β) that exhibit pH-responsive behavior, excellent cell membrane penetration, preferential tumor accumulation, and favorable biosafety with respect to immunogenicity and acute toxicity. As expected, IgP β effectively reduces the neoplastic PD-L1 burden through HSC70-dependent lysosomal degradation and durably rejuvenated the action of CTL in tumor microenvironment, surpassing antibody-based ICI therapy in potency of MSI-H colorectal cancer. The viable strategy presented here will expand the application of peptidic foldamers to the discovery of artificial protein drugs for targeted lysosomal degradation of membranal and cytoplasmic proteins, potentially revitalizing ICB therapy as a more comprehensive and effective treatment modality to enhance outcomes in patients with advanced cancer.
Methods
Synthesis of MPHP and pH-MPHP peptide
The peptides were synthesized on MBHA resin using an CS bio 336X automated peptide synthesizer, employing HBTU as the condensation agent and DIEA as the catalyst, following the solid phase synthesis technology of Fmoc peptides. Subsequently, after cleavage and deprotection in a reagent cocktail comprising 88% TFA, 5% phenol, 5% H2O, and 2% TIPS, the peptides were precipitated with cold ether. Finally, upon characterization by LC-MS (Waters SQD2), the peptides underwent purification to achieve homogeneity through preparative C18 reversed-phase HPLC utilizing acetonitrile and water containing a ratio of 1/1000 TFA as the purification reagent. The molecular weight of each peptide was determined via Electrospray Ionization Mass Spectrometry (ESI-MS) during mass spectrometry experiments employing a mobile phase consisting of an acetonitrile gradient ranging from 5% to 65%, supplemented with 0.1% acetic acid.
Synthesis of IgP α and IgP β
The MPHP or pH-MPHP peptides (2 mg) were completely dissolved in a solution comprising 500 μL NH2-PEG-SH (4 mg/mL) and 500 μL ethanol using ultrasonic oscillation. After complete dissolution, the peptide solution was combined with 2.25 mL of HEPES (100 mM, pH 7.0), 1.25 mL of ddH20, and 500 μL of HAuCl4 (10 mM). Simultaneously, another mixture was prepared by combining 2.25 mL of pH 7.0 HEPES, 2.25 mL of dH2O, and 500 μL of HAuCl4 (10 mM), which was then mixed with the peptide solution on a magnetic agitator at a temperature of 50 °C and a speed of 300 rpm. Finally, after a reaction time of approximately 5–10 min and removal of excess reactants through dialysis tubing with a cutoff at10K Da, successful preparationof IgP βwas achieved.
PDX/PDOX tumor of COAD
The human tumor tissues were obtained from The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China. Written informed consent was acquired from the patient. Tissues utilized in this study received approval from the committee for ethical review of research involving human subjects at Xi’an Jiaotong University. During the surgery to reduce the primary tumor, a specimen was carefully divided into approximately three pieces and promptly implanted into either the subserosa of the cecum or fossa iliaca of NSG female mice aged 4 to 5 weeks within two hours after resection. A total of 18 NSG female mice were employed in the PDX model, while 24 NSG female mice were used in the PDOX model. Experimental and control animals were bred separately. Once tumors reached a diameter of 1.5 cm, they were excised and passaged accordingly. The xenografts were meticulously collected for formalin-fixed-paraffin embedding (FFPE), rapidly frozen in liquid nitrogen, or subsequently implanted into another cohort of mice using identical procedures.
Statistical analysis & reproducibility
Data were analyzed and expressed as the Mean ± SD. Statistical differences were assessed using SPSS software and GraphPad Prism software, with *p < 0.05, **p < 0.01, and ***p < 0.001 considered to indicate significant differences. The results were replicated independently at least three times. Quantitative comparisons between samples on different gels/blots derived from the same experiment were processed simultaneously. No statistical method was used to determine sample size in advance, and no data were excluded from the analyses. The experiments were not randomized, and the investigators remained unblinded during both experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences are publicly accessible at https://ngdc.cncb.ac.cn/gsa/search?searchTerm=CRA017219. All the other data generated in this study are provided in the Supplementary Information/Source Data file. Source data are provided with this paper.
References
Krysko, D. V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12, 860–875 (2012).
Del Paggio, J. C. Cancer immunotherapy and the value of cure. Nat. Rev. Clin. Oncol. 15, 268–270 (2018).
Santoni, M., Montironi, R. & Battelli, N. Immune checkpoint blockade in advanced renal-cell carcinoma. N. Engl. J. Med. 379, 91–92 (2018).
Baumeister, S. H., Freeman, G. J., Dranoff, G. & Sharpe, A. H. Coinhibitory pathways in immunotherapy for cancer. Annu. Rev. Immunol. 34, 539–573 (2016).
Sharpe, A. H. & Pauken, K. E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 18, 153–167 (2018).
Yao, H. et al. Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nature Biomed. Eng. 3, 306–317 (2019).
Burr, M. L. et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature 549, 101–105 (2017).
Wang, H. et al. HIP1R targets PD-L1 to lysosomal degradation to alter T cell-mediated cytotoxicity. Nat. Chem. Biol. 15, 42–50 (2019).
Kornepati, A. V. R., Vadlamudi, R. K. & Curiel, T. J. Programmed death ligand 1 signals in cancer cells. Nat. Rev. Cancer 22, 174–189 (2022).
den Besten, W. & Lipford, J. R. Prospecting for molecular glues. Nat. Chem. Biol. 16, 1157–1158 (2020).
Banik, S. M. et al. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 584, 291–297 (2020).
Paiva, S.-L. & Crews, C. M. Targeted protein degradation: elements of PROTAC design. Curr. Opin. Chem. Biol. 50, 111–119 (2019).
He, W. et al. Turing milk into pro-apoptotic oral nanotherapeutic: De novo bionic chiral-peptide supramolecule for cancer targeted and immunological therapy. Theranostics 12, 2322–2334 (2022).
Yang, W., Liu, W., Li, X., Yan, J. & He, W. Turning chiral peptides into a racemic supraparticle to induce the self-degradation of MDM2. J. Adv. Res. 45, 59–71 (2023).
Yan, S. et al. A nano-predator of pathological MDMX construct by clearable supramolecular gold(I)-thiol-peptide complexes achieves safe and potent anti-tumor activity. Theranostics 11, 6833–6846 (2021).
Zheng, X. et al. De novo nano-erythrocyte structurally braced by biomimetic Au(I)-peptide skeleton for MDM2/MDMX predation toward augmented pulmonary adenocarcinoma immunotherapy. Small 17, e2100394 (2021).
Qi, J. et al. Semiconducting polymer nanoparticles with surface-mimicking protein secondary structure as lysosome-targeting chimaeras for self-synergistic cancer immunotherapy. Adv. Mater. 34, e2203309 (2022).
Kosorok, M. R. & Laber, E. B. Precision medicine. Ann. Rev. Stat. Appl. 6, 263–286 (2019).
Cardon, L. R. & Harris, T. Precision medicine, genomics and drug discovery. Hum. Mol. Genet. 25, R166–R172 (2016).
Lagassé, H. A. et al. Recent advances in (therapeutic protein) drug development. F1000Res 6, 113 (2017).
Burslem, G. M. et al. Towards “bionic” proteins: replacement of continuous sequences from HIF-1α with proteomimetics to create functional p300 binding HIF-1α mimics. Chem. Commun. (Camb.) 52, 5421–5424 (2016).
Kim, H. et al. Bioengineering strategies to generate artificial protein complexes. Biotechnol Bioeng 112, 1495–1505 (2015).
John, E. A., Massena, C. J. & Berryman, O. B. Helical anion foldamers in solution. Chem. Rev. 120, 2759–2782 (2020).
Gopalakrishnan, R., Frolov, A. I., Knerr, L., Drury, W. J. 3rd & Valeur, E. Therapeutic potential of foldamers: from chemical biology tools to drug candidates? J. Med. Chem. 59, 9599–9621 (2016).
Kent, S. B. Total chemical synthesis of proteins. Chem. Soc. Rev. 38, 338–351 (2009).
Chin, J. W. Reprogramming the genetic code. EMBO J. 30, 2312–2324 (2011).
Majeski, A. E. & Dice, J. F. Mechanisms of chaperone-mediated autophagy. Int. J. Biochem. Cell Biol. 36, 2435–2444 (2004).
He, W. et al. Self-assembly of therapeutic peptide into stimuli-responsive clustered nanohybrids for cancer-targeted therapy. Adv. Funct. Mater. 29, 1807736 (2019).
Chang, H.-N. et al. Blocking of the PD-1/PD-L1 interaction by a D-peptide antagonist for cancer immunotherapy. Angew. Chem. Int. Ed. 54, 11760–11764 (2015).
Jejurikar, B. L. & Rohane, S. H. Drug designing in discovery studio. Asian J. Res. Chem 14, 135–138 (2021).
Pawar, S. S. & Rohane, S. H. Review on discovery studio: An important tool for molecular docking. Asian J. Res. Chem 14, 86–88 (2021).
He, W. et al. Identification of amino acid residues critical for the B cell growth-promoting activity of HIV-1 matrix protein p17 variants. BBA-Gen. Subjects 1863, 13–24 (2019).
Yan, J., Ji, F., Yan, S., You, W. & He, W. A general-purpose nanohybrid fabricated by polymeric Au(I)-peptide precursor to wake the function of peptide therapeutics. Theranostics 10, 8513–8527 (2020).
Yan, J. et al. A hierarchical peptide–lanthanide framework to accurately redress intracellular carcinogenic protein–protein interaction. Nano Lett. 19, 7918–7926 (2019).
Yu, M. et al. Synthetic θ‐defensin antibacterial peptide as a highly efficient nonviral vector for redox‐responsive miRNA delivery. Adv. Biosyst. 1, 1700001 (2017).
Fan, X., Jin, W. Y., Lu, J., Wang, J. & Wang, Y. T. Rapid and reversible knockdown of endogenous proteins by peptide-directed lysosomal degradation. Nat. Neurosci. 17, 471–480 (2014).
Endicott, S. J., Boynton, D. N., Beckmann, L. J. & Miller, R. A. Long-lived mice with reduced growth hormone signaling have a constitutive upregulation of hepatic chaperone-mediated autophagy. Autophagy 17, 612–625 (2021).
Li, L., He, W., You, W., Yan, J. & Liu, W. Turing miRNA into infinite coordination supermolecule: a general and enabling nanoengineering strategy for resurrecting nuclear acid therapeutics. J. Nanobiotechnol. 20, 1–15 (2022).
He, W. et al. Resurrecting a p53 peptide activator - An enabling nanoengineering strategy for peptide therapeutics. J. Control. Release 325, 293–303 (2020).
Liu, J. et al. Biomimetic and self-assembled nanoclusters targeting beta-catenin for potent anticancer therapy and enhanced immunotherapy. Nano Lett. 19, 8708–8715 (2019).
Li, J. et al. Remodeling of the immune and stromal cell compartment by PD-1 blockade in mismatch repair-deficient colorectal cancer. Cancer Cell. 41, 1152–1169 (2023).
Llosa, N. J. et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 5, 43–51 (2015).
Chalabi, M. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 26, 566–576 (2020).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Overman, M. J. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 18, 1182–1191 (2017).
Altmann, K. H. et al. The state of the art of chemical biology. ChemBioChem 10, 16–29 (2009).
Yan, J. et al. Chiral protein supraparticles for tumor suppression and synergistic immunotherapy: an enabling strategy for bioactive supramolecular chirality construction. Nano Lett. 20, 5844–5852 (2020).
He, W. et al. Turning a Luffa protein into a self-assembled biodegradable nanoplatform for multitargeted cancer therapy. ACS Nano 12, 11664–11677 (2018).
Conibear, A. C. et al. Approaches to the stabilization of bioactive epitopes by grafting and peptide cyclization. Biopolymers 106, 89–100 (2016).
Bhardwaj, G. et al. Accurate de novo design of hyperstable constrained peptides. Nature 538, 329–335 (2016).
Acknowledgements
This work was supported by The National Key Research and Development Program of China (No. 2022YFE0133500), The National Natural Science Foundation of China (Nos. 82272782 and 32171256), Thousand Talents Plan of Shaanxi Province (For W.H.), “The Young Talent Support Plan” of Xi’an Jiaotong University (W.H.). We thank Instrument Analysis Center of Xi’an Jiaotong University for their assistance with TEM, DLS, FT-IR and XPS analysis. We also appreciate the help of proteomic analysis and Single cell RNA sequencing analysis from Tgene Biotech (Shanghai) Co., Ltd.
Author information
Authors and Affiliations
Contributions
D.L. and J.Y. designed the study and analyzed the data. D.L., F.M., J.W., and S.Y. performed the experiments. W.H. wrote the manuscript. J.Y. revised the manuscript. W.H. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Kwangmeyung Kim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, D., Yan, J., Ma, F. et al. Reinvigoration of cytotoxic T lymphocytes in microsatellite instability-high colon adenocarcinoma through lysosomal degradation of PD-L1. Nat Commun 15, 6922 (2024). https://doi.org/10.1038/s41467-024-51386-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51386-7
- Springer Nature Limited