Abstract
Regulatory T cells (Treg) are critical players of immune tolerance that develop in the thymus via two distinct developmental pathways involving CD25+Foxp3− and CD25−Foxp3lo precursors. However, the mechanisms regulating the recently identified Foxp3lo precursor pathway remain unclear. Here, we find that the membrane-bound lymphotoxin α1β2 (LTα1β2) heterocomplex is upregulated during Treg development upon TCR/CD28 and IL-2 stimulation. We show that Lta expression limits the maturational development of Treg from Foxp3lo precursors by regulating their proliferation, survival, and metabolic profile. Transgenic reporter mice and transcriptomic analyses further reveal that medullary thymic epithelial cells (mTEC) constitute an unexpected source of IL-4. We demonstrate that LTα1β2-lymphotoxin β receptor-mediated interactions with mTEC limit Treg development by down-regulating IL-4 expression in mTEC. Collectively, our findings identify the lymphotoxin axis as the first inhibitory checkpoint of thymic Treg development that fine-tunes the Foxp3lo Treg precursor pathway by limiting IL-4 availability.
Similar content being viewed by others
Introduction
Regulatory T cells (Treg) constitute a subset of CD4+ T cells that specifically express the transcription factor Foxp3 and CD25, also known as IL-2Rα. Naturally occurring CD25+Foxp3+ Treg critically prevent autoimmune and inflammatory disorders by maintaining self-tolerance through the suppression of autoreactive T cells that have escaped thymic selection. The majority of CD25+Foxp3+ Treg emerge in the thymus from 3 days after birth1. In particular, medullary thymic epithelial cells (mTEC) contribute to Treg development by expressing MHC class II and CD80/86 molecules as well as a broad range of self-antigens2,3,4. Treg development follows a “two-step” model in the thymic medulla5,6,7. The first step is driven by the stimulation of the T-cell receptor (TCR) and of CD28 in developing CD4+ single-positive (SP) thymocytes, leading to the generation of CD25+Foxp3− precursors (CD25+ TregP)5,6,8. TCR and CD28 signals govern the expression of tumor-necrosis factor receptor (TNFR) members, GITR and OX40, which promote CD25 expression9. The second step is driven by γ-chain cytokines, in particular IL-2 and IL-15, that convert CD25+ TregP into CD25+Foxp3+ mature Treg5. In addition to Foxp3 upregulation, IL-2 also modulates GITR and OX40 expression in mature Treg10. IL-2 was found to be provided by autoreactive CD4+ SP thymocytes11, whereas IL-15 is produced by mTEC12. More recently, a second CD25-Foxp3lo precursor (Foxp3lo TregP), lacking CD25 and expressing low levels of Foxp3, was identified13. Similarly to CD25+ TregP, this precursor has the ability to generate CD25+Foxp3+ mature Treg upon intrathymic transfer14,15. The differentiation of Foxp3lo TregP into mature Treg also follows the “two-step” model9,15. Although Foxp3lo TregP make a substantial contribution to the mature Treg pool, the mechanisms controlling this recently identified developmental pathway remain elusive. Peripheral Foxp3+ Treg were shown to express the membrane-bound lymphotoxin α1β2 (LTα1β2) heterocomplex, belonging to the TNF superfamily, which interacts with its unique cognate LTβ receptor (LTβR)16,17,18. However, it remains unknown whether LTα1β2 expression is induced in thymic Treg and whether it controls their generation.
Here, we show that LTα1β2 gradually increases during the “two-step” model of thymic Treg development. Its upregulation depends on TCR/CD28 stimulation in CD25+ and Foxp3lo TregP, and on IL-2 stimulation in CD25+Foxp3+ mature Treg. Interestingly, we observed an increased development of Foxp3lo TregP and mature Treg in Foxp3eGFPxLta−/− mice, indicating that Lta negatively regulates CD25+Foxp3+ Treg generation from Foxp3lo TregP. This phenotype was associated with an increased proliferation and survival of Foxp3lo TregP and mature Treg, as well as an altered metabolic profile. Strikingly, the maturational development of Foxp3lo TregP and CD25+Foxp3+ Treg increased in these mice. We show that IL-4 stimulation substantially promotes the conversion of Foxp3lo TregP into CD25+Foxp3+ mature Treg and that mTEC constitute an unexpected source of IL-4. We further demonstrate that the LTα1β2-LTβR axis negatively regulates the generation of CD25+Foxp3+ Treg by limiting IL-4 expression in mTEC. Altogether, this study reveals that LTα1β2-LTβR interactions with mTEC fine-tunes the Foxp3lo TregP developmental pathway in an IL-4-dependent manner.
Results
LTα1β2 expression is upregulated during thymic Treg development
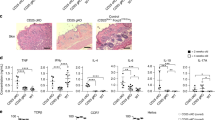
To characterize LTα1β2 expression during thymic Treg development, we used Rag2GFPxFoxp3Thy1.1 mice expressing the green fluorescent protein (GFP) and the membrane-bound Thy1.1 under the control of Rag2 and Foxp3 promoters, respectively19,20. These mice allowed us to discriminate newly generated thymocytes (Rag2GFP+) from more mature Treg that have been retained within the thymus or have recirculated from the periphery (Rag2GFP−)21,22,23. Among Rag2GFP+ thymocytes, we identified newly produced CD4+ SP thymocyte subsets: CD4+ Tconv (CD25−Foxp3−), CD25+ TregP (CD25+Foxp3−), Foxp3lo TregP (CD25−Foxp3lo) and mature Treg (CD25+Foxp3+) (Fig. 1a). The expression of the membrane-bound LTα1β2 heterocomplex, detected using the soluble LTβR-Fc receptor, was higher in CD25+ TregP and Foxp3lo TregP compared to CD4+ Tconv (Fig. 1b, c). This expression was even further increased in newly produced CD25+Foxp3+ Treg. In contrast, the soluble LTβR-Fc receptor did not bind to thymic Treg subsets from Lta−/− mice (Fig. 1b). We also used the differential expression of CCR6, which discriminates between newly produced Treg (CCR6−) and recirculating Treg (CCR6+) in the thymus4 and confirmed Lta and Ltb upregulation during Treg development using Foxp3eGFP reporter mice, in which an enhanced GFP reporter construct was introduced into the Foxp3 locus (Supplementary Fig. 1). Interestingly, the gradual upregulation of LTα1β2 expression during Treg development correlated with that of OX40 (Tnfrsf4) and GITR (Tnfrsf18) (Fig. 1d and Supplementary Fig. 1c). Furthermore, cells with the highest CD25 expression also had the highest Foxp3 expression in newly produced mature Treg and LTα1β2 level tightly correlated with CD25 and Foxp3 levels (Fig. 1e,f). These results show that LTα1β2 is gradually upregulated during Treg development in the thymus.
a Gating strategy used to analyze conventional CD4+ SP thymocytes (CD4+ Tconv, CD4+CD8−Rag2GFP+CD25−Foxp3−; black), CD25+ TregP (CD4+CD8−Rag2GFP+CD25+Foxp3−; blue), Foxp3lo TregP (CD4+CD8−Rag2GFP+CD25−Foxp3lo; green), and mature Treg (CD4+CD8−Rag2GFP+CD25+Foxp3+; red) in Rag2GFPxFoxp3Thy1.1 mice by flow cytometry. b Representative histograms and quantification of membrane-bound LTα1β2 expression, detected using the soluble LTβR-Fc receptor, in CD4+ Tconv, CD25+ TregP, Foxp3lo TregP, and mature Treg from Rag2GFPxFoxp3Thy1.1 mice and Lta−/− controls. c Geometric MFI (gMFI) of LTα1β2 expression in thymic Treg subsets (n = 10 from three independent experiments. d Correlation between LTα1β2 and OX40 or GITR expression in thymic Treg subsets (n = 9 from three independent experiments). e Gates represent the expression spectrum of CD25 and Foxp3 (left) in mature Treg that were analyzed for LTα1β2 expression (right). The gray histogram corresponds to mature Treg stained with only the secondary antibody. f Correlation between LTα1β2 and CD25 (left) or Foxp3 expression (right) in each gates (n = 10 from three independent experiments). Correlations were calculated using the parametric two-tailed Pearson correlation test for (d) and the non-parametric two-tailed Spearman correlation test for (f). Error bars show mean ± SEM, *p < 0.05, **p < 0.01 and ****p < 0.0001 using one-way ANOVA. Source data are provided as a Source Data File.
LTα1β2 is upregulated by instructive signals of the “two-step model” of Treg development
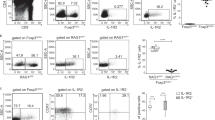
Since we observed a tight correlation between LTα1β2 and Foxp3 levels, we then asked whether LTα1β2 upregulation could be related to TCR stimulation. To address this question, we used the transcription factor Nur77 as a reliable readout of TCR signal strength24 and found a significant positive correlation between LTα1β2 and Nur77 in CD25+ TregP, Foxp3lo TregP and mature Treg (Fig. 2a and Supplementary Fig. 2a). We also analyzed LTα1β2 expression in Treg subsets stimulated in vitro with increasing doses of anti-CD3ε monoclonal antibody, which upregulated LTα1β2 expression in both CD25+ TregP and Foxp3lo TregP (Fig. 2b and Supplementary Fig. 2b). In contrast, CD3ε stimulation was unable to upregulate LTα1β2 expression in mature Treg. We next assessed the effect of CD28 costimulation on LTα1β2 expression. To this end, we used Cd28−/− mice, which showed decreased frequencies and numbers of CD25+ TregP, Foxp3lo TregP, and mature Treg (Supplementary Fig. 3a). Similarly to OX40 and GITR, LTα1β2 was reduced in these cells (Fig. 2c and Supplementary Fig. 3b,c), indicating that CD28 costimulation is implicated in LTα1β2 upregulation in Treg subsets.
a Correlation between LTα1β2 and Nur77 expression in CD25+ TregP (upper panel), Foxp3lo TregP (middle panel), and CD25+Foxp3+ mature Treg (lower panel) in CCR6− cells (n = 8 from two independent experiments). b Fold change in LTα1β2 expression measured in purified CCR6− CD25+ TregP, Foxp3lo TregP and mature Treg stimulated with 0.1 µg/mL (diamond, n = 11 for CD25+ TregP, n = 10 for Foxp3lo TregP and n = 9 for mature Treg), 1 µg/mL (triangle, n = 10 for CD25+ TregP, n = 10 for Foxp3lo TregP and n = 9 for mature Treg), 2.5 µg/mL (filled circle, n = 11 for CD25+ TregP, n = 9 for Foxp3lo TregP and n = 9 for mature Treg) or with 5 µg/mL anti-CD3 antibodies (open circle, n = 10 for CD25+ TregP, n = 7 for Foxp3lo TregP and n = 8 for mature Treg). Unstimulated cells (square, n = 11 for CD25+ TregP, n = 10 for Foxp3lo TregP, and n = 7 for mature Treg) were used as controls. Data are pooled from two independent experiments. c Representative histograms of LTα1β2 expression (left) and fold change (right) in CCR6− CD25+ TregP, Foxp3lo TregP, and mature Treg from WT (n = 6) and Cd28−/− (n = 5) mice. Data are pooled from two independent experiments. d, e Representative histograms (left) and quantification (right) of LTα1β2 expression levels in CD25+ TregP, Foxp3lo TregP and mature Treg from littermate controls (n = 6), Il15KOxRag2GFPxFoxp3Thy1.1 (Il15−/−) (n = 3) (d) or Il2KO xRag2GFPxFoxp3Thy1.1(Il2−/−) (n = 4) (e) mice. The gray histogram corresponds to mature Treg stained with only the secondary antibody. Data are pooled from two independent experiments. f Fold change in LTα1β2 expression in purified CCR6− CD25+ TregP, Foxp3lo TregP and mature Treg stimulated with 5 ng/mL (diamond, n = 7 for CD25+ TregP, n = 7 for Foxp3lo TregP and n = 7 for mature Treg), 20 ng/mL (triangle, n = 7 for CD25+ TregP, n = 7 for Foxp3lo TregP and n = 7 for mature Treg), 40 ng/mL IL-2 (circle, n = 7 for CD25+ TregP, n = 7 for Foxp3lo TregP and n = 7 for mature Treg) or unstimulated (square, n = 7 for CD25+ TregP, n = 6 for Foxp3lo TregP and n = 6 for mature Treg). Data are pooled from two independent experiments (n = 8). Correlations were calculated using the non-parametric two-tailed Spearman correlation test for (a). Error bars show mean ± SEM, *p < 0.05, **p < 0.01, and ***p < 0.001 using Kruskal–Wallis test for (b, f) and unpaired two-tailed Mann–Whitney U test for (c–e). Source data are provided as a Source Data File.
Given that IL-2 and IL-15 are crucial for the conversion of TregP into CD25+Foxp3+ mature Treg5,10,25, we hypothesized that these cytokines could also be implicated in LTα1β2 upregulation. To address their respective contribution, we used Il2KO or Il15KO mice backcrossed with Rag2GFPxFoxp3Thy1.1 mice to analyze newly generated Treg. In contrast to Il15KOxRag2GFPxFoxp3Thy1.1 (Il15−/−) mice (Fig. 2d), LTα1β2 expression was strongly altered in mature Treg from Il2KOxRag2GFPxFoxp3Thy1.1 (Il2−/−) mice (Fig. 2e). Because thymic IL-2 is important for Treg development and survival13,26, we next sought to demonstrate that IL-2 directly regulates LTα1β2 expression in mature Treg. To this end, purified CD25+ TregP, Foxp3lo TregP and mature Treg were stimulated in vitro with increasing doses of IL-2. Interestingly, LTα1β2 was upregulated in an IL-2 dose-dependent manner in mature Treg (Fig. 2f and Supplementary Fig. 2c). Altogether, these results show that LTα1β2 upregulation is initially induced by TCR/CD28 signals in TregP and further upregulated by IL-2 stimulation in mature Treg and thus, controlled by the signals of the “two-step model” of Treg development.
LTα1β2 limits Treg generation through the Foxp3lo TregP developmental pathway
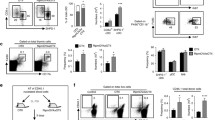
To test whether Lta could be implicated in Treg development, we backcrossed Lta−/− mice with Foxp3eGFP mice (Foxp3eGFPxLta−/− mice), in which membrane-bound LTα1β2 expression is fully abolished18. Remarkably, in contrast to CD25+ TregP, frequencies and numbers of Foxp3lo TregP and mature Treg were substantially higher in 6-week-old Foxp3eGFPxLta−/− mice compared to Foxp3eGFP controls (Fig. 3a, b). This phenotype was also observed in the thymus of 3-day-old neonates, indicating that Lta regulates the development of Foxp3lo TregP and mature Treg from their emergence (Fig. 3c, d). Moreover, 4-month-old adult Foxp3eGFPxLta−/− mice also showed an increased representation of these cells, suggesting that Lta controls Treg development throughout the lifespan. Since we previously reported an enhanced clonal deletion of CD4+ Tconv in Lta−/− mice27, we further analyzed the impact of Lta specifically on developing cells within the Treg lineage (Supplementary Fig. 4a). In accordance with the increased cellularity of Foxp3lo TregP and mature Treg (Fig. 3a–d), frequencies of these cells were enhanced in the Treg lineage of Foxp3eGFPxLta−/− mice (Supplementary Fig. 4a). In line with this phenotype, frequencies and numbers of Ki67+ proliferating cells were increased in Foxp3lo TregP and mature Treg subsets of Foxp3eGFPxLta−/− mice (Fig. 3e). Moreover, frequencies of annexin V+ were reduced in Foxp3lo TregP from Foxp3eGFPxLta−/− mice (Fig. 3f). Lower levels of the pro-apoptotic protein BIM, which is required for the apoptosis of autoreactive thymocytes28,29, were also observed in Foxp3lo TregP and mature Treg of these mice (Fig. 3g). In contrast, Ki67+ proliferating cells were slightly increased while annexin V+ cells and BIM levels were unchanged in CD25+ TregP (Supplementary Fig. 4c–e). Thus, the increase of Foxp3lo TregP and mature Treg in Foxp3eGFPxLta−/− mice likely relies on enhanced proliferation and survival.
a, b Representative flow cytometry profiles of CD25 and Foxp3 expression in CCR6−CD4+ SP thymocytes (a), frequencies and numbers (b) of CD25+ TregP, Foxp3lo TregP and mature Treg from the thymus of Foxp3eGFP (n = 13) and Foxp3eGFPxLta−/− (n = 12) mice. Data are pooled from four independent experiments. c, d Representative flow cytometry profiles, frequencies and numbers of CD25 and Foxp3 expression in CCR6−CD4+ SP thymocytes from 3 day- (n = 7 for Foxp3eGFP and n = 6 for Foxp3eGFPxLta−/−), 6 week- (n = 6 for Foxp3eGFP and n = 6 for Foxp3eGFPxLta−/−) and 4-month-(n = 5 for Foxp3eGFP and n = 4 for Foxp3eGFPxLta−/−) old mice. Data are pooled from 2 independent experiments. e Flow cytometry profiles, frequencies, and numbers of Ki67+ cells in CCR6− Foxp3lo TregP and mature Treg of Foxp3eGFP (n = 8) and Foxp3eGFPxLta−/− (n = 9) mice. Data are pooled from three independent experiments. f Representative annexin V staining and quantification in Foxp3lo TregP and mature Treg subsets from Foxp3eGFP (n = 8) and Foxp3eGFPxLta−/− (n = 8) mice. 7AAD+ dead cells were used as controls. The FMO is shown in Treg subsets from Foxp3eGFP mice. Data are pooled from two independent experiments. (g) BIM expression analyzed by flow cytometry in CCR6− Foxp3lo TregP and mature Treg subsets from Foxp3eGFP (n = 10) and Foxp3eGFPxLta−/− (n = 9) mice. The FMO is shown in Treg subsets from Foxp3eGFP mice. Histograms show the quantification of BIM gMFI. Data are pooled from three independent experiments. h-k t-SNE dimensional reduction of flow cytometry data using maturation markers (h) allowing the identification of Treg clusters in cells of the Treg lineage identified in Supplementary Fig. 4a (i), fluorescence intensity heatmap of the markers used for the t-SNE construction of each Treg cluster (j) and quantification of these clusters (k) in Foxp3eGFP (n = 6) and Foxp3eGFPxLta−/− (n = 4) mice. Data are pooled from two independent experiments. Error bars show mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 using unpaired two-tailed Student’s t test for (b), (e–f) and unpaired two-tailed Mann–Whitney U test for (d), (g), (k). Source data are provided as a Source Data File.
Given that cells of the Treg lineage are heterogeneous, we further analyzed their maturational state in Foxp3eGFPxLta−/− mice using a multiparametric flow cytometry analysis with developmental markers (Fig. 3h). We performed an unsupervised clustering that revealed a high maturational heterogeneity within the Treg lineage (Fig. 3i). Cell subsets were classified according to the relative expression of the maturational markers used (Fig. 3j). Subsets 1–5 corresponded to CD25+ TregP, subsets 6–8 to Foxp3lo TregP and subsets 9–10 to mature Treg (Fig. 3i, j). In Foxp3eGFPxLta−/− mice, CD25+ TregP subsets (clusters 1–4) were under-represented, while two immature Foxp3lo TregP subsets (clusters 6,7) were unchanged (Fig. 3k). In contrast, the Foxp3lo TregP cluster 8 expressing high levels of H2Kb and Qa2 and low levels of CD69 and CD24, likely corresponding to more mature transitional Foxp3lo TregP, was over-represented in Foxp3eGFPxLta−/− mice. Strikingly, we found an increased representation of mature Treg subsets (clusters 9, 10) in Foxp3eGFPxLta−/− mice. These results reveal a bias in the Treg maturational development via Foxp3lo TregP in Foxp3eGFPxLta−/− mice.
Because the LTα1β2-LTβR axis controls thymic medulla organization17,30, we analyzed the contribution of non-hematopoietic cells in Treg development by generating bone marrow (BM) chimeras in which lethally irradiated CD45.2 WT or Lta−/− recipients were reconstituted with CD45.1 Foxp3eGFP BM cells (Supplementary Fig. 5a). Six weeks later, similar frequencies and numbers of CD25+ TregP, Foxp3lo TregP, and mature Treg were observed in the thymus of WT and Lta−/− recipients (Supplementary Fig. 5b), indicating that the stromal environment of Lta−/− mice is unlikely implicated in the enhanced Treg development. Given that Lta is expressed by hematopoietic cells17, we next wondered whether a partial reintroducion of WT BM cells with Lta−/− BM cells could be sufficient to reverse the phenotype observed in Lta−/− mice. To this end, we generated mixed BM chimeras in which lethally irradiated CD45.1/2 WT recipients were reconstituted with BM cells from either CD45.1 Foxp3eGFP or Foxp3eGFPxLta−/− mice in competition with CD45.2 WT BM cells (Fig. 4a). Six weeks later, as opposed to CD25+ TregP, we found increased frequencies of Foxp3lo TregP and mature Treg in Foxp3eGFPxLta−/− + WT → WT chimeras (Fig. 4b). Moreover, we observed enhanced frequencies of Foxp3lo TregP and mature Treg by specifically analyzing cells of the Treg lineage (Fig. 4c). In line with these observations, we found slightly decreased frequencies of Ki67+ CD25+ TregP while Foxp3lo TregP proliferation increased (Fig. 4d). We next analyzed the maturational state of cells from the Treg lineage using a multiparametric flow cytometry analysis (Fig. 4e). We performed an unsupervised clustering that revealed that CD25+ TregP subsets (clusters 2, 5 and 6) were under-represented while clusters 7 and 8 corresponding to Foxp3lo TregP and clusters 10 corresponding to mature Treg were increased in Foxp3eGFPxLta−/− + WT → WT chimeras (Fig. 4f–h). Altogether, these data confirm that Lta expression in thymocytes negatively regulates the Foxp3lo TregP developmental pathway, which could not be rescued by the reintroduction of WT cells.
a Experimental set up: lethally irradiated CD45.1/2 WT recipients were reconstituted with CD45.2 WT + CD45.1 Foxp3eGFP or Foxp3eGFPxLta−/− (ratio 1:1) mixed BM cells. Six weeks later, CD45.1 CCR6− Treg were analyzed in the thymus of recipient mice. b Flow cytometry profiles and frequencies of CD25+ TregP, Foxp3lo TregP and mature Treg from CD45.1 Foxp3eGFP (n = 12) or Foxp3eGFPxLta−/− (n = 12) BM cells. Data are pooled from four independent experiments. c Representative flow cytometry profiles and frequencies of CD25+ TregP, Foxp3lo TregP, and mature Treg in the Treg cell lineage gate derived from CD45.1 Foxp3eGFP (n = 6) or Foxp3eGFPxLta−/− (n = 7) BM cells. Data are pooled from two independent experiments. d Frequencies of Ki67+ cells in CD25+ TregP (n = 9 for Foxp3eGFP and n = 10 for Foxp3eGFPxLta−/−), Foxp3lo TregP (n = 10) and mature Treg (n = 10) derived from CD45.1 Foxp3eGFP or Foxp3eGFPxLta−/− BM cells. Data are pooled from three independent experiments. e-h t-SNE dimensional reduction of flow cytometry data using maturation markers (e) allowing the identification of Treg clusters (f), fluorescence intensity heatmap of the markers used for the t-SNE construction of each Treg cluster (g), and quantification of these clusters (h) in cells derived from CD45.1 Foxp3eGFP (n = 4) or Foxp3eGFPxLta−/− (n = 5) BM cells. Data are pooled from two independent experiments. Error bars show mean ± SEM, *p < 0.05, **p < 0.01, and ****p < 0.0001 using unpaired two-tailed Student’s t test for (b) and unpaired two-tailed Mann–Whitney U test for (c), (d) and (h). Source data are provided as a Source Data File.
Lta expression controls the metabolic profile of Treg during their thymic development
To define the impact of Lta on Treg development, we performed 3′-end single-cell RNA sequencing (scRNA-seq) on purified Treg lineage cells from Foxp3eGFP and Foxp3eGFPxLta−/− mice (Fig. 5a). Our analysis revealed that these cells separated into five distinct clusters (Fig. 5b, c). Ccr7 expression was detected in all clusters, indicating that both TregP and mature Treg are located in the thymic medulla (Fig. 5d and Supplementary Fig. 6a). Clusters 0 and 1 exhibited an immature phenotype, characterized by the expression of Cd24a and Cd69, with no or poor Foxp3, Sell (CD62L) and S1pr1 expression. While cluster 0 expressed Il2rb (CD122), Il2rg (CD132) and Tnfrsf18 (GITR), cluster 1 also expressed Il2ra (CD25). This indicates that cluster 0 and 1 corresponded to Treg pre-precursors (CD122+CD25−Foxp3−)31 and CD25+ TregP, respectively. Cluster 2 expressed low levels of Foxp3 and Il2ra in addition to high levels of TCR-signaling associated genes (Nr4a1, Nr4a3, and Tnfrsf4) (Fig. 5c, d), suggesting a transitional state towards mature Treg (transitional Treg). Cluster 3 expressed low levels of Foxp3 while Il2ra was not detected, suggesting that cells from this cluster contained Foxp3lo TregP. All cells from cluster 3 expressed H2-K1 (H2Kb) while a fraction expressed Cd69 (Fig. 5d and Supplementary Fig. 6a). In line with this observation, Foxp3lo TregP contained mature CD69+H2Kb+ (M1) and CD69−H2Kb+ (M2) cells while CD25+ TregP contained semi-mature CD69+H2Kb− (SM), M1 and M2 cells (Supplementary Fig. 6b), indicating that Foxp3lo TregP have a more mature phenotype than CD25+ TregP. Accordingly, Foxp3lo TregP resemble mature Treg based on the differential expression of CD69 and H2Kb. Furthermore, we cannot exclude that cluster 3 included mature cells that could exit the thymus since high levels of Sell, Klf2, and S1pr1 were detected. In line with this observation, we found that Foxp3lo TregP expressed high level of CD62L by flow cytometry (Supplementary Fig. 6c). Cluster 4 expressed the highest levels of Foxp3, Il2ra, Il2rb, and Il2rg, suggesting that this cluster corresponded to CD25+Foxp3+ mature Treg (Fig. 5d and Supplementary Fig. 6a), consistently with a high expression of Sell, Klf2 and S1pr1.
a Gating strategy used to purify Treg lineage cells from Foxp3eGFP and Foxp3eGFPxLta−/− mice. b Merged UMAP visualization of Treg lineage cells colored by cell types from Foxp3eGFP and Foxp3eGFPxLta−/− mice. c Heatmap showing the expression level of the top 5 differentially expressed genes (adj-p-val<0.001) in each cluster. d Density scores of Treg markers, Foxp3, Il2ra, Il2rb and Il2rg, as well as maturation markers, Ccr7, Cd24a, Cd69, Tnfrsf18, H2-K1, Sell, Klf2 and S1pr1 on merged data from Foxp3eGFP and Foxp3eGFPxLta−/− mice. Scores were computed using the Nebulosa package in R. e UMAP visualization of Treg lineage cells from Foxp3eGFP (1433 cells) and Foxp3eGFPxLta−/− (1342 cells) mice. f Module score distributions per condition and cluster for various metabolic pathways. g Density scores of glycolysis-associated genes (Ldha, Pgk1, and Tpi1) and OXPHOS-associated genes (Ndufa4, Ndufc2, and Atp5b) on merged data from Foxp3eGFP and Foxp3eGFPxLta−/− mice. Scores were computed using the Nebulosa package in R. Statistical comparison of module scores between conditions for each cluster was performed using the Wilcoxon test with Benjamini-Hochberg multitest correction. Error bars show mean ± SD, *p < 0.05, **p < 0.01. Source data are provided as a Source Data File.
We next performed trajectory analysis using the Monocle 3 package32 to assess the developmental trajectory within the Treg lineage. Three major branches were identified, starting at roots Y1, Y30, and Y2 all of which converged to Y16 located in cluster 4 corresponding to mature Treg. (Supplementary Fig. 7a). The branch from Y1 to Y16 indicated a potential conversion of cells from cluster 0, to cluster 1, then to cluster 2, and finally to cluster 4. The Y30 to Y16 branch revealed another possible developmental route from cluster 0 directly to cluster 4. The branch from Y2 to Y16 indicated a short transition from cluster 3 to cluster 4. In line with the Seurat analysis (Fig. 5b–d), the progressive upregulation of Foxp3 and Il2ra was associated with an upregulation of genes related to terminal maturation (H2-K1, Sell and S1pr1) along the pseudotime axis (Supplementary Fig. 7b).
We then compared changes in distinct cellular pathways in the different clusters of Foxp3eGFP and Foxp3eGFPxLta−/− mice to analyze the impact of Lta on Treg development (Fig. 5e). We calculated module scores of gene sets taken from the KEGG database and found metabolic pathways substantially altered by Lta deficiency. Although metabolic reprogramming regulates Treg function in the periphery33,34, the metabolic profile of thymic Treg remains unknown. Our analysis revealed metabolic changes associated to Treg development (Fig. 5f and Supplementary Fig. 7c). Interestingly, the module score for the oxidative phosphorylation (OXPHOS) gene set increased from cluster 0 (CD122+CD25−Foxp3− pre-TregP) to cluster 1 (CD25+ TregP), then decreased in cluster 2 (Transitional Treg) and further decreased in cluster 4 (Mature Treg). We also found the module scores for glycolysis/gluconeogenesis, biosynthesis, and nucleotide metabolic pathways to be higher in cluster 1 compared to all other clusters, suggesting that CD25+ TregP have a high metabolic activity. In line with these observations, cluster 1 highly expressed both OXPHOS (Ndufa4, Ndufc2, and Atp5b) and glycolysis (Ldha, Pgk1, and Tpi1) hallmark genes (Fig. 5g), while clusters 3 and 4 expressed mainly OXPHOS hallmark genes. On the other hand, there is likely no implication of the fatty acid metabolism during Treg development suggested by the module score for this pathway (Fig. 5f). Compared to Foxp3eGFP mice, clusters 2, 3 and 4 of Foxp3eGFPxLta−/− mice showed an enhanced score for OXPHOS metabolism, indicating that Lta expression regulates the metabolic profile of the most mature Treg subsets (Fig. 5f and Supplementary Fig. 7d).
Given that scRNA-seq data suggest gene expression changes of OXPHOS-related genes during Treg development (Fig. 5f), we then analyzed mitochondrial activity. In line with the transcriptomic analysis, CD25+ TregP displayed higher mitochondrial activity compared to Foxp3lo TregP and mature Treg, as shown by MitoTracker Deep Red staining (Fig. 6a). CD25+ TregP and Foxp3lo TregP of Foxp3eGFPxLta−/− mice showed fewer active mitochondria compared to their respective control counterparts (Fig. 6b). Since this functional defect in mitochondrial polarization was unforeseen in scRNA-seq data, we further analyzed the metabolic profile of developing Treg using a functional assay. Given that low numbers of TregP can be retrieved from the thymus, we took advantage of the newly developed flow-cytometry-based method, SCENITH™ (Single-Cell Metabolism by Profiling Translation Inhibition)35, to analyze the metabolic profiles of TregP and mature Treg. This method is based on metabolism-dependent translation rates and puromycin incorporation into nascent proteins in response to specific metabolic inhibitors. Thus, SCENITH™ permits the assessment of glucose dependency, mitochondrial dependency, glycolytic capacity as well as fatty acid and amino acid oxidation capacity in multiple rare cell types simultaneously. In line with our transcriptomic (Fig. 5f) and MitoTracker analysis (Fig. 6a), CD25+ TregP showed higher levels of protein synthesis than Foxp3lo TregP and mature Treg (Supplementary Fig. 8a), further confirming that CD25+ TregP are more metabolically active. Strikingly, CD25+ TregP and Foxp3lo TregP showed a distinct metabolic profile (Fig. 6c and Supplementary Fig. 8b), with Foxp3lo TregP being more similar to mature Treg. Specifically, CD25+ TregP showed 50% dependency on both glucose and mitochondrial OXPHOS, while Foxp3lo TregP showed 85% mitochondrial dependency similar to the 75% mitochondrial dependency of mature Treg (Fig. 6c). These results are in accordance with the OXPHOS and glycolysis/gluconeogenesis module scores calculated using our scRNA-seq analysis at steady state (Fig. 5f). Interestingly, we found that Foxp3lo TregP and mature Treg from Foxp3eGFPxLta−/− mice exhibited an exacerbated glucose dependency (Fig. 6d and Supplementary Fig. 8c, d).
a, b Representative histogram and fold change in the gMFI of MitoTracker Deep Red (MDR) staining in CCR6− CD25+ TregP, Foxp3lo TregP and mature Treg from Foxp3eGFP (n = 9) mice (a) compared to Foxp3eGFPxLta−/− (n = 9) mice (b). FMO are shown in Foxp3eGFP Treg subsets. Data are pooled from two independent experiments. c, d SCENITH metabolic profiling of CD25+ TregP, Foxp3lo TregP, and mature Treg from Foxp3eGFP (n = 9) (c) and Foxp3eGFPxLta−/− (n = 8) (d) mice. Graphs show the percentage of glucose dependence, mitochondrial dependence, glycolytic capacity as well as fatty acid and amino acid (FAO and AAO) capacity of each cell subset. Data are pooled from three independent experiments. e The table summarizes the phenotype of semi-mature (SM), mature 1 (M1) and mature 2 (M2) CD4+ SP thymocytes. f, g SCENITH metabolic profiling depending on maturational state of CD25+ TregP, Foxp3lo TregP, and mature Treg from Foxp3eGFP (n = 9) and Foxp3eGFPxLta−/− (n = 6) mice. Data are pooled from three independent experiments. Error bars show mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 using one-way ANOVA for (a), (c) and (f), unpaired two-tailed Student’s t test for (b) and (d), unpaired two-tailed Mann–Whitney U test for (g). Source data are provided as a Source Data File.
Next, we further explored the metabolic profiles of the distinct maturational stages of TregP and mature Treg cells using CD69 and H2Kb developmental markers (Fig. 6e)36. In wild-type conditions, the mitochondrial dependence increased while the glycolytic capacity decreased during CD25+ TregP maturation (Fig. 6f). We further found a distinct metabolic profile for the M1 subset within CD25+ and Foxp3lo TregP, whereby M1 Foxp3lo TregP exhibited a higher mitochondrial dependence. Notably, CD25+ and Foxp3lo TregP M2 subsets had a similar metabolic profile compared to M1 and M2 mature Treg whose profile was characterized by a high mitochondrial dependency and a low glycolytic capacity. A similar profile was also observed in the Foxp3lo TregP M1 subset. These results suggest that the increase in mitochondrial dependency is tightly linked to Treg maturation. In Foxp3eGFPxLta−/− mice, CD25+ TregP SM cells showed an increased mitochondrial dependence while the M2 cells exhibited an increased glucose dependence (Fig. 6g). Moreover, the glucose dependence was also increased in Foxp3lo TregP and mature Treg at M1 and M2 stages. Thus, Foxp3lo TregP display a similar metabolic profile to the most mature Treg (M2) and Lta expression negatively regulates their glucose dependency. Overall, these findings show that LTα modulates the metabolic profile of Treg along their maturational development.
LTα1β2-regulated Treg development depends on IL-4 production by mTEC
We then investigated the underlying mechanism of LTα1β2-LTβR-regulated Treg development. We made the hypothesis that the LTα1β2-LTβR axis could regulate the expression of cytokines. Consistent with a previous report15, we found that Il4ra was preferentially expressed in Foxp3lo TregP compared to CD25+ TregP, and its expression was further upregulated in mature Treg at both mRNA and protein levels (Fig. 7a, b). In contrast, cells of the Treg lineage did not produce IL-4, ruling out an autocrine stimulation (Fig. 7a). This expression pattern suggests that IL-4 may support the conversion of Foxp3lo TregP into CD25+Foxp3+ mature Treg. To test this hypothesis, purified CD25+ TregP and Foxp3lo TregP were stimulated with increasing doses of IL-4. In contrast to CD25+ TregP, IL-4 stimulation promoted the robust conversion of Foxp3lo TregP into CD25+Foxp3+ mature Treg (Fig. 7c and Supplementary Fig. 9). Interestingly, we found increased Il4 expression in the thymus of Lta−/− mice, which was associated with enhanced development of IL-4-dependent Eomes+ memory-like CD8+ SP thymocytes37,38 (Fig. 7d, e). Furthermore, frequencies and numbers of phospho-Stat6+ cells were substantially enhanced, further highlighting an IL-4-rich environment in the thymus of Lta−/− mice (Supplementary Fig. 10a). Among these cells, frequencies of phospho-Stat6+ mature Treg were increased in Lta−/− mice (Supplementary Fig. 10b), suggesting that enhanced IL-4 expression affects mature Treg. In line with enhanced Foxp3lo TregP and mature Treg in Foxp3eGFPxLta−/− + WT → WT chimeras (Fig. 4), Il4 expression was higher in the thymus of those recipients, which was associated with increased frequencies of phospho-Stat6+ thymocytes (Fig. 7f and Supplementary Fig. 10c). Moreover, in line with the reintroduction of WT BM cells in these chimeras, IL-4 and phospho-Stat6 levels increased by only half of those observed in Lta−/− mice. This partial increase in Il4 expression was sufficient to enhance the frequencies of Foxp3lo TregP and mature Treg of CD45.2 WT origin (Fig. 7g). This indicates that the Foxp3lo TregP developmental pathway is highly sensitive to subtle variations in IL-4 levels. To investigate the role of IL-4 in vivo in Treg development, Lta−/− pups were injected with an anti-IL-4 neutralizing antibody during the emergence of TregP at post-natal day 0 and 3 (Fig. 7h). Five days after birth, Eomes+ memory-like CD8+ SP thymocytes were reduced, showing the effectiveness of IL-4 neutralization (Fig. 7i). Interestingly, frequencies and numbers of Foxp3lo TregP and CD25+Foxp3+ mature subsets were substantially reduced while CD25+ TregP were over-represented (Fig. 7j). These results suggest that IL-4 favors the Foxp3lo TregP developmental pathway at the expense of the CD25+ TregP pathway.
a UMAP visualization of Treg lineage cells colored by cell types (left), feature, and density UMAP representation of Il4ra (middle) and Il4 (right) expression from Foxp3eGFP mice. b Representative histogram and fold change in the gMFI of IL-4Rα in CD25+ TregP, Foxp3lo TregP, and mature Treg identified in CCR6−CD4+ SP thymocytes from Foxp3eGFP mice (n = 10). The FMO is shown in the CD25+Foxp3eGFP Treg subset. Data are pooled from three independent experiments. c Frequencies of CD25+Foxp3+ mature Treg generated from purified CD25+ TregP or Foxp3lo TregP stimulated with 0.5 ng/mL (diamond, n = 5), 1.0 ng/mL (triangle, n = 5), 100 ng/mL of IL-4 (circle, n = 5) or unstimulated (square, n = 5). Data are pooled from two independent experiments. d Relative expression of Il4 in the thymus of WT (n = 5) and Lta−/− (n = 8) mice. Data are pooled from two independent experiments. e Flow cytometry profiles and frequencies of Eomes+ CD8+ SP thymocytes from WT (n = 12) or Lta−/− (n = 12) mice. Data are pooled from two independent experiments. f Relative expression of Il4 in the thymus of mixed BM chimeras (Foxp3eGFP+WT mice, n = 8 and Foxp3eGFPxLta−/−+WT mice, n = 8). Data are pooled from two independent experiments. g Frequencies of CCR6− CD25+ TregP, Foxp3lo TregP, and mature Treg derived from CD45.2 BM cells (Foxp3eGFP+WT mice; n = 12 and Foxp3eGFPxLta−/−+WT mice; n = 14). Data are pooled from three independent experiments. h Experimental set-up: Lta−/− mice were injected i.p. with neutralizing anti-IL4 antibody or isotype control at postnatal day (PND) 0 and PND3. Pups were sacrificed at PND5. i, j Representative flow cytometry profiles, frequencies, and numbers of Eomes+ CD8+ SP thymocytes (i) and CCR6−CD4+ CD25+ TregP, Foxp3lo TregP, and mature Treg (j) from the thymus of Lta−/− pups injected with an isotype control (n = 5) or a neutralizing anti-IL4 antibody (n = 4). Data are pooled from two independent experiments. Error bars show mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 using one-way ANOVA for (b), Kruskal–Wallis test for (c), unpaired two-tailed Mann–Whitney U test for (d) and (i, j) and unpaired two-tailed Student’s t test for (e–g). Source data are provided as a Source Data File.
Although type 2 natural killer T cells (NKT2) are an important source of IL-4 in the thymus37,38,39, increased expression of Il4 in Lta−/− mice was unlikely due to NKT2 cell enrichment (Supplementary Fig. 10d), suggesting another cellular source of IL-4. We therefore explored whether other thymic cells may express Il4. Using a scRNA-seq dataset40, we found that Il4 was not expressed by thymic DC and macrophages (Fig. 8a). We next investigated the possibility that mTEC could produce this cytokine and found that IL-4 was mainly expressed by Aire+ mTEChi and late-Aire mTEC in scRNA-seq datasets (Fig. 8b). We confirmed by qPCR that the level of Il4 was higher in mTEC expressing high levels of the CD80 costimulatory molecule (mTEChi) than in mTEC expressing low levels of CD80 (mTEClo) and that the level in mTEChi was similar to that observed in NKT cells (Fig. 8c). Using IL-4 reporter mice on a C57BL/6 or BALB/c background, IL-4 expression was detected both in NKT cells and mTEC (Fig. 8d and Supplementary Fig. 10e), confirming that mTEC constitute a substantial source of IL-4 in the thymus. Given that the classical NF-κB member p65 (RelA) was reported to upregulate Il4 expression by binding to its promoter41, we assessed its expression in mTEChi of Lta−/− mice and found enhanced protein levels of p65 (Supplementary Fig. 10f). This suggests that the increased expression of p65 may lead to elevated Il4 expression in mTEChi of Lta−/− mice.
a UMAP visualization of DC and macrophage subsets colored by cell types (left). Density and feature UMAP representation of Il4 expression in DC and macrophage subsets (right). b UMAP visualization of TEC subsets colored by cell types (left). Il4 expression projected on UMAP visualization of TEC subsets (right). c Relative expression of Il4 in thymic NKT (n = 6), mTEClo (n = 9), and mTEChi (n = 9) from WT mice. Data are pooled from two independent experiments. d Confocal images of the thymus of 4get C57BL/6 mice showing IL-4eGFP (green), keratin 14 (red), and NK1.1 (blue) staining. Scale bar, 20 µm. Image representative of 3 mice. Error bars show mean ± SEM, **p < 0.01 using Kruskal–Wallis test for (c). Source data are provided as a Source Data File.
Given that mTEC control the generation of CD25+Foxp3+ Treg2,3,42,43,44, we explored the expression of LTβR within mTEC subsets by reanalysing TEC scRNA-seq datasets45,46. Ltbr was widely expressed in mTEC subsets (Supplementary Fig. 11a). We confirmed by flow cytometry this wide expression in mTEClo and mTEChi (Supplementary Fig. 11b,c). Next, we analyzed the TEC scRNA-seq with our thymic Treg scRNA-seq datasets to identify possible cell-cell interactions via the LTα1β2-LTβR axis using CellChat47. CD25+ TregP and Foxp3lo TregP likely interact with several mTEC subsets (Supplementary Fig. 11d). These interactions seemed to be stronger with transitional and mature Treg, consistent with the gradual increase of LTα1β2 expression during Treg development (Fig. 1c and Supplementary Fig. 1c).
Next, to determine the contribution of mTEC in LTα1β2-LTβR-regulated Treg development, we used OTII transgenic mice that possess CD4+ SP thymocytes expressing an MHCII-restricted TCR specific for the chicken ovalbumin (OVA). First, we verified that OTII cells transit through the CD25+ and Foxp3lo TregP developmental pathways. To this end, we took advantage of RipmOVAxOTIIxRag2−/− mice in which the Rat Insulin Promoter (Rip) drives the expression of the membrane-bound OVA (mOVA) form specifically in mTEC. In these transgenic mice, high-affinity interactions between OVA-expressing mTEC and OTII thymocytes are possible in vivo. Whereas Foxp3lo TregP and CD25+Foxp3+ mature Treg were undetectable in OTIIxRag2−/− mice, these two Treg subsets developed in RipmOVAxOTIIxRag2−/− mice, indicating that OTII thymocytes transit through Foxp3lo TregP toward mature Treg when the cognate self-antigen is expressed in mTEC (Fig. 9a). We then performed in vitro antigen-specific Treg conversion assays in which WT mTEC loaded with the OVA323-339 peptide were co-cultured with CD69+ semi-mature OTII CD4+ SP thymocytes, that expressed the membrane-bound LTα1β2 and were shown to contain cells with a high ability to differentiate into mature Treg42 (Fig. 9b, c). After 18 h of co-culture, mTEC presenting the cognate antigen had the capacity to induce Foxp3lo TregP, further confirming that OTII thymocytes transit via a Foxp3lo TregP stage (Fig. 9d). To further investigate the role of Lta, we performed this experiment with CD69+CD4+ SP thymocytes purified from OTII mice backcrossed on an Lta−/− background (OTIIxLta−/− mice). Interestingly, the conversion in Foxp3lo TregP increased while that of CD25+ TregP decreased with CD69+ CD4+ from OTIIxLta−/− mice compared to OTII mice. Moreover, blocking LTα1β2-LTβR interactions using the soluble LTβR-Fc receptor, also resulted in an increased Foxp3lo TregP conversion and a decreased CD25+ TregP conversion (Fig. 9e, f), indicating that the lymphotoxin axis limits the Foxp3lo TregP developmental pathway in favor of the CD25+ TregP pathway. As previously reported42, mTEC had the capacity to induce antigen-specific CD25+Foxp3+ mature Treg after 5 days of co-culture (Fig. 9g). We found a higher conversion in CD25+Foxp3+ mature Treg when OVA323-339-loaded mTEC were co-cultured with CD69+CD4+ SP thymocytes from OTIIxLta−/− mice compared to OTII mice. A similar enhanced Treg generation was observed irrespective of the mTEC maturation state (Fig. 9h). Moreover, blocking LTα1β2-LTβR interactions, also resulted in an increased mature Treg conversion after 5 days of co-culture (Fig. 9i). These results show that LTα1β2-LTβR interactions with mTEC act as a negative regulator of CD25+Foxp3+ mature Treg conversion via the Foxp3lo TregP developmental pathway. To further define whether the LTα1β2-LTβR axis controls the conversion of Foxp3lo TregP into CD25+Foxp3+ mature Treg, we backcrossed RipmOVAxOTIIxRag2−/− mice with the Foxp3eGFP reporter mice, which allowed us to purified both TregP. We performed antigen-specific co-culture assays using OVA323-339-loaded mTEC with CD25+ or Foxp3lo TregP purified from RipmOVAxOTIIxFoxp3eGFP mice and treated them or not with the soluble LTβR-Fc receptor (Fig. 9j and Supplementary Fig. 12). The addition in the co-culture of LTβR-Fc receptor caused a greater conversion into CD25+Foxp3+ mature Treg by Foxp3lo TregP, but not by CD25+ TregP (Fig. 9k, l). Altogether, these data reveal that the LTα1β2-LTβR axis acts as a feedback mechanism by which TregP can limit their own differentiation.
a Representative flow cytometry profiles, frequencies, and numbers of thymic CCR6−CD4+ CD25+ TregP, Foxp3lo TregP and mature Treg from OTIIxRag2−/− and RipmOVAxOTIIxRag2−/− mice (n = 5 from two independent experiments). b Treg conversion assay: OVA323-339-loaded mTEC were co-cultured with CD69+ semi-mature CD4+ thymocytes from OTII or OTIIxLta−/− mice. c LTα1β2 expression in CD69+ OTII CD4+ thymocytes compared to control (II ab). d Flow cytometry profiles and fold change in the frequencies of CD25+ and Foxp3lo TregP derived from CD69+ OTII or OTIIxLta−/− CD4+ thymocytes after 18 h of co-culture (n = 16 from two independent experiments). e Treg conversion assay: OVA323-339-loaded mTEC were co-cultured with CD69+ semi-mature CD4+ thymocytes pretreated or not with the blocking LTβR-Fc receptor. f Flow cytometry profiles and fold change in the frequencies of CD25+ and Foxp3lo TregP derived from CD69+ OTII CD4+ thymocytes pretreated or not with the blocking LTβR-Fc receptor after 18 h of co-culture (n = 9 from two independent experiments). g, h Flow cytometry profiles and fold change in the frequencies of CD25+Foxp3+ mature Treg derived from CD69+ OTII (n = 10) or OTIIxLta−/− (n = 9) CD4+ thymocytes co-cultured with OVA323-339-loaded mTEC (g), mTEClo or mTEChi (n = 5 for OTII and OTIIxLta−/−) (h) after 5 days. Data are pooled from three (g) and two (h) independent experiments. i Flow cytometry profiles and fold change in the frequencies of CD25+Foxp3+ mature Treg derived from CD69+ OTII CD4+ thymocytes pretreated (n = 7) or not (n = 8) with the blocking LTβR-Fc receptor after 5 days of co-culture. Data are pooled from two independent experiments. j Treg conversion assay: OVA323-339-loaded mTEC were co-cultured with CD25+ TregP or Foxp3lo TregP from RipmOVAxOTIIxFoxp3eGFP mice. TregP were pretreated or not with the blocking LTβR-Fc receptor. k, l Flow cytometry profiles (k) and fold change in the frequencies of mature Treg (l) derived from the conversion of pretreated or not CD25+ TregP or Foxp3lo TregP after 72 h or 24 h of co-culture, respectively (n = 7 from two independent experiments). m Relative expression of Il4 and Rela in co-cultures of OVA323-339-loaded mTEC and CD69+ semi-mature OTII or OTIIxLta−/− CD4+ thymocytes (n = 9 from two independent experiments). n Fold change in the frequencies of CD25+Foxp3+ mature Treg derived from CD69+ OTII or OTIIxLta−/− CD4+ thymocytes co-cultured with OVA323-339-loaded mTEC with (n = 6) or without (n = 10) a neutralizing anti-IL4 antibody. Data are pooled from two independent experiments. Error bars show mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 using unpaired two-tailed Mann–Whitney U test for (a), (g–i) and (l), unpaired two-tailed Student’s t test for (d), (f) and (m) and Kruskal–Wallis test for (n). Source data are provided as a Source Data File.
We next evaluated whether the LTα1β2-LTβR axis regulates Il4 expression in mTEC in these co-culture assays. Interestingly, we found higher levels of Il4 in OVA323-339-loaded mTEC co-cultured with CD69+CD4+ SP thymocytes from OTIIxLta−/− compared to OTII mice, demonstrating that Lta negatively regulates the expression of Il4 in mTEC (Fig. 9m). Consistently, Rela was also higher in co-cultures with CD69+CD4+ SP thymocytes from OTIIxLta−/− mice. To test the hypothesis that Il4 overexpression observed in the absence of Lta may be responsible for the enhanced conversion of CD69+CD4+ SP thymocytes into mature Treg (Fig. 9g), we used a neutralizing anti-IL-4 antibody in these co-culture assays. Strikingly, the increased conversion of CD69+CD4+ SP thymocytes from OTIIxLta−/− mice into CD25+Foxp3+ Treg was fully abolished by IL-4 neutralization (Fig. 9n). Overall, these results demonstrate that the LTα1β2-LTβR axis negatively regulates CD25+Foxp3+ Treg conversion by repressing IL-4 expression in mTEC.
Discussion
Recent advances have revealed that CD25+Foxp3+ mature Treg arise from two distinct developmental pathways involving CD25+ and Foxp3lo TregP7,14,15,48. Although the CD25+ TregP pathway is well studied, the more recently identified Foxp3lo TregP pathway remains poorly investigated. Our study reveals that the LTα1β2-LTβR axis plays an unexpected role in limiting CD25+Foxp3+ Treg generation via the Foxp3lo TregP developmental pathway.
Previous reports have found that the first step of Treg development is linked to the TCR signal strength, implicating transcription factors of the Nr4a family49. Concomitantly to TCR signaling, CD28 costimulation promotes Treg generation by inducing Foxp3 expression8. We observed that Cd28−/− mice have decreased numbers of both CD25+ TregP and Foxp3lo TregP, resulting in a drastic reduction of mature Treg. Our data show that the stimulation of TCR and CD28 also upregulates the expression of LTα1β2 in CD25+ TregP and Foxp3lo TregP. In addition to TCR/CD28 signals, γ-chain cytokines are important regulators of Treg development since Il2−/− and Il15−/− mice exhibit a severe reduction of TregP and mature Treg in their thymus10,50. Interestingly, we found that IL-2 controls LTα1β2 expression specifically in mature Treg. Accordingly, LTα1β2 and CD25 levels were tightly correlated and IL-2 in vitro stimulation led to a dose-dependent LTα1β2 upregulation in mature Treg. A recent report described that IL-2 also increases LTα1β2 expression in peripheral Treg51, suggesting a conserved regulatory mechanism between the thymus and the periphery.
Although Foxp3lo TregP leads to the generation of CD25+Foxp3+ Treg upon intrathymic transfer14,15, the underlying mechanisms that control this newly identified developmental pathway remain unknown. The generation of Foxp3eGFPxLta−/− mice revealed that Lta expression limits Treg development from Foxp3lo TregP. To our knowledge, LTα is the first identified molecule that fine-tunes the Foxp3lo TregP developmental pathway. Although CD25+ TregP express LTα1β2 to some extent, these cells remain poorly affected compared to Foxp3lo TregP in Foxp3eGFPxLta−/− mice. We show that the increased cellularity of Foxp3lo TregP and mature Treg in Foxp3eGFPxLta−/− mice was likely a direct consequence of enhanced proliferation and survival. Moreover, given that LTβR signaling was found to regulate the expression of chemokines involved in thymocyte trafficking17 and that the LTα1β2- LTβR axis has been implicated in Treg trafficking in a model of pancreatic islet allograft18,52, future investigations are expected to clarify whether Lta-deficiency could affect the intrathymic migration of Treg subsets. Multiparametric flow cytometry analyses based on maturational markers revealed an unexpected heterogeneity in both TregP and mature Treg. Our results highlight that CD25+ TregP is a highly heterogenous population, with cells at distinct maturational stages. Compared to CD25+ TregP, Foxp3lo TregP contain more mature cells at M1 and M2 stages, similarly to CD25+Foxp3+ mature Treg. Our transcriptomic and flow cytometry analyses highlight that CD25+ TregP and Foxp3lo TregP subsets contain not only precursors but also CD62L+ mature cells, which is a reliable marker for cells about to leave the thymus53. Therefore, cells commonly called TregP likely contain cells with the ability to emigrate, although this requires further investigations. The CD69+CD62L− Foxp3lo TregP population, displaying a mature phenotype characterized by high levels of H2Kb and Qa2, increased in Foxp3eGFPxLta−/− mice, suggesting that this putative transitional stage towards mature Treg is regulated by Lta. Altogether, our data identified LTα as a negative regulator of Treg maturational development.
A recent study performed scRNA-seq on thymic Treg using pre-sorted subpopulations from TCliβxTCRα+/−xFoxp3GFP mice and characterized CD25+TregP, Foxp3loTregP and mature de novo Treg54. In contrast, our cell-sorting strategy based on the entire Treg lineage allowed us to also capture CD122+ pre-TregP and transitional Treg states from Foxp3eGFP and Foxp3eGFPxLta−/− mice. Moreover, we characterized the metabolic profile in our scRNA-seq experiment during Treg development, which remained unknown to date. Interestingly, whereas CD25+ TregP rely on both glucose and OXPHOS mitochondrial dependencies, our transcriptomic and functional metabolic analyses revealed that like mature Treg, Foxp3lo TregP are highly dependent on OXPHOS activity and have a lower dependency on glucose metabolism. These findings are consistent with the fact that CD25+ TregP and Foxp3lo TregP have a distinct transcriptomic profile15,54. In Foxp3eGFPxLta−/− mice, the glucose dependency substantially increased in both TregP and mature Treg, indicating that Lta regulates their metabolic profile. This higher glucose dependency may be linked to the enhanced survival and/or proliferation of TregP and mature Treg observed in Foxp3eGFPxLta−/− mice. Further investigations will elucidate whether the distinct metabolic profiles of CD25+ TregP and Foxp3lo TregP depend on their responsiveness to γ-chain cytokines and influence their epigenetic landscape. Understanding the intricate interplay between the two TregP and their respective stromal niches, which could modulate their metabolic profiles and influence their differentiation, represents an exciting area for future research.
Treg conversion assays using co-cultures of CD69+ semi-mature OTII CD4+ SP thymocytes with mTEC that express LTβR revealed that the LTα1β2-LTβR axis acts as a negative regulator of CD25+Foxp3+ mature Treg conversion. We found an enhanced generation of Foxp3lo TregP from CD69+ CD4+ SP thymocytes in the absence of Lta in CD4+ SP thymocytes or when LTα1β2-LTβR interactions were blocked with the soluble LTβR-Fc receptor. In line with these observations, this resulted in an increased conversion into CD25+Foxp3+ mature Treg. Furthermore, Treg conversion assays using co-cultures with TregP showed that the LTα1β2-LTβR axis acts as an inhibitory feedback by which Foxp3lo TregP can limit their own differentiation into CD25+Foxp3+ mature Treg. In agreement with a previous study15, we found that Foxp3lo TregP express higher levels of IL-4Rα than CD25+ TregP both by scRNA-seq and flow cytometry. Moreover, Foxp3lo TregP have a higher ability to respond to IL-4 stimulation than CD25+ TregP. Remarkably, Il4 expression was higher in Lta−/− mice, which correlated with a greater frequency of Eomes+ memory-like CD8+ SP thymocytes and phospho-Stat6+ cells. In vivo IL-4 neutralization resulted in a substantial reduction of Foxp3lo TregP and CD25+Foxp3+ mature Treg whereas CD25+ TregP were over-represented, indicating that IL-4 favors the Foxp3lo TregP pathway at the expense of the CD25+ TregP developmental pathway. Future studies are expected to define the respective contribution of TregP and mature Treg in LTα1β2-controlled IL-4 production by mTEC.
ScRNA-seq, qPCR and IL-4eGFP reporter mice revealed that mTEC are a valuable source of IL-4 in addition to NKT2. Strikingly, mTEC produced a larger amount of IL-4 upon antigen-specific co-cultures with CD69+CD4+ SP thymocytes purified from OTIIxLta−/− mice compared to OTII mice, indicating that Lta expression negatively regulates Il4 expression in mTEC. This higher production correlated with a greater conversion into mature Treg, which was abrogated by a neutralizing IL-4 antibody. The classical NF-κB member p65 has the ability to bind to the P4/PRE-I and P1 sites of the Il4 promoter and upregulate its transcription41,55. Whereas the non-classical NF-κB pathway is preferentially activated by lymphotoxin signaling at steady state27,56, our data indicate a preferential usage of the classical NF-κB pathway characterized by an upregulation of p65 when lymphotoxin signaling is disrupted. Since IL-4 is regulated by the classical NF-κB member p65, which was overexpressed in Lta−/− mice, this cytokine upregulation in Lta−/− mice is likely induced by the classical NF-κB pathway. Interestingly, previous studies have shown that IL-4 acts as a proliferation and survival factor of peripheral Treg57,58,59. Thus, IL-4 may not only induce mature Treg conversion but also promote their survival and proliferation. In line with this hypothesis, we found reduced expression of the pro-apoptotic protein BIM in Foxp3lo TregP and mature Treg of Foxp3eGFPxLta−/− mice. Whereas IL-2 is crucial for the CD25+ TregP developmental pathway10,50, our findings show that IL-4 preferentially regulates the Foxp3lo TregP developmental pathway, indicating that these two cytokines play a non-overlapping role in Treg development. IL-15, also implicated in Treg development, is expressed by mTEC10,12,14. Therefore, by their ability to produce both IL-15 and IL-4, mTEC likely constitute a privilege niche that controls Treg development.
In summary, this study shows that instructive signals of the “two step model” induce the gradual upregulation of the membrane-bound LTα1β2 during Treg development. Importantly, we identify that LTα1β2-LTβR interactions act as an inhibitory checkpoint that limits the Foxp3lo TregP developmental pathway by negatively regulating IL-4 production in mTEC (Supplementary Fig. 13). Interestingly, human thymi, routinely removed during pediatric cardiac surgery, constitute a novel source of stable Treg for cell therapy that can be purified in large quantities60,61. Given that CD25+ and Foxp3lo Treg P-derived Treg likely exhibit a differential ability to protect against distinct autoimmune disorders15,62, targeting LTα1β2-LTβR interactions may therefore be used to foster the Foxp3lo TregP pathway in order to treat specific pathologies. Our findings are, thus, expected to pave the way for therapeutic strategies tailored to treat distinct autoimmune and inflammatory disorders.
Methods
Mice
All mice - CD45.1, CD45.2 and CD45.1/2 WT (Charles River), CD45.2 Lta−/−63, Rag2−/−64, Cd28−/−65, OTII66, Rip-mOVA67, CD45.1 Foxp3eGFP68, Rag2GFP19, Foxp3Thy1.120, Il15KO69 and Il2KO (B6.129P2-Il2tm1Hor/J, JAX laboratories) - were on a pure C57BL/6 background. The IL-4eGFP reporter (4get) mice70 were on a C57BL/6 or BALB/c background. CD45.2 Lta−/− mice were backcrossed with CD45.1 Foxp3eGFP mice (CD45.1 Foxp3eGFPxLta−/− mice). OTII mice were backcrossed on a Rag2−/− background (OTIIxRag2−/− mice) and OTIIxRag2−/− on a Lta−/− background (OTIIxRag2−/−xLta−/− mice). Rip-mOVA mice were backcrossed with OTIIxRag2−/− mice (Rip-mOVAxOTIIxRag2−/−). Rip-mOVAxOTIIxRag2−/− were backcrossed with Foxp3eGFP mice (Rip-mOVAxOTIIxFoxp3eGFP). Foxp3Thy1.1 mice were backcrossed with Rag2GFP mice (Rag2GFPxFoxp3Thy1.1 mice) and Rag2GFPxFoxp3Thy1.1 mice on a Il15KO or Il2KO background (Il15KOxRag2GFPxFoxp3Thy1.1 or Il2KOxRag2GFPxFoxp3Thy1.1 mice). All mice were maintained under specific pathogen free conditions at the CIML (Marseille, France). Mice were housed under a standard 12 h/12 h light-dark cycle, with a controlled ambient temperature of 22 °C ± 2 °C, and relative humidity between 45% and 65%. Standard food and water were given ad libitum. For all experiments, male and female mice were sex- and age-matched. Unless specified, adult mice were used between 6 and 8 weeks of age. Chimeras were generated between 6 and 12 weeks of age. All experiments were done in accordance with National and European laws for laboratory animal welfare (EEC Council Directive 2010/63/UE) and approved by the Marseille Ethical Committee for Animal Experimentation no.14.
Bone marrow chimeras
Bone marrow chimeras were generated by co-injecting intravenously (i.v.) 2 × 106 BM cells from CD45.2 WT mice with 2 × 106 BM cells from either CD45.1 Foxp3eGFP or Foxp3eGFPxLta−/− mice (ratio 1:1) into lethally irradiated CD45.1/2 WT recipients (two doses of 500 rads, 12 h apart, X-ray using a RS-2000 Irradiator, Rad Source Technologies). Similarly, 5 × 106 BM cells from CD45.1 Foxp3eGFP mice were injected into lethally irradiated CD45.2 WT or Lta−/− recipients. Mice were analyzed 6 weeks post-reconstitution.
Thymic cell isolation
For Treg isolation, thymi were mechanically dissociated on a 70 µm cell strainer. CD4+ SP thymocytes were pre-enriched by depleting CD8+ and CD11c+ cells using biotinylated anti-CD8α (clone 53.6.7; Biolegend) and anti-CD11c (clone N418; Biolegend) antibodies with anti-biotin microbeads and ran on an AutoMACS Pro separator using the Deplete program (Miltenyi Biotec). For TEC isolation, thymi were digested 10 min at 37 °C in HBSS medium containing 50 µg/ml of LiberaseTM (Roche) and 100 µg/ml DNase I (Roche) and were then subjected to vigorous pipetting. This step was repeated until complete tissue digestion. Cells were filtered through a 70 μm strainer to remove clumps. CD45+ hematopoietic cells were depleted using biotinylated anti-CD45 antibodies (clone 30-F11; Biolegend) with anti-biotin microbeads, and run on an AutoMACS Pro separator using the DepleteS program (Miltenyi Biotec). CD69+CD4+ SP thymocytes, TregP, mature Treg, NKT, and mTEC subsets were cell-sorted using a FACSAria III cell sorter (BD Biosciences).
In vivo IL-4 neutralization experiments
Lta−/− neonates received an i.p. injection of either isotype control (clone RTK2071, Biolegend) or neutralizing anti-mouse IL-4 antibody (clone 11B11, BioXcell) on the day they were born (100 µg) and 3 days later (100 µg). Mice were analyzed 5 days post-birth.
In vitro antigen-specific Treg conversion assays
Co-culture assays between mTEC and CD69+CD4+ SP thymocytes to assess Treg conversion were performed71. Briefly, 1 × 103 cell-sorted total mTEC (CD45−EpCAM+Ly51loUEA-1hi), mTEChi (CD45−EpCAM+Ly51loUEA-1hiCD80+) and mTEClo (CD45-EpCAM+Ly51loUEA-1hiCD80−) from WT thymi were loaded or not for 90 min with OVA323-339 peptide (5 µg/ml, PolyPeptide) at 37 °C. Cells were then co-cultured with 5 × 103 purified CCR6−CD25−CD69+CD4+CD8− SP thymocytes from OTIIxRag2−/− mice pre-incubated or not during 1 h at 37 °C with recombinant LTβR-Fc (2 μg/ml, RnD systems) or from OTIIxRag2−/−xLta−/− mice in a complete medium containing D-MEM (ThermoFisher Scientific), 10 % FBS (Sigma Aldrich), 2 mM L-glutamine, 1 mM sodium pyruvate, 2.10−5 M 2-mercaptoethanol, 100 IU/ml penicillin, and 100 μg/ml streptomycin (all from ThermoFisher Scientific). In addition, total mTEC were co-cultured with 5 × 103 purified CD25+ TregP or Foxp3lo TregP from Rip-mOVAxOTIIxFoxp3eGFP mice pre-incubated or not during 1 h at 37 °C with recombinant LTβR-Fc (2 μg/ml, RnD systems). Alternatively, 10 µg/mL of neutralizing anti-mouse IL-4 antibody (clone 11B11, BioXcell) was added in the co-cultures of OVA323–339-loaded mTEC with CCR6−CD25−CD69+CD4+CD8− SP from OTIIxRag2−/− or OTIIxRag2−/−xLta−/− mice. The conversion into CD25+ TregP, Foxp3lo TregP, or CD25+Foxp3+ mature Treg was analyzed by flow cytometry 18 h, 24 h, 72 h, or 5 days later, as indicated in the legends.
In vitro TregP conversion assays with IL-4 stimulation
1 × 104 purified CD25+ TregP or Foxp3lo TregP from Foxp3eGFP mice were incubated in complete RPMI medium supplemented with different doses of mouse IL-4 (0.5, 1 or 100 ng/mL; Peprotech). After 72 h, cells were harvested, stained with anti-CD4 and anti-CD25 antibodies, and analyzed by flow cytometry for the conversion into CD25+Foxp3eGFP+ mature Treg.
In vitro activation of Treg cell subsets
For CD3ε stimulation, 2 × 104 purified CD25+ TregP, Foxp3lo TregP or CD25+Foxp3+ Treg from Foxp3eGFP mice were cultured with different doses (0.1, 1, 2.5 or 5 µg/mL) of coated anti-CD3ε antibody (clone 2C11, BD Biosciences) in a complete RPMI medium containing soluble anti-CD28 antibody (0.5 µg/mL, clone 37.51, Biolegend) in the presence of a low dose of IL-2 (5 ng/mL, Immunotools). For IL-2 stimulation, 2 × 104 purified CD25+ TregP, Foxp3lo TregP, or mature Treg from Foxp3eGFP mice were cultured with a low dose of coated anti-CD3ε antibody (2 µg/mL, clone 2C11, BD Biosciences) in a complete RPMI medium containing soluble anti-CD28 antibody (0.5 µg/mL, clone 37.51, Biolegend) in the presence or not of different doses (5, 20 or 40 ng/mL) of IL-2 (Immunotools). After 16 h, cells were harvested and analyzed for LTα1β2 expression by flow cytometry.
Flow cytometry
Cells were stained with standard procedures using antibodies listed in Supplementary Data 1. For intracellular staining, cells were fixed, permeabilized, and stained with the Foxp3 staining kit according to the manufacturer’s instructions (ThermoFisher Scientific). Fluorescence minus one (FMO) and secondary antibody controls were used to set positive staining gates. NKT cells were detected using CD1d tetramers loaded with PBS-57 or unloaded (obtained from the National Institutes of Health Tetramer Core Facility). For LTα1β2 staining, cells were incubated with LTβR-Fc (1 μg/106 cells, R&D systems) for 45 min on ice, and staining was visualized using an APC-conjugated donkey anti-human IgG F(ab’)2 antibody (Jackson ImmunoResearch). For all panels, we inferred the background levels observed in the respective cell subsets from Lta−/− mice. To evaluate mitochondrial activity, thymocytes were stained with MitoTracker Deep Red (100 nM) (Thermo Fisher) for 30 min at 37 °C in complete RPMI medium. For unbiased clustering of the cells from the Treg lineage, data from all samples were concatenated and analyzed using the t-SNE plugin of the FlowJo software (BD Life Sciences, version 10.8.1). The PhenoGraph plugin was used for the unsupervised discrimination of Treg subsets (with a k-nearest neighbor value of 130 for Fig. 3i and 120 for Fig. 4f), which were then visualized on the t-SNE dimensional reduction and quantified. The color code representing the expression level of each marker in heatmaps was normalized on the median intensity value for a given marker. Levels of LTα1β2 expression were measured in thymic Treg subsets from Rag2GFPxFoxp3Thy1.1 (Fig. 1b–f), Rag2GFPxFoxp3Thy1.1xIl15−/− and Rag2GFPxFoxp3Thy1.1xIl2−/− (Fig. 2d, e) mice on a BD LSR Fortessa at Infinity (Toulouse, France). The other flow cytometry experiments were conducted using FACSCanto II, BD LSR II, and Symphony cytometers (BD Biosciences) at CIML (Marseille, France). Data were analyzed using the FlowJo software (BD Life Sciences).
SCENITH™ metabolic profiling
SCENITH kit containing all reagents (including anti-puromycin, clone R4743L-E8) and protocols were obtained (www.scenith.com) and the Treg metabolic profile was analyzed35. Briefly, 4 × 106 thymocytes were plated at 40 × 106 cells/ml in complete medium in a 96-well plate and treated with Puromycin (10 µg/mL) and DMSO (Co), 2-deoxy-glucose (2DG; 100 mM), oligomycin (O; 1 µM), a combination of 2DG and oligomycin (DGO) or harringtonine (H; 2 µg/mL) for 40 min at 37 °C, 5 % CO2. Cells were washed in cold FACS buffer and incubated with a BD HorizonTM Fixable Viability Stain 700 (BD Biosciences). Then, cells were stained with primary-conjugated antibodies for 25 min at 4 °C in FACS Buffer and were fixed and permeabilized with the Foxp3 staining kit according to the manufacturer’s instructions (ThermoFisher Scientific). Intracellular staining with anti-puromycin and anti-Foxp3 antibodies was performed by incubating cells during 1 h at 4 °C in Permeabilization Buffer (ThermoFisher Scientific).
Immunofluorescence staining
20-µm-thick-frozen thymic sections embedded in O.C.T (Sakura Finetek) were stained72. Briefly, sections were fixed with 2% paraformaldehyde (Merck), saturated with 3% BSA, and 0.01 % Triton X100 in 0.1 M Tris HCl buffer. Sections were then stained for 45 min with anti-keratin 14 and anti-NK1.1 Alexa Fluor 647 (clone PK136, Biolegend) in hybridization buffer (1% BSA and 0.02% Triton X100 in 0.1 M Tris-HCl, pH 7.4). Keratin 14 staining was revealed with a Cy3-conjugated anti-rabbit antibody (ThermoFisher Scientific). Sections were counterstained with DAPI (1 µg/mL; Biolegend) and mounted with Mowiol (Calbiochem). Confocal microscopy was performed with an LSM 880 confocal microscope (Carl Zeiss Microscopy) and analysed using Zen Blue (3.5.093).
RT-qPCR
Total RNA was isolated with TRIzol (ThermoFisher Scientific) and cDNA was synthesized with random oligo-dT primers and Superscript II reverse transcriptase (ThermoFisher Scientific). qPCR was performed with SYBR Premix Ex Taq master mix (Takara) on an ABI 7500 fast real-time PCR system (Applied Biosystem). Reactions were conducted in duplicates. Results were normalized to Actin mRNA. Primer sequences are provided in Supplementary Data 2.
scRNA-seq library preparation
CD8- and CD11c-depleted thymocytes from Foxp3eGFP and Foxp3eGFPxLta−/− mice were stained with fluorescently labeled antibodies (Fig. 5a) and barcoded using TotalSeq-A antibodies (BioLegend). FACS sorted Live Dead−CD4+CD8−CCR6−CD3ε+ expressing either CD25, Foxp3eGFP, or both were pooled and subsequently prepared for single-cell sequencing using the Chromium Single Cell 3′ kit v3 (10X Genomics) with a targeted number of 10,000 total cells. Libraries were prepared according to the manufacturer’s instructions. The concentration and quality of cDNA libraries were respectively determined using the Qubit dsDNA HS Assay Kit (ThermoFisher Scientific) for the Qbit fluorometer and the High Sensitivity DNA Analysis Kit (Agilent) for the bioanalyzer. Libraries were sequenced on the Illumina NextSeq2000 sequencer.
Data analysis for scRNA-seq analysis
mRNA and Cell Hashing FASTQ raw files were processed using Cell Ranger v6.0.1 (10X genomics Inc.) software with default parameters to perform alignment, filtering, barcode counting, and unique molecular identifier (UMI) counting. Reads were aligned to the mm10 genome. A total number of 4,798 cells were identified with a mean of 44,015 reads per cell and a median of 1,992 genes per cell. Data sets were analysed using the R package Seurat 4.0.573. First, cells were demultiplexed (with the Cell Hashing tags) to their original sample groups using the HTODemux function from Seurat R package with automated threshold finding to define the best quantile. 302 doublet cells and 4 negative cells were identified and removed. Quality control (QC) was performed to remove poor quality cells. We observed co-variables using the ShIVA interface and set the following thresholds74: cells with less than 603 or more than 4128 detected genes, cells with less than 851 or more than 21,686 detected UMI, cells with more than 9.94% mitochondrial gene expression, and cells with less than 11.5% ribosomal gene expression were removed. Expression data was normalized using the NormalizeData function of the Seurat R package (logNormalize method and scale factor of 10,000). We centered the expression data from these factors using the Seurat R package ScaleData function (centering true and scaling false).
Feature selection was performed using the Seurat R package FindVariableGene function; the variance-stabilizing transform (vst) algorithm and 2,000 genes were selected. Principal component analysis (PCA) was ran using Seurat RunPCA function on those 2,000 most variable genes. UMAP dimensionality reduction was ran using Seurat RunUMAP function on the first 50 principal components from PCA. Clustering was done with Seurat FindClusters based on the first 30 principal components from PCA dimensionality reduction and with an initial resolution parameter set to 1.1. Markers for each cluster were identified using Wilcoxon Rank Sum test from Seurat FindAllMarkers with the log fold change threshold of 0.1 and an adjusted FDR threshold of 0.001. Cell-cycle scores and cell phases were calculated using CellCycleScoring with s.genes and g2m.genes from the Seurat package. After cell type analysis using marker genes, we identified several clusters to be contaminants, in proliferation or recirculating cells and excluded them. PCA, UMAP, and clustering (with resolution 0.8) were performed again on the filtered dataset.
Heatmaps of gene expression data per cluster were created using the Heatmap function of the ComplexHeatmap (v2.10.0)75 R package. Expression was presented as the mean of the expression of cells inside each cluster. Values were scaled to make them more comparable. Module scores of gene set list from the KEGG database were calculated using the AddModuleScore function from the Seurat R package. The Nebulosa package was used to visualize features on UMAP embeddings using kernel density estimation76.
Trajectory analysis were performed using Monocle 332. Cells were clustered using the Monocle 3 cluster_cells function with a resolution set to 0.003. Trajectories were computed using the Monocle 3 learn_graph and order_cells function. Gene set scores were computed as previously described and plotted along the pseudotime trajectories. All regression lines were computed using the ggplot2 geom_smooth function, setting the method to “loess”.
Statistical analysis
All statistical analyses were performed in GraphPad Prism 9.5.1 and were considered statistically different at a p-value < 0.05. Unpaired Student’s t test, Mann–Whitney U test, one-way ANOVA, or Kruskal–Wallis test were calculated. Normal distribution of the data was assessed using d’Agostino-Pearson omnibus normality test. Correlation was calculated using the parametric two-tailed Pearson correlation test or the non-parametric two-tailed Spearman correlation test. Statistical comparison of module scores between conditions for each cluster was performed using the Wilcoxon test with Benjamini-Hochberg multitest correction. *p < 0.05; **p < 0.01; ***p < 0.001, ****p < 0.0001. Error bars represent mean ± SEM.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The scRNA-seq data generated in this study have been deposited in the GEO database under accession number GSE237589. All data supporting the findings of this study are found within the paper and its Supplementary Information. Source data are provided with this paper.
Code availability
Scripts used to analyze sequencing data have been deposited on GitHub (https://github.com/CIML-bioinformatic/MIlab_LTaTreg-Thymus) and Zenodo (https://doi.org/10.5281/zenodo.12705747)77.
References
Fontenot, J. D., Dooley, J. L., Farr, A. G. & Rudensky, A. Y. Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med. 202, 901–906 (2005).
Aschenbrenner, K. et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat. Immunol. 8, 351–358 (2007).
Perry, J. S. A. et al. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity 41, 414–426 (2014).
Charaix, J. et al. Recirculating Foxp3+ regulatory T cells are restimulated in the thymus under Aire control. Cell. Mol. Life Sci. 79, 355 (2022).
Lio, C. W. J. & Hsieh, C. S. A two-step process for thymic regulatory T cell development. Immunity 28, 100–111 (2008).
Klein, L., Robey, E. A. & Hsieh, C. S. Central CD4+ T cell tolerance: deletion versus regulatory T cell differentiation. Nat. Rev. Immunol. 19, 7–18 (2019).
Santamaria, J. C., Borelli, A. & Irla, M. Regulatory T cell heterogeneity in the thymus: impact on their functional activities. Front Immunol. 12, 1–8 (2021).
Tai, X., Cowan, M., Feigenbaum, L. & Singer, A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat. Immunol. 6, 152–162 (2005).
Mahmud, S. A. et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat. Immunol. 15, 473–481 (2014).
Apert, C. et al. IL-2 and IL-15 drive intrathymic development of distinct periphery-seeding CD4+Foxp3+ regulatory T lymphocytes. Front Immunol. 13, 965303 (2022).
Hemmers, S. et al. IL-2 production by self-reactive CD4 thymocytes scales regulatory T cell generation in the thymus. J. Exp. Med. 216, 2466–2478 (2019).
Cui, G. et al. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc. Natl Acad. Sci. USA 111, 1915–1920 (2014).
Tai, X. et al. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity 38, 1116–1128 (2013).
Marshall, D., Sinclair, C., Tung, S. & Seddon, B. Differential requirement for IL-2 and IL-15 during bifurcated development of thymic regulatory T cells. J. Immunol. 193, 5525–5533 (2014).
Owen, D. L. et al. Thymic regulatory T cells arise via two distinct developmental programs. Nat. Immunol. 20, 195–205 (2019).
Gommerman, J. L. & Browning, J. L. Lymphotoxin/LIGHT, lymphoid microenvironments and autoimmune disease. Nat. Rev. Immunol. 3, 642–655 (2003).
Borelli, A. & Irla, M. Lymphotoxin: from the physiology to the regeneration of the thymic function. Cell Death Differ. 28, 2305–2314 (2021).
Brinkman, C. C. et al. Treg engage lymphotoxin beta receptor for afferent lymphatic transendothelial migration. Nat. Commun. 7, 12021 (2016).
Yu, W. et al. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature 400, 682–687 (1999).
Liston, A. et al. Differentiation of regulatory Foxp3 T cells in the thymic cortex. Proc. Natl Acad. Sci. USA 105, 11903–11908 (2008).
McCaughtry, T. M., Wilken, M. S. & Hogquist, K. A. Thymic emigration revisited. J. Exp. Med. 204, 2513–2520 (2007).
Thiault, N. et al. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat. Immunol. 16, 628–634 (2015).
Yang, E. J., Zou, T., Leichner, T. M., Zhang, S. L. & Kambayashi, T. Both retention and recirculation contribute to long-lived regulatory T-cell accumulation in the thymus. Eur. J. Immunol. 44, 2712–2720 (2014).
Moran, A. E. et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011).
Apert, C., Romagnoli, P. & van Meerwijk, J. P. M. IL-2 and IL-15 dependent thymic development of Foxp3-expressing regulatory T lymphocytes. Protein Cell 9, 322–332 (2018).
Bayer, A. L., Lee, J. Y., de la Barrera, A., Surh, C. D. & Malek, T. R. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J. Immunol. 181, 225–234 (2008).
Lopes, N., Charaix, J., Cédile, O., Sergé, A. & Irla, M. Lymphotoxin α fine-tunes T cell clonal deletion by regulating thymic entry of antigen-presenting cells. Nat. Commun. 9, 1–16 (2018).
Bouillet, P. et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415, 922–926 (2002).
Gray, D. H. D. et al. The BH3-Only proteins bim and puma cooperate to impose deletional tolerance of organ-specific antigens. Immunity 37, 451–462 (2012).
Irla, M. et al. Three-dimensional visualization of the mouse thymus organization in health and immunodeficiency. J. Immunol. 190, 586–596 (2013).
Schuster, M., Plaza-Sirvent, C., Visekruna, A., Huehn, J. & Schmitz, I. Generation of Foxp3+CD25− regulatory T-cell precursors requires c-rel and IκBNS. Front Immunol. 10, 1583 (2019).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
Shi, H. & Chi, H. Metabolic control of treg cell stability, plasticity, and tissue-specific heterogeneity. Front Immunol. 10, 2716 (2019).
Newton, R., Priyadharshini, B. & Turka, L. A. Immunometabolism of regulatory T cells. Nat. Immunol. 17, 618–625 (2016).
Argüello, R. J. et al. SCENITH: a flow cytometry-based method to functionally profile energy metabolism with single-cell resolution. Cell Metab. 32, 1063–1075.e7 (2020).
Xing, Y., Wang, X., Jameson, S. C. & Hogquist, K. A. Late stages of T cell maturation in the thymus involve NF-κB and tonic type i interferon signaling. Nat. Immunol. 17, 565–573 (2016).
Lee, Y. J., Holzapfel, K. L., Zhu, J., Jameson, S. C. & Hogquist, K. A. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 14, 1146–1154 (2013).
Lai, D. et al. KLF13 sustains thymic memory-like CD8+ T cells in BALB/c mice by regulating IL-4-generating invariant natural killer T cells. J. Exp. Med. 208, 1093–1103 (2011).
Wang, H. et al. Myeloid cells activate iNKT cells to produce IL-4 in the thymic medulla. Proc. Natl Acad. Sci. USA 116, 22262–22268 (2019).
Zhou, T. A. et al. Thymic macrophages consist of two populations with distinct localization and origin. Elife 11, e75148 (2022).
Li-Weber, M., Giasi, M. & Krammer, P. H. Involvement of Jun and Rel proteins in up-regulation of interleukin-4 gene activity by the T cell accessory molecule CD28. J. Biol. Chem. 273, 32460–32466 (1998).
Wirnsberger, G., Mair, F. & Klein, L. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proc. Natl Acad. Sci. USA 106, 10278–10283 (2009).
Malchow, S. et al. Aire enforces immune tolerance by directing autoreactive T cells into the regulatory T cell lineage. Immunity 44, 1102–1113 (2016).
Malchow, S. et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 339, 1219–1224 (2013).
Michelson, D. A., Hase, K., Kaisho, T., Benoist, C. & Mathis, D. Thymic epithelial cells co-opt lineage-defining transcription factors to eliminate autoreactive T cells. Cell 185, 2542–2558.e18 (2022).
Wells, K. L. et al. Combined transient ablation and single cell RNA sequencing reveals the development of medullary thymic epithelial cells. Elife 9, 1–80 (2020).
Jin, S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Irla, M. Instructive Cues of Thymic T Cell Selection. Annu. Rev. Immunol. 26, 95–119 (2022).
Sekiya, T. et al. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat. Immunol. 14, 230–237 (2013).
Fontenot, J. D., Rasmussen, J. P., Gavin, M. A. & Rudensky, A. Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005).
Piao, W. et al. Regulatory T cells condition lymphatic endothelia for enhanced transendothelial migration. Cell Rep. 30, 1052–1062.e5 (2020).
Saxena, V. et al. Treg tissue stability depends on lymphotoxin beta-receptor- and adenosine-receptor-driven lymphatic endothelial cell responses. Cell Rep. 39, 110727 (2022).
James, K. D. et al. Endothelial cells act as gatekeepers for LTβRdependent thymocyte emigration. J. Exp. Med. 215, 2984–2993 (2018).
Owen, D. L., Rue, R. S., La, Munro, S. A. & Farrar, M. A. Tracking regulatory T cell development in the thymus using single-cell RNA sequencing/TCR sequencing. J. Immunol. 209, 1300–1313 (2022).
Li-Weber, M. & Krammer, P. H. Regulation of IL4 gene expression by T cells and therapeutic perspectives. Nat. Rev. Immunol. 3, 534–543 (2003).
Irla, M., Hollander, G. & Reith, W. Control of central self-tolerance induction by autoreactive CD4+ thymocytes. Trends Immunol. 31, 71–79 (2010).
Pandiyan, P. & Lenardo, M. J. The control of CD4+CD25+Foxp3+ regulatory T cell survival. Biol. Direct 3, 6 (2008).
Zhou, J. Y., Alvarez, C. A. & Cobb, B. A. Integration of il-2 and il-4 signals coordinates divergent regulatory T cell responses and drives therapeutic efficacy. Elife 10, 1–27 (2021).
Yang, W. C. et al. Interleukin-4 supports the suppressive immune responses elicited by regulatory T cells. Front. Immunol. 8, 1508 (2017).
Dijke, I. E. et al. Discarded human thymus is a novel source of stable and long-lived therapeutic regulatory T cells. Am. J. Transplant. 16, 58–71 (2016).
Bernaldo-de-Quirós, E. et al. First-in-human therapy with Treg produced from thymic tissue (thyTreg) in a heart transplant infant. J. Exp. Med. 220, e20231045 (2023).
Wyss, L. et al. Affinity for self antigen selects Treg cells with distinct functional properties. Nat. Immunol. 17, 1093–1101 (2016).
De Togni, P. et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 264, 703–707 (1994).
Shinkai, Y. et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 (1992).
Morin, S. O. et al. In the absence of its cytosolic domain, the CD28 molecule still contributes to T cell activation. Cell. Mol. Life Sci. 72, 2739–2748 (2015).
Barnden, M. J., Allison, J., Heath, W. R. & Carbone, F. R. Defective TCR expression in transgenic mice constructed using cDNA- based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Kurts, C. et al. Constitutive class i-restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 184, 923–930 (1996).
Wang, Y. et al. Th2 lymphoproliferative disorder of lat Y136F mutant mice unfolds independently of TCR-MHC engagement and is insensitive to the action of Foxp3+ regulatory T cells. J. Immunol. 180, 1565–1575 (2008).
Kennedy, M. K. et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191, 771–780 (2000).
Mohrs, M., Shinkai, K., Mohrs, K. & Locksley, R. M. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 15, 303–311 (2001).
Borelli, A., Zamit, C. & Irla, M. Medullary thymic epithelial cell antigen-presentation assays. Bio Protoc. 13, e4865 (2023).
Sergé, A., Bailly, A. L., Aurrand-Lions, M., Imhof, B. A. & Irla, M. For3D: Full organ reconstruction in 3D, an automatized tool for deciphering the complexity of lymphoid organs. J. Immunol. Methods 424, 32–42 (2015).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021).
Aussel, R. et al. ShIVA: a user-friendly and interactive interface giving biologists control over their single-cell RNA-seq data. Sci. Rep. 13, 14377 (2023).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Alquicira-Hernandez, J. & Powell, J. E. Nebulosa recovers single-cell gene expression signals by kernel density estimation. Bioinformatics 37, 2485–2487 (2021).
Spinelli, L. CIML-bioinformatic/MIlab_LTaTreg-Thymus: article publication release. Zenodo https://doi.org/10.5281/zenodo.12705747 (2024).
Acknowledgements
We are grateful to Jacques Nunes and Geoffrey Guittard (CRCM, Marseille, France) for providing us with Cd28−/− mice, to Bernard Malissen (CIML, Marseille, France) for Foxp3eGFP mice, to Joost P.M. van Meerwijk (Inifinity, Toulouse, France) for Rag2GFPxFoxp3Thy1.1, Il2−/− and Il15−/− mice and to David Voehringer and Andreas Ruhl (FAU, Erlangen, Germany) for 4get mice. We also thank the National Institutes of Health Tetramer Facility for PBS57:CD1d tetramers. We thank the CIML animal facility, flow cytometry, PICSL imaging facility (ImagImm), and Genomics platform for technical support. We are grateful to Benoit Salomon (Infinity, Toulouse, France), Lena Alexopoulou (CIML, Marseille, France), and Arnauld Sergé (LAI, Marseille, France) for helpful discussions. We thank Serge van de Pavert (CIML, Marseille, France) for help in preparing the Supplementary Fig. 13. This work was supported by the ARC Foundation (PJA20171206491 to M.I.), Inserm CoPoC—proof of concept (N° MAT-PI-17326-A-01 to M.I.), Agence de Biomédecine (21GREFFE009 to M.I.) and the Agence Nationale de la Recherche (grant ANR-22-CE18-0045-01 Reality to M.I.). This work was supported by the France Génomique national infrastructure, funded as part of the “Investissements d’Avenir” program managed by the Agence Nationale de la Recherche (contract ANR-10-INBS-0009). We also acknowledge financial support from n° ANR-10-INBS-04-01 France Bio Imaging. A.B. was supported by a PhD fellowship from the Ministère de l’Enseignement Supérieur et de la Recherche et de l’Innovation (MESRI) and by the Fondation ARC pour la Recherche sur le Cancer (ARCDOC42022010004424). The Supplementary Fig. 13 was created with BioRender.com.
Author information
Authors and Affiliations
Contributions
A.B., J.C.S, C.A., C.Z., and M.I. conducted the experiments, analyzed and interpreted the data. L.S. and J.C. analyzed scRNA-seq data. R.J.A. provided assistance with data interpretation, discussion on SCENITH and scRNA-seq data and reviewed the manuscript. P.P. provided us anti-puromycin antibody. A.B., J.C.S., and M.I. wrote the manuscript. M.I. initiated, supervised, and conceived the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare the existence of a financial/non financial competing interest. M.I. holds a patent related to Treg genetically modified for the lymphotoxin alpha gene (PCT/EP2020/063346; WO2020229546A1). There are restrictions to the commercial use of SCENITHTM due to pending patent application (PCT/EP2020/060486;WO2020212362A1 to R.J.A.). All other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Derk Amsen and the other anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Borelli, A., Santamaria, J.C., Zamit, C. et al. Lymphotoxin limits Foxp3+ regulatory T cell development from Foxp3lo precursors via IL-4 signaling. Nat Commun 15, 6976 (2024). https://doi.org/10.1038/s41467-024-51164-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51164-5
- Springer Nature Limited