Abstract
Developing high-performance Pt-based catalysts with low Pt loading is crucial but challenging for CO oxidation at temperatures below 100 °C. Herein, we report a Pt-based catalyst with only a 0.15 wt% Pt loading, which consists of Pt–Ti intermetallic single-atom alloy (ISAA) and Pt nanoparticles (NP) co-supported on a defective TiO2 support, achieving a record high turnover frequency of 11.59 s–1 at 80 °C and complete conversion of CO at 120 °C. This is because the coexistence of Pt–Ti ISAA and Pt NP significantly alleviates the competitive adsorption of CO and O2, enhancing the activation of O2. Furthermore, Pt single atom sites are stabilized by Pt–Ti ISAA, resulting in distortion of the TiO2 lattice within Pt–Ti ISAA. This distortion activates the neighboring surface lattice oxygen, allowing for the simultaneous occurrence of the Mars-van Krevelen and Langmuir–Hinshelwood reaction paths at low temperatures.
Similar content being viewed by others
Introduction
Due to their unique catalytic properties, Pt-based catalysts are widely used in many industrial fields, such as hydrogenation/dehydrogenation, fuel cells/electrolyzers, and automobile exhaust purification systems. CO is one of the most poisonous gases in the exhaust, so its catalytic oxidation has received more attention in the last several decades in industrial applications and academic research. To meet the more rigorous emission regulations in the future1,2,3, developing CO oxidation catalysts that can work well at exhaust temperatures below 150 °C is necessary and pressing4,5. Despite advancements in developing low-temperature exhaust catalysts, such as Au nanoparticles6 and Co3O47, the harsh working environment of automotive catalysts leads to the deterioration of these nano-catalysts. Pt remains the catalyst component most frequently used in exhaust purification systems because of its good reactivity and chemical stability8,9.
There are challenges in designing effective catalysts, with current strategies primarily relying on trial and error methods, resulting in high costs and limited success rates. Understanding the mechanisms involved can help modify and optimize catalyst structures to improve efficiency and enable rational design. For CO oxidation on Pt-based catalysts, two reaction mechanisms have been proposed: the Mars-van Krevelen (MvK) mechanism and the Langmuir–Hinshelwood (L–H) mechanism10,11,12. The MvK mechanism typically occurs on reducible metal oxide supports via the activation of surface lattice oxygen species13, while the L–H mechanism assumes the reaction happens on the catalyst surface via co-adsorbed CO and molecular O214. However, activating oxygen (O2) is difficult, as CO strongly adsorbs on the Pt surface, making O2 activation a crucial step in the L–H mechanism11,15. Introducing a second reducible metal16,17,18 or increasing the oxygen vacancy (Ov) content19,20 can promote the O2 activation step, but oxidation under reaction conditions can lead to catalyst deactivation15,21. Recent research shows that simultaneous activation of O2 and surface lattice oxygen in Cu1/TiO2 can improve its catalytic CO oxidation performance, but this is limited to high temperatures above 200 °C22. To overcome these challenges, we propose a catalyst design that involves accelerating O2 adsorption and activation without introducing a second reducible metal. This will allow both reaction pathways to occur concurrently on a single Pt metal, activating both molecular oxygen and surface lattice oxygen at low temperatures.
In this work, we design a new Pt-based catalyst, which consists of both Pt single-atoms and Pt nanoparticles co-anchored on H2-reduced TiO2 (TiO2-R). In this unique structure, the Pt single-atoms are surrounded by Ti and appear as Pt-Ti intermetallic single-atom alloy (ISAA). We call this catalyst PtPt-TiISAA-NP/TiO2-R. The Pt-Ti ISAA and Pt NP in this catalyst act as dual active centers. This design effectively reduces the competitive adsorption between CO and O2 and enhances the adsorption and activation of O2. The TiO2 lattice distortion induced by Pt-Ti ISAA activates the adjacent surface lattice oxygen, thus simultaneously activating molecular oxygen and surface lattice oxygen. As a result, this catalyst improves CO oxidation activity by 1–3 orders of magnitude compared to the reported Pt-based catalysts in the literature.
Results
Characterization of the catalysts
PtPt-TiISAA+NP/TiO2-R with a 0.15 wt% Pt loading was synthesized as depicted in Fig. 1a. Typically, a certain amount of commercial TiO2 was reduced in H2 to generate TiO2-R enriched with Ov, followed by impregnation of Pt precursor, calcination in air, and reduction in H2. Two control samples of PtNP/TiO2 and PtNP/TiO2-R were prepared with identical Pt loading and using TiO2 and TiO2-R as the respective supports. White TiO2 changed to gray TiO2-R after hydrogen treatment (Supplementary Fig. 1). The removal of oxygen anions in TiO2 usually generates Ov and/or Ti3+ centers. The electron paramagnetic resonance (EPR) spectrum of TiO2 support (Supplementary Fig. 2) shows two EPR signals, including an isotropic signal at giso = 2.003 corresponding to the formed Ov with trapped single electrons and an axial signal at g = 1.992 associated with the creation of Ti3+ sites23,24. Ti3+ and Ov signals show a significant increase in intensity after the reduction with H2 (TiO2-R) at 600 °C, suggesting that TiO2 was partially reduced, agreeing with the H2 temperature-programmed reduction (H2-TPR) curve of TiO2 (Supplementary Fig. 3). For comparison, the Raman spectrum of TiO2 shows six distinct Raman active vibrations at 144, 198, 401, 515, 519, and 637 cm−1 (Supplementary Fig. 4)25,26. In contrast, the Raman spectrum of TiO2-R displays slightly broadened peaks and reduced peak intensities due to a considerable number of defects within the lattice and on the surface27,28. Additionally, the vibrational modes of the anatase phase are observed in both TiO2 and TiO2-R, indicating that the crystalline phase was unchanged after the H2 treatment.
a Schematic illustration of the synthesis process of the PtPt-TiISAA+NP/TiO2-R catalyst. b AC-HAADF-STEM images and c EELS spectra of the PtPt-TiISAA+NP/TiO2-R, d, e enlarged high-resolution HAADF-STEM images taken from the selected areas in (c), and f interatomic distances in different orientations. The red circles indicate the Pt-Ti ISAA, while the blue and yellow spheres represent Ti and Pt atoms, respectively. g k2-weighted Fourier transform spectra from EXAFS and h Normalized Pt L3-edge XANES spectra of Pt foil, PtO2, PtNP/TiO2-R, and PtPt-TiISAA+NP/TiO2-R. i XPS spectra of Pt 4f.
Inductively coupled plasma-optical emission spectroscopy results indicate that the mass contents of Pt are comparable, approximately 0.15, 0.15, and 0.17 wt% in PtNP/TiO2-R, PtPt-TiISAA+NP/TiO2-R, and PtNP/TiO2, respectively. X-ray diffraction patterns (Supplementary Fig. 5) of PtNP/TiO2-R, PtPt-TiISAA+NP/TiO2-R, and PtNP/TiO2 reveal the sole presence of the anatase phase of TiO2 (JCPDS No. 71–1166)29, without any observable peaks corresponding to Pt species diffraction. The high-resolution transmission electron microscopy images of the three samples (Supplementary Figs. 6–8) demonstrate a uniform interplanar distance of 0.35 nm, which corresponds to the (101) plane of anatase TiO230. The mapping images suggest the uniform distributions of Pt throughout the entire architecture (Supplementary Figs. 9–11). Scanning transmission electron microscope-second electron images show that PtNP/TiO2-R, PtPt-TiISAA+NP/TiO2-R, and PtNP/TiO2 have similar morphology (Supplementary Figs. 12–14), suggesting that the morphology of TiO2 remained unchanged after the reduction process. For all the catalysts, Pt nanoparticles are clearly observed on the supports (Fig. 1b and Supplementary Figs. 15 and 16). For Pt/TiO2 and Pt/TiO2-R, the average Pt particle sizes are very close, which are 2.9 nm and 2.6 nm, respectively (see Supplementary Fig. 17), probably related to the anatase structure of the two TiO2 supports (Supplementary Fig. 4). The measured electron energy loss spectroscopy (EELS) spectra at Ti L2,3 edges verify that Ti species are present on Pt particle surface. Compared to the titanium signal of TiO2, the energy loss edge position of Ti species on Pt particles is shifted down by 1.6 eV, indicating the formation of Pt–Ti intermetallic alloy31 (Fig. 1c). Figure 1d presents an enlarged Aberration-corrected high-angle-annular-dark-field scanning transmission electron microscopy (AC-HAADF-STEM) image of the area marked by the red dashed boxes in Fig. 1b. Notably, distinct atomic arrangements are observed on the surface of ISAA Pt–Ti, as shown in Fig. 1e. The periodic variation in Z-contrast between two adjacent atoms demonstrates the intermetallic nature of ISAA Pt–Ti. This observation indicates that these bright spots, unequivocally identified as Pt atoms, are isolated by the Ti atoms that are visible as dim spots. This variation is directly associated with the disparity in atomic numbers between Pt and Ti atoms and the distorted crystal lattice of TiO2 (Fig. 1e). This phenomenon is similar to the ISAA In-Pd bimetallene reported by Yu et al., in which Pd single atoms were surrounded by In atoms32. Furthermore, the interatomic distance between the two closest Pt atoms is 0.29 nm (Fig. 1f), which notably surpasses the Pt–Pt bond length (0.27 nm) in Pt NP, indicating that the bright spots, unequivocally identified as Pt atoms, are isolated by the Ti atoms that are present as dim spots33. The observed atomic distributions of Pt and Ti in the catalyst surface layer are ascribed to the body-centered cubic (bcc) B2 structure, which belongs to the CsCl type and the Pm-3m space group. In this arrangement, Pt atoms are isolated by Ti atoms (Supplementary Fig. 18). Supplementary Fig. 19a displays the features of the Fast Fourier Transform (FFT) pattern for region d in Fig. 1b, which arises from the crystal planes of Pt (002), (1\(\bar{1}\)1), and (\(\bar{1}\)11), confirming the existence of metallic Pt in PtPt-TiISAA+NP/TiO2-R. Moreover, the (001), (101), and (100) superlattice spots of the particles (region e in Fig. 1b) are observed in the FFT pattern (Supplementary Fig. 19b), indicating the formation of Pt–Ti ISAA. The formation of this structure in multiple places (Supplementary Fig. 20) demonstrates its prevalence in PtPt-TiISAA+NP/TiO2-R. A similar phenomenon of epitaxial growth has been observed in the Au/TiO2 catalyst34, where gold atoms preferentially attach on the specific surface sites of rutile TiO2 (110) and form an epitaxial and coherent hetero-interface. No Pt–Ti ISAA structure was detected in the Pt/TiO2 catalyst (PtNP/TiO2-H2) treated at 300 °C in a hydrogen atmosphere (Supplementary Fig. 21). This finding, when considered alongside the preceding analysis, underscores the crucial role of Ov in the TiO2 support and the subsequent calcination in a hydrogen environment after Pt deposition in the formation of the Pt–Ti ISAA structure. Specifically, the Pt–Ti ISAA structure was formed during the calcination process in hydrogen after loading Pt onto the TiO2 support. To investigate whether the cohesion between Pt atoms in larger NPs surpassed the Pt–Ti interactions that resulted in the disappearance of Pt–Ti ISAA, a sample of 0.5 wt% PtPt-TiISAA+NP/TiO2-R was prepared. The results, as presented in Supplementary Fig. 22, reveal that the Pt-Ti ISAA and Pt NPs can still coexist even in the presence of the larger Pt particles (~20 nm).
Figure 1g shows the Pt L3-edge k3χ(k) Fourier-transformed (FT) curves of PtNP/TiO2-R, PtPt-TiISAA+NP/TiO2-R, and the reference Pt foil and PtO2. The FT spectrum of PtNP/TiO2-R exhibits a peak at 2.46 Å assigned to Pt–Pt coordination. However, a new peak appeared at 1.63 Å attributed to Pt–O coordination is also observed for PtPt-TiISAA+NP/TiO2-R, which was generated by the oxidation of Pt NP in air during the sample transfer measurement process, and the FT curve is characterized by a prominent peak at 2.31 Å, notably contracted in position as compared to the Pt–Pt coordination peaks of PtNP/TiO2-R. This is due to the partial reduction of Pt species to the intermetallic Pt–Ti phase, as well as the relatively lighter metallic properties of Ti compared to Pt, since there is no other metallic element lighter than Pt in PtPt-TiISAA+NP/TiO2-R22. We fitted EXAFS using least squares to determine the coordination number in each sample (Supplementary Figs. 23–31 and Supplementary Table 1). The PtNP/TiO2-R data can be characterized by Pt–O and Pt–Pt coordination at distances of 1.98 and 2.72 Å, with an average coordination number of 1.1 and 8.3, respectively. In PtPt-TiISAA+NP/TiO2-R, the Pt–Pt coordination number decreases, accompanied by the concurrent emergence of the Pt–Ti bonds with a bond length of 2.57 Å and an average coordination number of 5.6.
Figure 1h shows the normalized Pt L3-edge X-ray absorption near-edge structure (XANES) spectra of PtNP/TiO2-R and PtPt-TiISAA+NP/TiO2-R, along with the reference spectra of Pt foil and PtO2. In the case of PtNP/TiO2-R, its white-line intensity closely resembles that of the Pt foil, while for PtPt-TiISAA+NP/TiO2-R, the XANES spectrum shows higher white-line intensity than that of the PtNP/TiO2-R and Pt foil due to the existence of the Pt–O coordination (Fig. 1g). Figure 1i presents the Pt 4f X-ray photoelectron spectroscopy (XPS) spectra of all the samples. The peaks at 74.8 and 71.5 eV belong to Pt0,35. Compared to PtNP/TiO2-R and PtNP/TiO2, the peak Pt0 of PtPt-TiISAA+NP/TiO2-R shifts to significantly lower binding energy, further demonstrating an increased electron density at Pt atoms in this sample36. The AC-HAADF-STEM analyses, along with the XAFS and XPS spectroscopic observations, confirm the transformation of part of the Pt metal in the PtNP/TiO2-R NP into ISAA Pt–Ti after the H2 treatment.
From the Ti 2p spectra (Supplementary Fig. 32), the lower binding energies of PtPt-TiISAA+NP/TiO2-R and PtNP/TiO2-R compared to PtNP/TiO2 are attributed to the reduction of Ti from +4 valence to a lower valence state, suggesting the presence of Ov in TiO2. Defects of Ov and adsorbed O2− ions are detected by EPR measurement (Fig. 2a). For PtNP/TiO2, only a signal with g = 2.002 attributed to Ov can be observed37. Compared to PtNP/TiO2, PtPt-TiISAA+NP/TiO2-R, and PtNP/TiO2-R exhibit distinct peaks at gz = 2.045, gy = 2.031 and gx = 2.006, which are associated with adsorbed O2− ions. Notably, PtPt-TiISAA+NP/TiO2-R has the highest concentrations of Ov and O2− ions23. The O2– signal arises from a single electron transfer from the Ti 3d orbitals to the adsorbed O2. A higher concentration of Ov results in increased unpaired electron transfer, enhancing the adsorption of oxygen molecules. Additionally, the PtPt-TiISAA+NP/TiO2-R exhibits a more pronounced O2– signal than the PtNP/TiO2-R catalyst and the TiO2-R support, indicating the interaction between isolated Pt species and the TiO2 support. The thus altered electronic properties of the Pt species also enhance the adsorption and activation capacities of O2. In all, the presence of isolated Pt and Ov serves as two significant factors contributing to the enhanced formation of chemisorbed O2–.
Temperature-programmed desorption of CO (CO-TPD) was also conducted to examine the desorption of CO on PtNP/TiO2-R, PtPt-TiISAA+NP/TiO2-R, and PtNP/TiO2 (Fig. 2b). The desorption temperature of CO follows the trend: PtNP/TiO2 > PtNP/TiO2-R > PtPt-TiISAA+NP/TiO2-R, indicating that the existence of Pt single atom and Ov weakens the CO adsorption. The temperature-programmed desorption of O2 (O2-TPD) analysis is performed to investigate the activated oxygen species. As shown in Fig. 2c, all PtNP/TiO2-R, PtPt-TiISAA+NP/TiO2-R, and PtNP/TiO2 samples show a prominent peak below 200 °C, attributed to the desorption of the physisorbed O2. The desorption peak observed in the moderate temperature range (200–300 °C) can be assigned to the surface-adsorbed oxygen species usually associated with Ov22. In particular, the desorption peaks of PtPt-TiISAA+NP/TiO2-R suggest that significantly more pronounced surface-adsorbed oxygen species existed in it than in PtNP/TiO2-R and PtNP/TiO2. The desorption peak at 300 °C can be attributed to the bulk oxygen species in the lattice (Olatt)22. Compared to PtNP/TiO2, PtPt-TiISAA+NP/TiO2-R shows lower desorption temperatures of Olatt species, indicating that the Olatt species in PtPt-TiISAA+NP/TiO2-R had higher mobility, attributed to the combined effect of Ov and Pt-Ti ISAA. In brief, the EPR, CO-TPD, and O2-TPD results confirm that Pt-Ti ISAA and Ov weaken the adsorption of CO and realize simultaneous dual activation of chemisorbed O2 and surface lattice oxygen.
The oxygen species of the catalysts were further characterized by H2-TPR analysis (Fig. 2d). A major reduction peak is observed for Pt/TiO2-R at 243 °C, which is attributed to the reduction of surface lattice oxygen near Pt (Pt-O-Ti bond). For PtPt-TiISAA+NP/TiO2-R, a peak at 186 °C is observed, likely caused by a new type of active surface lattice oxygen (lattice oxygen near Pt–Ti ISAA). The lattice oxygen near Pt–Ti ISAA in PtPt-TiISAA+NP/TiO2-R may oxidize CO adsorbed on Pt readily5.
Figure 3a shows the In situ diffuse reflectance Fourier transform infrared spectroscopy (DRIFTS) spectra of CO chemisorption on all catalysts at 100 °C. The adsorption of CO on PtNP/TiO2-R generates a prominent vibration peak at 2069 cm–1 and a weak peak at 2119 cm–1. The main peak at 2069 cm–1 can be ascribed to CO linearly bonded to Pt0 sites38, while the peak at 2119 cm–1 is attributed to the adsorption of CO on Pt2+,39. This observation aligns with the analysis results of AC-HAADF-STEM and EXAFS, indicating the exclusive existence of Pt NPs. Compared to PtNP/TiO2-R, PtPt-TiISAA+NP/TiO2-R has an additional peak at 2097 cm–1, attributed to CO adsorption on single Ptδ+ atoms, indicating the existence of Pt SA. This agrees with the AC-HAADF-STEM and EXAFS results that Pt NP coexists with individual Pt atoms in the PtPt-TiISAA+NP/TiO2-R sample. For CO adsorption on the sample PtNP/TiO2, only two weak peaks are observed at 2119 and 2069 cm–1, respectively. Additionally, the CO adsorption on TiO2 and TiO2-R supports alone is minimal (Supplementary Fig. 33).
a In situ DRIFTS spectra of the CO adsorption for all catalysts. b–d In situ DRIFTS spectra of the CO oxidation over various catalysts at different temperatures. The reaction conditions: 20 mg of Cat.; CO = 1 vol%, O2 = 16 vol %, N2 balance; v = 100 mL min−1. In situ NAP-XPS of e O 1s and (f and g) C 1s spectra of PtPt-TiISAA+NP/TiO2-R at 150 °C: in UHV, under CO, under O2, under O2 and CO conditions. h Raman spectra of PtPt-TiISAA+NP/TiO2-R. i Schematic of CO oxidation on PtPt-TiISAA+NP/TiO2-R. j Raman spectra of PtNP/TiO2-R.
Figures 3b–d show the in situ DRIFTS spectra of PtNP/TiO2-R, PtPt-TiISAA+NP/TiO2-R, and PtNP/TiO2. The measurements were taken under a continuous flow of the reaction gas (VO2/VCO = 16) at different reaction temperatures. The strength of the CO adsorption on the Pt species in PtNP/TiO2-R increases gradually with the rise in temperature, indicating a slow reaction of the CO adsorbed on Pt. At the temperature of 75 °C, carbonate or formate species are observed22, with peak intensity increasing as temperature rises to a maximum at 125 °C, then dropping. Carbonate or formate species are active reaction intermediates closely associated with catalytic activity. In addition, the intensity of CO adsorption on Pt species in PtPt-TiISAA+NP/TiO2-R gradually increased with increasing temperature, reaching a maximum at 125 °C, followed by a decrease. Notably, CO adsorption on Pt0 nearly vanished at 200 °C. Furthermore, the amount of carbonate or formate species decreased with temperature and eventually disappeared completely at 200 °C. Interestingly, no CO band attributed to Ptδ+ was found in PtNP/TiO2-R when O2 and CO were co-fed at 50 °C, and the catalyst was not catalytically active at this temperature. It is because the Ptδ+ sites were mainly occupied for O2 activation rather than reacting with O2, leading to the disappearance of the CO band associated with Ptδ+. In contrast, for PtNP/TiO2, both the CO adsorption peaks and the carbonate or formate peaks gradually become more robust with the increase in temperature. DRIFTS results show that the CO adsorbed on PtPt-TiISAA+NP/TiO2-R can rapidly convert to reactive carbonate species during CO oxidation and can subsequently be consumed by Pt-activated O2. These results indicate that active carbonate species existed extensively on the catalyst surface during CO oxidation, further confirming the involvement of abundant active oxygen species. The CO adsorbed on PtPt-TiISAA+NP/TiO2-R quickly converts to active carbonate species during CO oxidation, and these species can then readily react with O2 activated by Pt, compared to the other two catalysts.
Figure 3e shows the O 1s near ambient pressure XPS (NAP-XPS) spectra measured under different atmospheric conditions. In ultra-high vacuum (UHV) conditions, the measured predominant peak at 530.0 eV for PtPt-TiISAA+NP/TiO2-R corresponds to Olatt40. Upon introducing CO into the chamber, the intensity of the Olatt signal is significantly weaker than that under UHV conditions. When the reaction condition was changed to O2 + CO, a new peak at 531.5 eV emerged following exposure to an O2 atmosphere, which can be attributed to the formation of Oads41. This suggests that in the absence of injected O2, CO can react with the surface lattice Olatt via the MvK mechanism, followed by the adsorption of additional O2 on the reacted surface lattice oxygen22. Meanwhile, transitioning from a pure CO to a CO + O2 environment decreases the signal of CO32– and CO2– reaction intermediates (Fig. 3f). The abundant O2 promotes CO oxidation by accelerating the replenishment of reactive oxygen species over PtPt-TiISAA+NP/TiO2-R. Upon initial exposure of PtPt-TiISAA+NP/TiO2-R to O2, the two peaks associated with CO32– and CO2– decreased notably, and no significant O 1s peak of Oads was due to the further complete oxidation of CO32– and CO2– intermediates to CO2 (Fig. 3g and Supplementary Fig. 34). CO32– and CO2– were not regenerated when CO passed again due to the oxidation of Pt0. However, the change in Pt valence could not be observed due to the low Pt loading (Supplementary Fig. 35). However, when CO and O2 were introduced simultaneously, Pt0 was not oxidized because of the competitive adsorption. Pt0 has a preferential adsorption for CO and a weaker adsorption ability for O2.
In the Raman spectra of PtPt-TiISAA+NP/TiO2-R (Fig. 3h), the peaks observed at 638, 511, and 395 cm−1 are assigned to the vibration modes of Eg, A1g + B1g doublet, and B1g, respectively42. In contrast, the Eg, A1g + B1g, and B1g vibrational modes of PtPt-TiISAA+NP/TiO2-R shift to lower wavenumbers after exposure to CO, indicating the weakened bond strength of O–Ti(Pt)–O. The weakened bond suggests a higher mobility of Olatt in TiO2, thus contributing to the reverse oxygen spillover during CO oxidation on the surface of the PtPt-TiISAA+NP/TiO2-R catalyst (Fig. 3i)19. In the case of PtNP/TiO2-R (Fig. 3j), there is no discernible change in peak positions upon adding CO, indicating that the strength of the O–Ti–O bond remains unaltered during the CO oxidation reaction at 150 °C. In conclusion, in situ DRIFTS results demonstrate that CO is more readily adsorbed on Pt NP than on Pt SA in the Pt–Ti ISAA. Pt SA provides a suitable site for the activation of oxygen species. The in situ NAP-XPS and Raman results indicate the involvement of the surface lattice oxygen in the vicinity of Pt–Ti ISAA during the reaction, demonstrating that CO oxidation over PtPt-TiISAA+NP/TiO2-R catalyst involves the MvK mechanism.
Performance of CO oxidation on the catalysts
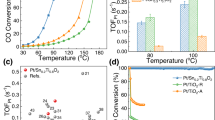
The catalytic CO oxidation performance and the participation of active oxygen species were studied by using CO as the probe molecule. Figure 4a shows the CO oxidation curves of PtNP/TiO2-R, PtPt-TiISAA+NP/TiO2-R, and PtNP/TiO2 under a high WHSV of 60,000 mL g–1 h–1, utilizing gas mixtures comprising 1 vol% CO and 4 vol% O2 balanced with N2. PtPt-TiISAA+NP/TiO2-R exhibited significantly superior catalytic activity for CO oxidation. For PtPt-TiISAA+NP/TiO2-R, the CO conversion was 50% at 106 °C, which is substantially lower by 118 °C and 162 °C than PtNP/TiO2-R (T50 = 224 °C) and PtNP/TiO2 (T50 = 268 °C), respectively. In particular, PtPt-TiISAA+NP/TiO2-R achieved a much higher CO conversion in the low-temperature range (80–150 °C) than PtNP/TiO2-R and PtNP/TiO2. PtNP/TiO2-R demonstrates superior catalytic performance compared to PtNP/TiO2. It is because of the increased concentration of Ov (Fig. 2a), which facilitates more unpaired electron transfer and enhances the adsorption of oxygen molecules. Furthermore, PtPt-TiISAA+NP/TiO2-R exhibits a more significant interaction between the Pt species and the TiO2 support than observed in PtNP/TiO2-R (Fig. 1i). This interaction leads to modified electronic properties of Pt, which in turn further improve the adsorption and activation of O2. The catalytic tests of TiO2 and TiO2-R supports indicate that the activity of supports below 250 °C is negligible (Supplementary Fig. 36). For the three catalysts examined, a decrease in the oxygen concentration was observed to correlate with a reduction in CO conversion. The strong correlation between the catalyst activity and the O2 concentration is indicative of the L–H mechanism that governs CO oxidation, which is applicable to all three catalysts. Notably, in the case of PtPt-TiISAA+NP/TiO2-R, the temperature required for 50% CO conversion rises by 35 °C (from 90 °C to 125 °C) as the oxygen content diminishes from 16% to 1% (Fig. 4b). This increase is significantly less pronounced than that observed for PtNP/TiO2-R (110 °C) (Fig. 4c) and PtNP/TiO2 (94 °C) (Fig. 4d), which is attributed to the presence of the MvK pathway on PtPt-TiISAA+NP/TiO2-R. To assess the impact of hydrogen treatment on catalytic performance, we evaluated the catalytic activity of PtNP/TiO2-H2, which was obtained via treatment in hydrogen at 300 °C for 30 min (Supplementary Fig. 37). The results indicated a negligible improvement in performance compared to PtNP/TiO2. This finding supports the conclusion that the enhanced performance of PtPt-TiISAA+NP/TiO2-R over PtNP/TiO2-R is not due to alterations in the valence state of Pt induced by hydrogen treatment. Instead, the superior performance is attributed to the presence of the Pt–Ti ISAA structure in PtPt-TiISAA+NP/TiO2-R. As depicted in Fig. 4e, the measured apparent activation energy (Ea) value of CO oxidation for PtNP/TiO2-R, PtPt-TiISAA+NP/TiO2-R, and PtNP/TiO2 is 82.39, 34.08, and 208.93 kJ mol−1, respectively. The intensity of CO adsorption on the catalyst surface influences the subsequent dissociation of CO and the desorption energy barrier of CO2, which ultimately leads to the change of the activation energy barrier of CO. CO-TPD analysis reveals that the presence of Pt–Ti ISAA and Ov diminishes CO adsorption (Fig. 2b), thereby reducing the Ea for PtPt-TiISAA+NP/TiO2-R to the lowest value among the catalysts. Density Functional Theory (DFT) calculations will further discuss the activation energy barrier of CO over different catalysts. To quantify the activity of PtPt-TiISAA+NP/TiO2-R, we measured its TOF and compared it with the values reported for catalysts in the literature (Fig. 4f and Supplementary Table 2). It should be noted that the TOF value of PtPt-TiISAA+NP/TiO2-R obtained in this work is among the best of all the reported catalysts (Pt NP/TiO243, Pt/CNT-60044, Ptn/CeCu4, Pt1/CeOx-TiO245, Pt/Sn0.2Ti0.8O219, Pt/CeO246, Pt-O-Pt/CeO247, and Pt/m-Al2O348). Moreover, PtPt-TiISAA+NP/TiO2-R exhibits excellent cycling stability due to the presence of Pt–Ti ISAA, showing no decline in activity after five cycles (Fig. 4g). We further evaluated the long-term catalytic performance at 110 °C (Fig. 4h). PtPt-TiISAA+NP/TiO2-R maintained a ~87% CO conversion at 110 °C during the 100 h test period.
a CO conversion as a function of reaction temperature over all catalysts. Reaction conditions: 0.4 g of Cat., 1 vol% CO, 4 vol% O2, balanced with N2; v = 400 mL min−1, GHSV = 60,000 mL g−1 h−1, b–d Activity test on all catalysts with different O2 concentrations. Reaction conditions: 0.4 g of Cat., v = 400 mL min−1, GHSV = 60,000 mL g−1 h−1, e Apparent activation energies (Ea) over different catalysts. f In comparison with the catalysts listed in Supplementary Table 2. g The cycling test of the CO oxidation activity of PtPt-TiISAA+NP/TiO2-R from 80 to 160 °C. Cyclic CO oxidation reaction condition: 0.4 g of Cat., a gas mix containing 1 vol% CO and 16 vol% O2 balanced with N2, v = 400 mL min−1. h A long-term test of PtPt-TiISAA+NP/TiO2-R (0.4 g of Cat., 110 °C, 1 vol% CO, 16 vol% O2, balanced with N2; v = 400 mL min−1, GHSV = 60,000 mL g−1 h−1).
DFT study on the CO oxidation pathways
Based on the nearly identical catalyst structures of PtNP/TiO2-R and PtNP/TiO2, the main difference being the varying concentrations of oxygen vacancies, and considering the experimental data on different oxygen concentrations, it is clear that these catalysts follow a similar reaction pathway. Consequently, we have selected PtNP/TiO2 and PtPt-TiISAA+NP/TiO2-R as model catalysts to calculate the reaction energies for each basic phase of CO oxidation as it occurs along the L–H and MvK pathways on their surfaces, as depicted in Fig. 5 and Supplementary Fig. 38. To align our established DFT computational model more closely with the catalyst structures during actual reactions, we employed a CO-covered NP model to depict the catalyst surface under CO oxidation conditions more accurately. In pursuit of determining the energetically optimal CO and O2 coverage for the two Pt/TiO2 models, we assessed the CO binding energy on the Pt NP of Pt10/TiO2. Our analysis reveals that within the 8 CO saturated adsorption structure, O2 can effectively compete for adsorption at the ninth Pt site, as its binding energy (Ebind) (−1.80 eV) surpasses that of the ninth CO molecule (−1.48 eV), particularly evident in the case of PtPt-TiISAA+NP/TiO2-R. This observation corroborates with previously reported findings49. The adsorption energies of O2 on both catalysts are comparable, measuring −1.80 eV for CO oxidation reactions along the L–H pathway on either catalyst (Fig. 5a). Although the energy barrier for CO2 formation is marginally higher for PtPt-TiISAA+NP/TiO2-R compared to PtNP/TiO2, the critical rate-determining step is the subsequent desorption of CO2. Notably, PtPt-TiISAA+NP/TiO2-R shows a superior capacity for CO2 desorption, which facilitates easier CO2 removal and thus facilitates the overall reaction.
In the course of the MvK pathway, the CO adsorption energy of the Pt NP on PtPt-TiISAA+NP/TiO2-R is −1.48 eV, which is less negative than the −1.80 eV observed for the Pt NP on PtNP/TiO2 (Fig. 5b). This suggests a reduction in the CO adsorption energy barrier due to the presence of single atoms. This finding aligns with the CO-TPD results. Furthermore, the adsorption energy of the Pt SA on PtPt-TiISAA+NP/TiO2-R for CO is −2.03 eV, indicating that the Pt SA within the Pt-Ti ISAA configuration possesses the capability to adsorb CO effectively. The rate-determining step on PtNP/TiO2-R is the premier oxidation of CO by surface lattice oxygen, necessitating overcoming an energy barrier of 1.59 eV. In contrast, for the PtPt-TiISAA+NP/TiO2-R system, the surface lattice oxygen in proximity to the Pt–Ti ISAA markedly reduces the energy barrier of the initial step to 1.47 eV via oxidation with the CO adsorbed on Pt SA. The activation threshold for the foremost formation of CO2 on PtPt-TiISAA+NP/TiO2-R is adequately low, thereby facilitating the reaction at comparatively subdued temperatures, agreeing with the experimental results. This is because Pt substitution instigates lattice deformation, thus reducing the energy barrier for generating oxygen vacancies. For PtNP/TiO2, the inaugural CO2 molecule is synthesized through the interaction of CO with the lattice oxygen at the surface. The ensuing desorption of CO2 gives rise to an oxygen vacancy at the surface, which is subsequently replenished via the adsorption of O2. Interestingly, the energy requisite for the secondary CO oxidation reaction on the PtNP/TiO2 catalyst is 0.98 eV, significantly higher than its counterpart on PtPt-TiISAA+NP/TiO2-R (0.61 eV). In this step, the extruded O atoms of the O2 molecules oxidize the second CO again, directly precipitate to form the second CO2 molecules, and the remaining oxygen atoms fill the oxygen vacancies and restore the initial structure of the catalyst. These findings contribute to a comprehensive understanding of catalytic CO oxidation processes and pave the way for designing improved catalysts for other oxidation reactions.
Discussion
We have developed a highly efficient Pt-based catalyst PtPt-TiISAA-NP/TiO2-R for CO oxidation by designing a unique structure with dual active centers of Pt NP and Ti-isolated Pt atoms anchored on oxygen-deficient TiO2. This catalyst achieves a record high turnover frequency (TOF) of 11.59 s–1 at 80 °C in the CO oxidation in air, surpassing that of PtNP/TiO2, PtNP/TiO2-R, and other Pt-based catalysts reported in the literature. It is found that the TiO2 lattice distortion induced by Pt SA in the Pt–Ti ISAA activates the adjacent surface lattice oxygen, allowing the Mars-van Krevelen (MvK) reaction path to prevail at low temperatures. Furthermore, the synergistic effect of Pt nanoparticles and isolated Pt atoms weakens the competitive adsorption of CO and O2. It accelerates the adsorption and activation process of O2, thereby enhancing the catalytic performance. This work provides valuable insights into the reaction mechanisms and lays a solid foundation for designing highly efficient Pt catalysts for low-temperature abating pollutants in exhaust gases.
Methods
Chemicals and materials
Anatase TiO2 (99.8%), ethanol (99.9%), and the solution of H2PtCl6 (8.0 wt%) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.
Synthesis of TiO2-R support
The TiO2 sample was reduced at 600 °C for 3 h with pure H2 gas to obtain the defective TiO2 (TiO2-R) with surface oxygen vacancies.
Preparations of PtNP/TiO2-R and PtPt-TiISAA+NP/TiO2-R catalysts
1 g of TiO2-R was added to 50 mL of ethanol, stirred, and continuously sonicated for 60 min. Then, 50 mg of H2PtCl6 solution was added to the above slurry dropwise. After stirring for 12 h, the yellow powder was collected by centrifugation, dried, and further calcined in a muffle furnace at 300 °C for 2 h. The obtained catalyst is denoted as PtNP/TiO2-R, and its Pt loading is 0.15 wt%, determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES).
PtPt-TiISAA+NP/TiO2-R was obtained by treating PtNP/TiO2-R in H2 atmosphere at 300 °C for 30 min.
Preparations of 0.5 wt%PtPt-TiISAA+NP/TiO2-R
H2PtCl6 solution (0.05 g) was dissolved into 50 mL of ethanol, and then TiO2-R (2.00 g) was added to the above solution. The mixture was stirred at 50 °C until ethanol evaporated and then calcined at 300 °C for 2 h after dried at 50 °C overnight, followed by reduction at 300 °C in H2 for 30 min. This catalyst was denoted 0.5 wt%PtPt-TiISAA+NP/TiO2-R.
Preparations of PtNP/TiO2 and PtNP/TiO2-H2 catalysts
PtNP/TiO2 was synthesized with the same method as PtNP/TiO2-R on pristine TiO2 as the support.
PtNP/TiO2-H2 was obtained by treating PtNP/TiO2 in H2 atmosphere at 300 °C for 30 min.
Characterization
The microstructures of the catalysts are characterized by transmission electron microscopy (TEM) (JEM-2010F, JEOL, Tokyo, Japan) and energy dispersive spectroscopy (EDS), at an operating voltage of 200 kV.
Aberration-corrected scanning transmission electron microscopy (AC-STEM) and EELS analyses were conducted utilizing a probe-corrected Hitachi HF5000 S/TEM, with operation at 200 kV. The microscope is furnished with bright field (BF) and high-angle annular-dark field (HAADF) detectors to facilitate high spatial resolution STEM imaging experiments. Additionally, the microscope features a secondary electron detector and dual Oxford Instruments XEDS detectors (2 × 100 mm2) with a collection angle of 2.02 sr. Further aberration-corrected scanning transmission electron microscopy analyses were carried out employing a Thermo Fisher ThemisZ S/TEM, operating at 300 keV. This instrument is equipped with a HAADF detector and a segmented DF4 detector to enable high spatial resolution STEM-HAADF and STEM-iDPC imaging experiments. The incorporated Super-X detector covers a total area of 120 mm2 and a solid angle of 0.7 sr.
The crystalline phases of the samples were analyzed through X-ray diffraction (XRD) using a PANalytical X’Pert PRO MPD instrument with Cu Kα radiation (λ = 1.5418 Å).
CO temperature-programmed desorption (CO-TPD) and O2 temperature-programmed desorption (O2-TPD) experiments were carried out using the Quanta chrome Automated Chemisorption Analyzer equipped with a TCD detector (chemBET pulsar TPR/TPD). In the CO-TPD experiment, a quantity of 0.05 g of catalyst was used. The catalyst was treated in a CO/N2 (5/95) atmosphere for 60 min, followed by the removal of physically adsorbed CO by purging with N2 for 2 h. Then, the sample was heated up to 600 °C with a ramping rate of 10 °C min−1 under a flow of N2 (30 mL min−1). For O2-TPD, a 0.05 g catalyst was used. The catalyst was treated in O2/Ar (10/90) for 60 min, followed by the removal of physically adsorbed O2 by purging with Ar for 2 h. Finally, the sample was heated to 600 °C with a ramping rate of 10 °C min−1 in an Ar flow (30 mL min−1).
Raman spectra were acquired on a Renishaw inVia Raman spectrometer with an excitation wavelength of 532 nm (5 mW) and a spectral range spanning from 100 to 1000 cm−1.
The ICP-OES analysis was conducted using the Perkin-Elmer Optima 5300DV instrument. Before the analysis, a quantity of 0.05 g of sample was dissolved in 3 ml of a 3 M nitric acid solution at room temperature. Following suitable dilution until to achieve a concentration below 100 ppm, the solution was then assayed for Pt content.
The surface compositions of the catalysts were analyzed using X-ray photoelectron spectroscopy (XPS) with Al Kalpha radiation (hν = 1486.6 eV) on a Model VG ESCALAB 250 spectrometer manufactured by Thermo Electron in the U.K.
The X-band electron paramagnetic resonance (EPR) spectra results were obtained using a JEOL JES-RE2X electron spin resonance spectrometer at 70 K (BRUKER E500). The g values were standardized with 2,2-diphenyl-1-picrylhydrazyl (g = 2.0036) as the reference compound.
In situ DRIFTS analysis was conducted utilizing a Nicolet 6700 FTIR spectrophotometer (Thermo Fisher, Germany) equipped with a Harrick cell and a liquid nitrogen-cooled MCT detector. Initially, the catalysts and KBr were physically blended in a 1:10 weight ratio and pre-treated in a stream of pure N2 (100 mL min–1) at 200 °C for 1 h, followed by cooling to the desired test temperature. Background spectra were acquired during the cooling process in a pure N2 (100 mL min–1) atmosphere and subtracted from the corresponding spectra. The DRIFTS test was conducted under the following conditions: 16 vol% CO + 16 vol% O2, with an equilibrium gas of N2 and a total flow rate of 50 mL min–1. All spectra were obtained by averaging 32 scans in the 600–4000 cm–1 range at a resolution of 4 cm–1.
The extended X-ray absorption spectroscopy (XAS) measurements at lower potentials at beamline BL14W1 were conducted at the Shanghai Synchrotron Radiation Facility (SSRF). The XAS data were analyzed utilizing the IFEFFIT software package, with energy calibration and spectral normalization carried out using Athena software50.
Catalytic measurement
The CO oxidation activity tests were conducted in a continuous flow fixed-bed quartz microreactor with an inner diameter of 8 mm using prepared catalysts (400 mg, 20–40 mesh). In all activity tests, the total flow rate of the feed gas was maintained at 400 mL/min, and the gas hourly space velocity (GHSV) was set at 60,000 mL g–1 h–1. The reaction gas mixture consists of 1% CO, 16% O2, and N2 as the equilibrium gas. Continuous monitoring of CO concentrations at the inlet and outlet was performed using a portable compound gas analyzer (PTM600, Shenzhen Elantech Electronics Co., Ltd.). The conversion of CO was determined using Eq. (1):
Where X represents the CO conversion, and NCO,inlet, and NCO,outlet denote the inlet and outlet concentration of CO (ppm).
The catalytic velocities were ascertained through the turnover frequency of CO conversion over Pt sites (TOFPt (s−1)), which can be calculated using the following equation19:
Where XCO is CO conversion (<15%), FCO (μmol s−1) is the molar gas flow rate of CO, and NPt (μmol) is the total number of Pt atoms on the catalyst. It should be noted that all the catalysts used contained 0.15 wt% Pt. In order to ensure a fair evaluation of catalyst activities at the same Pt usage, all Pt atoms were taken into the TOF calculation.
Following the Arrhenius equation, the apparent activation energies (Ea in kJ/mol) over the catalysts were determined based on the data obtained at a CO conversion below 15%.
Computational detail
All DFT calculations were done through the Vienna ab initio simulation package (VASP)51, with the Perdew–Burke–Ernzerhof (PBE) functional employed in this study52. The core electrons were treated with projector augmented-wave (PAW) pseudopotentials53. A plane wave cutoff energy of 450 eV was adopted. The k-point was set to the Γ-point. The geometric relaxation convergence criterion was 0.02 eV/Å. Additionally, van der Waals interactions were corrected using the DFT-D3 method54. The transition states for all elementary reactions were located utilizing the CI-NEB (Climbing Image Nudged Elastic Band) method55. The activation energy (Ea) was determined using the equation Ea = ETS − ER. In this equation, ETS denotes the energy of the transition state system, while ER represents the energy of the reactants. This calculation employed the TiO2 (100) surface, consisting of four layers, to explore the potential reaction mechanism. The atoms in the bottom two layers of the TiO2 slabs were held fixed, whereas the remaining atoms were allowed to relax in their respective positions. The Pt10 cluster56 and Pt1 single-atom catalysts were also generated to represent the active phases of this research. The vacuum region was set as 13 Å. To better understand the reaction mechanism, it was considered the adsorption of 8 CO molecules and 1 O2 molecule on the surface of the Pt10 cluster in the L–H mechanism. In contrast, in the MvK mechanism calculation, the adsorptions of 9 CO molecules on the Pt10 cluster and 1 CO molecule on the Pt1 surface were considered.
Data availability
The data that support the plots within this paper and another finding of this study are available from the corresponding author upon request. Source data are provided as a Source Data file. Source data are provided in this paper.
References
Zammit, M. et al. Future automotive aftertreatment solutions: the 150 °C challenge workshop report (U.S. DRIVE Report, Southfield, MI, 2013).
Getsoian, A., Theis, J. R., Paxton, W. A., Lance, M. J. & Lambert, C. K. Remarkable improvement in low temperature performance of model three-way catalysts through solution atomic layer deposition. Nat. Catal. 2, 614–622 (2019).
Emissions, G. G. Fuel efficiency standards for medium-and heavy-duty engines and vehicles–phase 2. Environ. Prot. Agency EPA-HQ-OAR-2014-082, Fed. Register 81, 73478–74274 (2016).
Liu, X. et al. Activation of subnanometric Pt on Cu-modified CeO2 via redox-coupled atomic layer deposition for CO oxidation. Nat. Commun. 11, 4240 (2020).
Nie, L. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Min, B. K. & Friend, C. M. Heterogeneous gold-based catalysis for green chemistry: low-temperature CO oxidation and propene oxidation. Chem. Rev. 107, 2709–2724 (2007).
Xie, X., Li, Y., Liu, Z., Haruta, M. M. & Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 458, 746–749 (2009).
Qu, J. et al. Eye-readable detection and oxidation of CO with a platinum-based catalyst and a binuclear rhodium complex. Angew. Chem. Int. Ed. 58, 12258–12263 (2019).
Wang, A. & Olsson, L. The impact of automotive catalysis on the united nations sustainable development goals. Nat. Catal. 2, 566–570 (2019).
Beniya, A. & Higashi, S. Towards dense single-atom catalysts for future automotive applications. Nat. Catal. 2, 590–602 (2019).
Allian, A. D. et al. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 133, 4498–4517 (2011).
Kale, M. J. & Christopher, P. Utilizing quantitative in situ FTIR spectroscopy to identify well-coordinated Pt atoms as the active site for CO oxidation on Al2O3-supported Pt catalysts. ACS Catal. 6, 5599–5609 (2016).
Ruiz Puigdollers, A., Schlexer, P., Tosoni, S. & Pacchioni, G. Increasing oxide reducibility: the role of metal/oxide interfaces in the formation of oxygen vacancies. ACS Catal. 7, 6493–6513 (2017).
Beniya, A. et al. CO oxidation activity of non-reducible oxide-supported mass-selected few-atom Pt single-clusters. Nat. Commun. 11, 1888 (2020).
Fu, Q. et al. Interface-confined ferrous centers for catalytic oxidation. Science 328, 1141–1144 (2010).
Wang, Y. et al. Electron penetration triggering interface activity of Pt-graphene for CO oxidation at room temperature. Nat. Commun. 12, 5814 (2021).
Mu, R. et al. Synergetic effect of surface and subsurface Ni species at Pt−Ni bimetallic catalysts for CO. Oxid. J. Am. Chem. Soc. 133, 1978–1986 (2011).
Kim, J. et al. Adsorbate-driven reactive interfacial Pt-NiO1−x nanostructure formation on the Pt3Ni(111) alloy surface. Sci. Adv. 4, 3151 (2018).
Chen, J. et al. Reverse oxygen spillover triggered by CO adsorption on Sn-doped Pt/TiO2 for low-temperature CO oxidation. Nat. Commun. 14, 3477 (2023).
Liu, Y. et al. Surface oxygen vacancies on reduced Co3O4 (100): superoxide formation and ultra-low-temperature CO oxidation. Angew. Chem. Int. Ed. 60, 16514–16520 (2021).
Fu, Q. et al. Reversible structural transformation of FeOx nanostructures on Pt under cycling redox conditions and its effect on oxidation catalysis. Phys. Chem. Chem. Phys. 15, 14708–14714 (2013).
Fang, Y. et al. Dual activation of molecular oxygen and surface lattice oxygen in single atom Cu1/TiO2 catalyst for CO oxidation. Angew. Chem. Int. Ed. 61, 202212273 (2022).
He, Y. et al. Operando identification of dynamic photoexcited oxygen vacancies as true catalytic active sites. ACS Catal. 13, 191–203 (2023).
Chiesa, M., Giamello, E. & Che, M. EPR characterization and reactivity of surface-localized inorganic radicals and radical ions. Chem. Rev. 110, 1320–1347 (2010).
Bi, X. et al. Tuning oxygen vacancy content in TiO2 nanoparticles to enhance the photocatalytic performance. Chem. Eng. Sci. 234, 116440 (2021).
Xie, H., Li, N., Chen, X., Jiang, J. & Zhao, X. Surface oxygen vacancies promoted photodegradation of benzene on TiO2 film. Appl. Surf. Sci. 511, 145597 (2020).
Zheng, X. et al. Construction of Fe-doped TiO2−x ultrathin nanosheets with rich oxygen vacancies for highly efficient oxidation of H2S. Chem. Eng. J. 430, 132917 (2022).
Choudhury, B., Verma, R. & Choudhury, A. Oxygen defect assisted paramagnetic to ferromagnetic conversion in Fe doped TiO2 nanoparticles. RSC Adv. 4, 29314–29323 (2014).
Wan, J. et al. Defect effects on TiO2 nanosheets: stabilizing single atomic site Au and promoting catalytic properties. Adv. Mater. 30, 1705369 (2018).
Wang, T. et al. Synergistic Pd single atoms, clusters, and oxygen vacancies on TiO2 for photocatalytic hydrogen evolution coupled with selective organic oxidation. Small 17, 2006255 (2021).
Zhang, X. et al. Pt3Ti intermetallic alloy formed by strong metal–support interaction over Pt/TiO2 for the selective hydrogenation of acetophenone. ACS Catal. 13, 4030–4041 (2023).
Xie, M. et al. Intermetallic single-atom alloy In–Pd bimetallene for neutral electrosynthesis of ammonia from nitrate. J. Am. Chem. Soc. 145, 13957–13967 (2023).
He, W. et al. Structural evolution of anatase-supported platinum nanoclusters into a platinum-titanium intermetallic containing platinum single atoms for enhanced catalytic CO oxidation. Angew. Chem. Int. Ed. 62, 202213365 (2023).
Shibata, N. et al. Interface structures of gold nanoparticles on TiO2 (110). Phys. Rev. Lett. 102, 136105 (2009).
Zhang, B.-W. et al. Atomic structural evolution of single-layer Pt clusters as efficient electrocatalysts. Small 17, 2100732 (2021).
Ji, Y. et al. Isolating contiguous Ir atoms and forming Ir–W intermetallics with negatively charged Ir for efficient NO reduction by CO. Adv. Mater. 34, 2205703 (2022).
Rabee, A. I. M. et al. Role of interfacial oxygen vacancies in low-loaded Au-based catalysts for the low-temperature reverse water gas shift reaction. Appl. Catal. B 321, 122083 (2023).
Xie, S. et al. Pt atomic single-layer catalyst embedded in defect-enriched ceria for efficient CO oxidation. J. Am. Chem. Soc. 144, 21255–21266 (2022).
Jeong, H. et al. Controlling the oxidation state of Pt single atoms for maximizing catalytic activity. Angew. Chem. Int. Ed. 59, 20691–20696 (2020).
Zhang, T. et al. The dual-active-site tandem catalyst containing Ru single atoms and Ni nanoparticles boosts CO2 methanation. Appl. Catal. B 323, 122190 (2023).
Zhang, T. et al. Enhancing the low-temperature CO2 methanation over Ni/La-CeO2 catalyst: the effects of surface oxygen vacancy and basic site on the catalytic performance. Appl. Catal. B 312, 121385 (2022).
Ohsaka, T., Izumi, F. & Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 7, 321–324 (1978).
Hojo, H., Gondo, M., Yoshizaki, S. & Einaga, H. Atomic and electronic structure of Pt/TiO2 catalysts and their relationship to catalytic activity. Nano Lett. 22, 145–150 (2022).
Chen, W. et al. Molecular-level insights into the electronic effects in platinum-catalyzed carbon monoxide oxidation. Nat. Commun. 12, 6888 (2021).
Yoo, M. et al. A tailored oxide interface creates dense Pt single-atom catalysts with high catalytic activity. Energy Environ. Sci. 13, 1231–1239 (2020).
Tan, W. et al. Fine-tuned local coordination environment of Pt single atoms on ceria controls catalytic reactivity. Nat. Commun. 13, 7070 (2022).
Wang, H. et al. Surpassing the single-atom catalytic activity limit through paired Pt-O-Pt ensemble built from isolated Pt1 atoms. Nat. Commun. 10, 3808 (2019).
Zhang, Z. et al. Thermally stable single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation. Nat. Commun. 8, 16100 (2017).
Oh, S. et al. Oxygen activation on the interface between Pt nanoparticles and mesoporous defective TiO2 during CO oxidation. J. Chem. Phys. 151, 234716 (2019).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Fu, N. et al. Controllable conversion of platinum nanoparticles to single atoms in Pt/CeO2 by laser ablation for efficient CO oxidation. J. Am. Chem. Soc. 145, 9540–9547 (2023).
Acknowledgements
This research was funded by the National Key R&D Program of China (No.2023YFC3707001, T.Z. and F.S.), National Natural Science Foundation of China (52322004, X.L., 22072090 and 22272106 X.L.), and Open Research Fund of State Key Laboratory of Mesoscience and Engineering (MESO-23-D13, F.S.). Thanks to Professor Jie Zeng from Anhui University of Technology for valuable suggestions and revisions.
Author information
Authors and Affiliations
Contributions
T.Z., P.Z., and X.L. designed the study. T.Z., Y.J., and Z.Z. wrote the paper. J.T. performed most of the reactions. Y.Z. carried out the DRIFTs analysis. J.G. carried out the stability test. Z.S. and W.C. did the XAS resolution. Q.H., G.C., and X.L. did the AC-STEM characterization. G.X., T.Z., and F.S. did the most data analysis. All authors performed certain experiments and discussed and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, T., Zheng, P., Gao, J. et al. Simultaneously activating molecular oxygen and surface lattice oxygen on Pt/TiO2 for low-temperature CO oxidation. Nat Commun 15, 6827 (2024). https://doi.org/10.1038/s41467-024-50790-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-50790-3
- Springer Nature Limited