Abstract
Cohesin mediates sister chromatid cohesion to enable chromosome segregation and DNA damage repair. To perform these functions, cohesin needs to be protected from WAPL, which otherwise releases cohesin from DNA. It has been proposed that cohesin is protected from WAPL by SORORIN. However, in vivo evidence for this antagonism is missing and SORORIN is only known to exist in vertebrates and insects. It is therefore unknown how important and widespread SORORIN’s functions are. Here we report the identification of SORORIN orthologs in Schizosaccharomyces pombe (Sor1) and Arabidopsis thaliana (AtSORORIN). sor1Δ mutants display cohesion defects, which are partially alleviated by wpl1Δ. Atsororin mutant plants display dwarfism, tissue specific cohesion defects and chromosome mis-segregation. Furthermore, Atsororin mutant plants are sterile and separate sister chromatids prematurely at anaphase I. The somatic, but not the meiotic deficiencies can be alleviated by loss of WAPL. These results provide in vivo evidence for SORORIN antagonizing WAPL, reveal that SORORIN is present in organisms beyond the animal kingdom and indicate that it has acquired tissue specific functions in plants.
Similar content being viewed by others
Introduction
Eukaryotic cells perform a complex series of events in order to equally distribute the replicated genome among their daughter cells. DNA replication is not immediately followed by karyokinesis and the newly formed sister chromatids are physically linked for long periods of time until their disjunction during mitosis or meiosis1,2. Sister chromatid cohesion (SCC) is mediated by the cohesin complex, which is thought to topologically entrap DNA helices from both newly replicated sisters3,4. While SCC promotes chromosome biorientation and DNA damage repair, cohesin can also extrude loops of DNA and facilitate distant intra-chromatid interactions, supporting further roles in chromatin organization and gene expression5.
Cohesin’s core subunits have been identified and characterized in all branches of the eukaryotic kingdom including yeast and plants6,7. As a member of the Structural Maintenance of Chromosome (SMC) protein family, cohesin is formed by a heterodimer of SMC1 and SMC3. These proteins fold back on themselves at the hinge domain, where they interact with each other, to form long antiparallel coiled-coil structures. At the other end, their ATPase head domains are bridged together by an α-kleisin subunit, RAD21 (also known as Scc1 or Mcd1) or its meiotic counterparts REC8 and RAD21L8,9,10. These heterotrimeric ring-like structures crucially depend on the recruitment of SCC3 (SA or STAG proteins) to fulfil their chromatin-related functions. SCC3 contributes to cohesin loading, maintenance on chromosomes and its subsequent release from DNA11,12,13,14,15,16. Together, these four proteins form the cohesin core complex.
In addition to SCC3, two further HAWK proteins (HEAT repeat proteins Associated With Kleisin), SCC2 (also known as NIPBL or Mis4) and PDS517, bind to kleisin in a mutually exclusive manner to regulate cohesin behaviour18,19. SCC2 is needed to stimulate cohesin’s ATPase activity16,18,20,21 and has been proposed to load cohesin onto DNA11,22. In vitro experiments have shown that NIPBL is further required for cohesin-mediated loop extrusion20,21. PDS5 and WAPL can disrupt the interaction between the SMC3 and kleisin subunits, thereby releasing cohesin from chromatin23,24,25,26. While cohesin shows a highly dynamic behaviour through cycles of association and release from chromatin, especially during G1, a fraction of cohesin becomes stably bound to DNA after replication and mediates SCC27,28. Establishment of cohesion during DNA replication requires acetylation of two lysine residues on SMC3 by the conserved acetyltransferase Eco1/CTF729,30,31,32,33. In yeast, Pds5 is required for the acetylation process and for stabilizing cohesin on chromatin25. Inactivation of cohesin loading during G1 induces complete cohesin dissociation from DNA in a Wpl1-dependent manner, whereas if inactivation takes place during G2, some cohesin remains chromatin-bound28. In A. thaliana, mutation of four of the five PDS5 genes leads to mild defects in meiosis and to severe deficiencies in development, fertility and somatic homologous recombination (HR)34. Inactivation of both copies of WAPL in A. thaliana only mildly affects overall plant development and fertility35, but rescues the dramatic somatic deficiencies associated with loss of CTF736,37.
In vertebrates and Drosophila, an additional protein factor, Sororin, is recruited to the cohesin complex in a replication and SMC3-acetylation-dependent manner38,39,40,41,42,43. Sororin promotes SCC until the onset of anaphase by displacing WAPL from PDS5 and counteracting its releasing effects40. Both WAPL and Sororin bind to PDS5 through conserved FGF and YSR motifs40,44.
In somatic cells, Sororin accumulates on chromatin between S and G2 phases and becomes dispersed in the cytoplasm after nuclear envelope breakdown except at centromeric regions where it persists until metaphase40,42, consistent with its function in promoting SCC43,45. This suggests that Sororin, as the cohesin complex, is removed from chromosomes in a stepwise manner46. First, the so-called prophase pathway removes chromosomal arm cohesin in a non-proteolytic manner during the first stages of mitosis and meiosis. This process largely depends on WAPL and phosphorylation of STAG213,23,47,48. Sororin phosphorylation has been proposed to participate in both processes: Cdk1-phosphorylated Sororin may act as a docking protein and recruit Polo-like kinase 1 (Plk1) to mediate STAG2 phosphorylation49. Besides, Aurora B and Cdk1 phosphorylate Sororin on several sites and destabilise its association with PDS5, thereby promoting WAPL-mediated removal of cohesion50,51. At centromeres, the Shugoshin-PP2A complex protects cohesin from the prophase pathway by keeping Sororin and cohesin subunits in a dephosphorylated state51,52,53. During the metaphase-to-anaphase transition, the anaphase-promoting complex/cyclosome (APC/CCdc20) targets phosphorylated Securin for degradation to promote the separase-mediated cleavage of the phosphorylated kleisin subunit42,54,55,56.
Current data suggest that the main function of Sororin is to counteract the activity of WAPL. While WAPL appears conserved across kingdoms, including yeasts and land plants, no conserved WAPL antagonist has been described so far. SMC3 acetylation has been proposed to be sufficient to counteract the function of WAPL in organisms thought to lack Sororin, like yeast and plants37,57. In Drosophila melanogaster, the Sororin-related protein Dalmatian has been characterized40. Dalmatian combines protein functions of Sororin and Shugoshin to promote and protect cohesion41. Recently, a meiosis I-specific WAPL antagonist (SWI1), that shares no sequence homology to Sororin, has been characterized in A. thaliana58.
To identify possible homologues of Sororin we performed a thorough bioinformatics analysis. Our searches revealed putative Sororin relatives in various lower and higher eukaryotes. Here we show that S. pombe Sor1 is required for efficient sister chromatid cohesion and that wpl1 deletion partially suppresses defects caused by the sor1Δ mutation. We also demonstrate that Sor1 physically interacts with the cohesin subunit Psm3 (SMC3) and Pds5. We furthermore show, that the A. thaliana Sororin homologue (AtSORORIN) is essential for vegetative development and microsporogenesis. Lack of AtSORORIN leads to tissue-specific reduction or loss of SCC and chromosomal mis-segregation. Consistent with AtSORORIN’s proposed function, these somatic phenotypes can be alleviated by loss of WAPL. Atsororin mutant plants are sterile, affected in male meiosis with chromatids displaying premature loss of cohesion and splitting of sister-centromeres at anaphase I. Interestingly, the meiotic defects cannot be alleviated by loss of WAPL. Taken together, we provide the first organismal in vivo evidence for Sororin antagonizing WAPL function and demonstrate that Sororin is an evolutionary conserved cohesin regulator that has acquired additional functions in plants.
Results
S. pombe Sor1 and A. thaliana AtSORORIN share sequence similarities with metazoan Sororin proteins
To identify possible orthologs of Sororin, we performed a comprehensive bioinformatics analysis using sensitive remote homology searches. Our searches revealed putative Sororin proteins in both lower and higher eukaryotes including various yeast and plant species. They all show only weak overall sequence conservation with their vertebrate counterparts but they share various characteristic features. The S. pombe (SPAC9E9.05) and the A. thaliana (At3g56250) gene candidates which both encode short proteins were analysed in detail. Vertebrate Sororin and Wapl proteins interact with Pds5 through their YSR and FGF motifs40,44. Whereas SPAC9E9.05 has a putative FGF motif, such sequence is not present in the plant candidate. A KEN box targets vertebrate Sororin and Drosophila Dalmatian for APC/CCdh1-dependent degradation, but has not been found in either the plant (At3g56250) or the yeast (SPAC9E9.05) candidates. Similar to metazoan Sororin, the proteins encoded by SPAC9E9.05 and At3g56250 have a conserved motif, referred to as the Sororin domain, preceded by a K/R-rich domain at their C-termini (Fig. 1a). The Sororin domain has been implicated in interactions with STAG2 and contains two conserved phenylalanine residues important for the maintenance of sister chromatid cohesion (Fig. 1b)59,60.
a Domain architecture of Sororin and putative Sororin orthologs. The Sororin domain is shown in blue, the cluster of positively charged residues (lysine, arginine) in red, the KEN box in magenta and the FGF motif in yellow. The domain graphs were created with the help of the domain illustrator (DOG 2.0112). b Alignment of the C-terminal Sororin domain. UniProt accessions are provided next to the species names. Residues mutated in this study are indicated by asterisks. Secondary structure prediction of H. sapiens and S. pombe are shown on the top and bottom, respectively, where alpha helices are in red, and beta strands in green.
The S. pombe Sororin candidate, SPAC9E9.05, has so far been annotated as a poorly characterized Schizosaccharomyces specific protein61. Interestingly, a SPAC9E9.05 deletion mutant was identified in a screen for mutants that showed negative synthetic growth interaction with the cohesion-defective mutants eso1-G799D (Eso1 is the S. pombe ortholog of Esco1/2 and CTF762) and mis4-242 (Mis4 is the S. pombe ortholog of NIPBL63), suggesting that SPAC9E9.05 may be involved in regulation of sister chromatid cohesion64. Given the similarity of S. pombe SPAC9E9.05 and Arabidopsis At3G56250 with metazoan Sororin and the data presented below, we decided to name their encoding genes sor1 (Sororin-like 1) and AtSORORIN, respectively. Using this extended information we compiled Sororin-like sequences and further cohesion regulators (WAPL23 and Haspin65) from evolutionary distant species to illustrate that each has a common ancestor and was lost in some lineages during evolution (Supplementary Fig. 1).
S. pombe Sor1 is a nuclear protein involved in sister chromatid cohesion
If S. pombe Sor1 was functionally related to mammalian Sororin, then it should be present in the nucleus. Nuclear localization of Sor1 was previously observed when expressed under the control of a strong nmt1 promoter66. To analyse Sor1 localization, we expressed Sor1-GFP from its native promoter. In an asynchronously growing culture, Sor1-GFP localized to the nucleus in most cells (Supplementary Fig. 2a). Immunostaining experiments confirmed the nuclear localization of Sor1-Flag during all tested cell cycle stages (Supplementary Fig. 2b). Western blot analysis showed that Sor1-Pk protein levels are lower in nitrogen starved/G1 cells as compared to cycling cells which are mostly in G2 (Supplementary Fig. 2c).
To assess the role of Sor1 in regulation of cohesion, we analysed sister chromatid cohesion at the centromeric region (cen2-GFP) of chromosome 2. In metaphase, sor1Δ mutant cells showed a small, but significant, increase of split sister centromeres (Fig. 2a), indicative of a cohesion defect between sister centromeres. However, the role of Sor1 in sister chromatid cohesion is not essential because we observed no defects in chromosome segregation in sor1Δ cells (Fig. 2b). In mammalian cells, Sororin is dispensable for sister chromatid cohesion in the absence of WAPL40. We therefore analysed sister chromatid cohesion in cells lacking Wpl1, the fission yeast ortholog of WAPL62. Interestingly, the increase in split sister centromeres in sor1Δ mutant cells was prevented in sor1Δ wpl1Δ double mutants (compared to wild type), suggesting that similarly to mammalian cells wpl1 deletion reduces the sister chromatid cohesion defect caused by the sor1Δ mutation (Fig. 2a).
a sor1Δ cells show a weak cohesion defect which is partially suppressed by wpl1Δ. Wild type and sor1Δ haploid cells expressing cen2-GFP were fixed and stained with antibodies against tubulin and GFP and sister chromatid cohesion was analysed in metaphase cells. Nuclei were visualized by Hoechst staining. Means +/− standard deviations are shown. Unpaired t-test was performed for statistical analysis (**p < 0.01; ns—not significant). b Negative synthetic growth interaction in eso1ts sor1Δ and mis4ts sor1Δ double mutants are associated with chromosome segregation defects. Wild type, sor1Δ, eso1-G799D (eso1-ts), eso1-G799D sor1Δ, mis4-242 (mis4-ts) and mis4-242 sor1Δ haploid cells expressing cen2-GFP were fixed and stained with antibodies against tubulin and GFP. Nuclei were visualized by Hoechst staining. Samples were examined under the fluorescence microscope, and segregation of chromosome 2 marked by cen2-GFP was scored in late anaphase cells. Lagging chromosomes were identified as Hoechst-staining bodies between the poles of the spindle in late anaphase. Means +/− standard deviations are shown. Unpaired t-test was performed for statistical analysis (*p < 0.05; **p < 0.01). c Pds5-Myc co-immunoprecipitates with Sor1-Pk. Protein extracts were prepared from cycling wild-type cells and cells expressing Sor1-Pk, Pds5-myc or both Sor1-Pk and Pds5-Myc, as indicated. Proteins bound to anti-V5 agarose beads, which bind the Pk tag on Sor1, were analysed for Pds5-Myc by Western blotting using anti-Myc antibody. The experiment was repeated twice with similar results. d Psm3-GFP co-immunoprecipitates with Sor1-Pk and this interaction is weakened by mutating conserved Sor1 residue D303. Protein extracts were prepared from cycling wild-type cells and cells expressing Sor1-Pk, Psm3-GFP or both Sor1-Pk and Psm3-GFP, as indicated. Proteins bound to anti-GFP agarose beads, which bind the GFP tag on Psm3, were analysed for Sor1-Pk by Western blotting using anti-V5 antibody. Mutant protein Sor1-D303A-Pk co-immunoprecipitated with the Psm3-GFP protein less efficiently, as compared to wild-type Sor1-Pk. The experiment was repeated twice with similar results. e The four conserved residues in the Sororin domain are important for the Sor1 function. Strains with the indicated mutations were grown on YES medium for one day. Serial dilutions were spotted onto YES plates and incubated for 3 days at 25 °C or 30 °C. While expression of a wild type Sor1 rescued the growth defect of the eso1-G799D sor1Δ double mutant (eso1-ts sor1-wt), mutant Sor1 proteins carrying F299A, V302A, D303A or Y305A substitutions did not rescue the growth defect of eso1-G799D sor1Δ double mutants (eso1-ts sor1-F299A, eso1-ts sor1-V302A, eso1-ts sor1-D303A, eso1-ts sor1-Y305A).
Deletion of sor1 showed negative synthetic growth interaction with both eso1-G799D and mis4-242 mutations but the cause of these defects is unknown64. We asked whether defective segregation of chromosomes contributes to this growth defect. Indeed, we observed a higher frequency of lagging chromosomes associated with a higher rate of chromosome mis-segregation in eso1-G799D sor1Δ and mis4-242 sor1Δ double mutants as compared to single mutants (Fig. 2b). This observation is consistent with the role of Sor1 in sister chromatid cohesion regulation.
In telophase and G1, mammalian Sororin is targeted by APC/C for degradation42. We therefore tested whether the fission yeast Sor1 is an APC/C substrate, despite the lack of a defined KEN box. We added in vitro translated Sor1-HA to interphase Xenopus egg extracts in the presence of cycloheximide followed by addition of Cdh1 to activate APC/CCdh1. We also added in vitro translated Sor1-HA to meiotic metaphase-arrested CSF extracts in the presence of cycloheximide followed by addition of CaCl2 to activate APC/CCdc20. As expected, activation of APC/CCdc20 led to a rapid degradation of the APC/CCdc20 substrate Cyclin B2 and also endogenous Xenopus Sororin was degraded within few minutes after activation of APC/CCdh1. However, we did not observe degradation of S. pombe Sor1 by either APC/CCdh1 or APC/CCdc20 (Supplementary Fig. 2d).
Conserved residues in the Sororin domain are important for Sor1 function and association with cohesin
Mammalian Sororin physically interacts with cohesin and Pds5 and these interactions are essential for Sororin’s function40,59,60. If the fission yeast Sor1 was an ortholog of metazoan Sororin, Sor1 should interact with cohesin and/or Pds5. We indeed observed that Pds5-Myc co-immunoprecipitated with Sor1-Pk and Sor1-Pk co-immunoprecipitated with Psm3-GFP (Fig. 2c, d).
The Sororin domain is required for sister chromatid cohesion and association of Sororin with cohesin in mammalian cells59,60. To test whether the Sororin domain of S. pombe Sor1 is important for its association with cohesin in fission yeast, we analysed the ability of Psm3-GFP to immunoprecipitate mutant protein Sor1-D303A-Pk, in which a conserved aspartic acid residue D303 in the Sororin domain has been replaced by alanine. Sor1-D303A-Pk co-immunoprecipitated less efficiently with the Psm3-GFP protein, compared to wild-type Sor1-Pk, suggesting that the conserved residue D303 in the Sororin domain of Sor1 is important for the association of Sor1 with cohesin (Fig. 2d).
We then asked whether the interaction between S. pombe Sor1 and cohesin is functionally relevant. As expected, expression of a wild-type Sor1 rescued the growth defect of the eso1-G799D sor1Δ double mutant to the level of the eso1-G799D single mutant. However, expression of the Sor1-D303A mutant, which weakens the interaction between Sor1 and cohesin, did not restore the growth defect of eso1-G799D sor1Δ double mutants (Fig. 2e). Mutating three other conserved residues in the Sororin domain of Sor1 (F299A, V302A and Y305A) resulted in a similar phenotype (Fig. 2e). The observed mutant phenotype was not due to lack of Sor1 expression as all four Sor1 mutant proteins (Sor1-D303A-TAP, Sor1-F299A-TAP, Sor1-V302A-TAP and Sor1-Y305A-TAP) were expressed, although at reduced levels (Supplementary Fig. 2e).
Taken together, we show that fission yeast Sor1 shares similarity with metazoan Sororin proteins. Sor1 is associated with the cohesin complex and sor1Δ mutant cells show defects consistent with the role of Sor1 in regulation of sister chromatid cohesion. Conserved residues at the C-terminus of Sor1 are important for the Sor1 function and its association with cohesin. Unlike metazoan Sororin proteins, Sor1 is not essential for sister chromatid cohesion, suggesting that fission yeast possesses mechanisms that are able to compensate for the absence of Sor1. Our results are consistent with the notion that Sor1 is an ortholog of Sororin in the fission yeast S. pombe.
A. thaliana SORORIN is essential for vegetative development and microsporogenesis
Our findings obtained in S. pombe motivated us to analyse a Sororin candidate in a non-vertebrate higher eukaryote. The A. thaliana SORORIN gene candidate (At3g56250) consists of four exons and codes for a relatively small protein (222 amino acids). Using CRISPR-Cas9 technology we generated a 5 bp deletion in its first exon, creating a premature stop codon (Fig. 3a). The deletion was confirmed by sequencing the genomic region and also the mRNA transcripts (converted to cDNA) of Atsororin –/– plants (Supplementary Fig. 3a). According to the nature of the CRISPR-Cas9 mediated short deletion, the AtSORORIN gene expression in wild-type and Atsororin –/– mutant plants was not significantly different in all analysed tissues (Supplementary Fig. 3b). Heterozygous Atsororin +/– plants appear like wild type with only minimally reduced seed numbers, but homozygous mutants display a prominent dwarf phenotype, have few and short siliques and epinastic rosette leaves with short petioles that grow around an undersized stem (Fig. 3b). This dramatic phenotype can be complemented with a transgene containing the wild-type gene, including all up- and down-stream regulatory sequences and introns (Supplementary Fig. 3c, d), corroborating that the mutation in the AtSORORIN gene indeed caused the observed aberrations. Plant roots and shoots develop from meristems, which are formed by actively dividing cells that self-renew and differentiate into new tissue. Root development is severely affected by the lack of AtSORORIN. Atsororin mutant plant roots grow significantly shorter than those of wild type, and they completely lose the characteristic layered cellular organization (Fig. 3c, d). Moreover, mutant plants are sterile since their short siliques do not develop viable seeds (Fig. 3e).
a Schematic representation of AtSORORIN (AT3G56250) gene, with 5’ and 3’ UTRs (grey boxes), introns (black lines) and exons (black boxes), open reading frame (ATG/TAA, black), Cas9 target site (black triangle) and premature stop codon in mutant plans (TAA, red) indicated. b The severe growth restriction of homozygous Atsororin mutants plants (seedlings, scale bar = 5 mm; mature plants, scale bar = 5 cm) is alleviated by loss of WAPL (Atsororin wapl1-1 wapl2 triple mutants). Wild-type plants, Atsororin, wapl1-1 wapl2 double mutants and Atsororin wapl1-1 wapl2 triple mutants were grown side-by-side for comparison. c Images of root tips and entire seedlings (small pictures) of plants grown on media plates for two weeks. Root growth restriction (red bars) and loss of characteristic layering of the root meristem in Atsororin mutant plants are evident. These deficiencies are rescued by loss of WAPL (wapl1-1 wapl2 double mutants). All plants were grown side-by-side for comparison. Scale bar = 1 mm. d Quantification of root growth of wild-type plants (n = 139), Atsororin –/– plants (n = 23), wapl1-1 wapl2 plants (n = 113) and Atsororin wapl1-1 wapl2 plants (n = 113) grown on media plates for two weeks. Error bars represent standard deviations of the mean. Unpaired Mann-Whitney test has been applied (*p < 0.05; **p < 0.01; ****p < 0.0001; ns—difference not significant). e Loss of fertility in Atsororin mutant plants is only partially rescued by WAPL inactivation. Wild type plants (n = 74), Atsororin –/– plants (n = 52), wapl1-1 wapl2 plants (n = 144) and Atsororin wapl1-1 wapl2 plants (n = 166) were grown side-by-side and genotypes are indicated. Error bars represent standard deviations of the mean. Unpaired Mann-Whitney test has been applied (****p < 0.0001). Images show representative, opened siliques and developing seeds. Atsororin wapl1-1 wapl2 triple mutant plants have siliques with some seeds, which are mostly bigger than those formed in wild-type plants. Scale bar = 1 mm. f Genotypes of offspring of self-pollinated AtSORORIN +/– plants. The homozygous Atsororin –/– genotype is strongly underrepresented (chi-square analysis, p value indicated). g Genotyping the offspring of reciprocal crosses between AtSORORIN +/– and wild-type plants indicates that only male, but not female, gametogenesis, is affected by the Atsororin mutation (two-sided Fisher’s exact test, p values indicated). h Flower architecture is not affected by the lack of AtSORORIN whereas anther growth and pollen viability are severely disturbed. Atsororin single mutants (n = 20) and Atsororin wapl1-1 wapl2 triple mutants (n = 17) develop smaller anthers with few viable pollen grains than wild type (n = 8) and wapl1-1 wapl2 mutants (n = 12). All plants were grown side-by-side and genotypes are indicated. The term “Atsororin mutant” refers to the homozygous mutant allele configuration if not explicitly stated otherwise. Scale bar flowers = 1 mm, scale bar anthers = 200 μm.
Heterozygous, self-pollinated Atsororin +/– plants have less than 4% homozygous Atsororin –/– offspring, representing a significant deviation from the expected Mendelian segregation ratio (Fig. 3f). Reciprocal crosses between Atsororin +/– heterozygous mutant plants and wild type plants revealed that the distortion of segregation ratios is exclusively caused by the male generative cells (Fig. 3g). In fact, the morphology of Atsororin –/– mutant anthers is abnormal, their size decreased and the amount of shed pollen strongly reduced. A test for pollen viability (Alexander staining) showed that unlike wild-type plants, Atsororin –/– mutants produce only very few pollen grains of which only very few are viable (Fig. 3h). In Atsororin +/– heterozygous mutant plants the regularly sized anthers develop a variable number of non-viable pollen grains. We assume that most pollen grains lacking AtSORORIN die prior to fertilization, in line with the transmission distortion reported above (Supplementary Fig. 3e).
Loss of WAPL rescues Atsororin-associated defects
In mammalian cells, Sororin is needed to counteract the cohesin-releasing activity of Wapl, and therefore deficiencies related to loss of Sororin can be suppressed by loss of Wapl40. Arabidopsis wapl1-1 wapl2 double mutants exhibit normal vegetative growth and only a mild reduction in fertility (Fig. 3b)35. The Atsororin-associated somatic defects can be suppressed by the wapl1-1 wapl2 double mutant, underlining that Arabidopsis SORORIN is a bona fide relative of its vertebrate counterpart. In the Atsororin wapl1-1 wapl2 triple mutant normal growth of the aerial plant parts and of the roots is restored (Fig. 3b–d).
WAPL inactivation only leads to a limited rescue of the fertility defect observed in Atsororin mutants. Atsororin wapl1-1 wapl2 anthers are nearly as small as those of Atsororin single mutants and only very few viable pollen grains are formed (Fig. 3h). Correspondingly, the triple mutant produces only very few seeds, but still significantly more than the Atsororin single mutant (wild type 55 ± 4 seeds/silique (n = 74), wapl1-1 wapl2 36 ± 9 seeds/silique (n = 144; p < 0.0001), Atsororin 0.096 ± 0.35 seeds/silique (n = 52; p < 0.0001); Atsororin wapl1-1 wapl2 5 ± 4 seeds/silique (n = 166; p < 0.0001) (Fig. 3e).
AtSORORIN is essential in a sub-set of tissues
The data, especially the epistatic relation to WAPL, suggested that the gene product of AtSORORIN acts in a similar manner as its vertebrate counterpart. We anticipated that the most obvious molecular phenotype of Atsororin mutants should be pre-mature loss of sister-chromatid cohesion. To analyse chromosome numbers and sister chromatid cohesion we prepared mitotic cell nuclei samples and specifically stained centromeres (via fluorescent in situ hybridization, FISH).
Indeed, interphase nuclei from roots of Atsororin mutant plants contain on average 16.82 ± 3.68 centromere signals (n = 34). This is significantly more compared to wild type (10.02 ± 0.1458 centromere signals; n = 93; p < 0.0001), wapl1-1 wapl2 double mutants (10.32 ± 1.66 centromere signals; n = 73; p < 0.0001) and Atsororin wapl1-1 wapl2 triple mutants (10.49 ± 1.83 centromere signals; n = 59; p < 0.0001) (Fig. 4a, b). We attribute the severe mis-organisation of cells in the Atsororin mutant roots (Fig. 3c) (Supplementary movies 1–4) and the arbitrary chromosome numbers in interphase nuclei to massive chromosome mis-segregation due to pre-mature loss of cohesin. Since the homozygous Atsororin mutant plants are under-represented and the mutant root material is scarce and experimentally difficult to process we could only obtain a few cells at metaphase. While in wild-type 10 doublet signals can be seen, in Atsororin plants individual chromatids are arranged at the metaphase plate. The anticipated pre-mature loss of SCC leads to random segregation of chromatids during anaphase in Atsororin mutants. Importantly, Atsororin wapl1-1 wapl2 triple mutants are much less affected than the Atsororin single mutant (Figs. 3c and 4a, b).
DNA was stained with DAPI (magenta) and fluorescence in situ hybridization (FISH) was performed to detect centromeric regions (green). a Spreads of root cell nuclei. Interphase, metaphase and anaphase stages were analysed for wild-type plants and Atsororin, wapl1-1 wapl2 and Atsororin wapl1-1 wapl2 mutants. Scale bar = 10 μm. b Quantification of centromeric-FISH signals in interphase root nuclei. Atsororin mutants (n = 34) show a significantly higher number of signals than wild type (n = 93), wapl1-1 wapl2 (n = 73) and Atsororin wapl1-1 wapl2 (n = 59) (Fisher’s exact test; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns—difference not significant). c Spreads of somatic cell nuclei from inflorescences. Interphase, prophase, prometaphase, metaphase and anaphase stages were analysed for wild-type plants and Atsororin, wapl1-1 wapl2 and Atsororin wapl1-1 wapl2 mutants. Magnifications of signals at the sister centromeres are provided for prophase and prometaphase stages. Scale bar = 10 μm. d Quantification of centromeric-FISH signals observed in nuclei of cells from inflorescences at interphase. Quantification was performed on wild-type plants (n = 224) and Atsororin (n = 266), wapl1-1 wapl2 (n = 238) and Atsororin wapl1-1 wapl2 mutants (n = 236) (Fisher’s exact test; ****p < 0.0001; ns—difference not significant). e Measurements of the physical distance between FISH signals of sister chromatid centromeres during prophase. Atsororin mutants (n = 39) show a significant increase in distance between sister centromeres when compared to wild type (n = 45), wapl1-1 wapl2 (n = 37) and Atsororin wapl1-1 wapl2 (n = 50). Error bars represent standard deviations of the mean. Unpaired t-test was performed (*p < 0.05; ***p < 0.001; ****p < 0.0001). f Measurements of the physical distance between FISH signals at sister chromatid centromeres during prometaphase. Atsororin mutants (n = 55) show a significant increase in distance between sister centromeres when compared to wild type (n = 42), wapl1-1 wapl2 (n = 46) and Atsororin wapl1-1 wapl2 (n = 49). Error bars represent standard deviations of the mean. Unpaired t-test was performed (****p < 0.0001; ns—difference not significant).
Somatic interphase cell nuclei isolated from leaves of Atsororin mutant plants, had a close to regular number of chromosomes (10.3 ± 0.5746 centromere signals; n = 53), which is still significantly different when compared to wild-type plants (10 centromere signals; n = 84; p < 0.0001), wapl1-1 wapl2 double mutants (10 centromere signals; n = 68; p < 0.0001) or Atsororin wapl1-1 wapl2 triple mutants (10 centromere signals; n = 82; p < 0.0001) (Supplementary Fig. 3f, g).
We also prepared somatic cells from inflorescences, containing a large number of actively dividing cells that can be readily processed and analysed (Fig. 4c). As for the leaf cells, we established first the number centromeric signals of interphase nuclei. We found, similar to the numbers obtained from leaf cells and in contrast to the ones obtained from root cells, that most cells contain the correct number of chromosomes in Atsororin mutants (10.26 ± 1.25 centromere signals; n = 266) but still significantly different when compared to wild type (10 centromere signals; n = 224; p < 0.0001), wapl1-1 wapl2 double mutants (10.01 centromere ± 0.09 centromere signals; n = 238; p < 0.0001) or Atsororin wapl1-1 wapl2 triple mutants (10.09 centromere signals; n = 236; p < 0.0001) (Fig. 4d).
Since a large number of actively dividing cells in anaphase could be observed in the inflorescence tissue, we were also in the position to monitor chromosome segregation. In accordance with the mild aberrations of chromosome numbers in interface nuclei, we observed mostly regular chromosome disjunction in Atsororin nuclei from inflorescences (97% symmetric disjunction, n = 133) with 10 separating chromosomes at either side of the division plane. Those Atsororin plants (13/133) that carried 11 chromosomes in all cells, most likely obtained via a gamete with a supernumerary chromosome, showed regular disjunction. In this sense, the occurrence of symmetric divisions was not significantly different from wild-type plants (n = 112, p = 0.2525) and wapl1-2 wapl2 (n = 119, p = 0.9999) and Atsororin wapl1-2 wapl2 (n = 118, p = 0.6246) mutants. Moreover, staining for SCC3 in somatic cells of inflorescences of wild-type and Atsororin mutants showed no difference in SCC3 abundance on chromosomes during prophase and interphase, indicating normal distribution and abundance of cohesin in these cells in Atsororin mutants (Supplementary Fig. 4a).
We also measured the inter-sister centromere distance during prophase and prometaphase (Fig. 4c, e, f) in somatic cells from inflorescences. Post S-phase 10 doublet signals can be seen in all genotypes tested. While the mutation in AtSORORIN does not lead to complete loss of cohesion between sister chromatids, the distance between the 10 centromeric doublet signals is significantly increased compared to wild type (Prophase: 457.6 nm in Atsororin, n = 39; 378.9 nm in wild type, n = 45; p < 0.0001. Prometaphase: 699 nm in Atsororin, n = 55; 562.9 nm in wild type, n = 42; p < 0.0001). The sister-centromere distance is, as anticipated, significantly shortened in wapl1-2 wapl2 mutants compared to wild type (Prophase: 294.2 nm in wapl1-2 wapl2, n = 37; p < 0.0001. Prometaphase: 481.4 nm in wapl1-2 wapl2, n = 46; p < 0.01). During prophase, the Atsororin wapl1-2 wapl2 triple mutants have a centromeric distance that is not different from wild type (349.2 nm, n = 50; p = 0.2695), significantly shorter than the Atsororin single mutant (457.6 nm, p < 0.0001) and increased when compared to wapl1-2 wapl2 mutants (294.2 nm, p < 0.0001). At prometaphase the centromeric distance of Atsororin wapl1-2 wapl2 is as tight as in the wapl1-2 wapl2 mutant (446.1 nm in Atsororin wapl1-2 wapl2, n = 49; p = 0.4004). It is interesting to note that in wapl1-2 wapl2 double mutants, cohesion of sister chromatid arms is maintained in prometaphase since no individual arms can be distinguished. This also holds true in the Atsororin wapl1-2 wapl2 triple mutant background.
Taken together, we conclude that both AtSORORIN and WAPL impact sister-chromatid cohesion, and that AtSORORIN is not the exclusive antagonist of WAPL activity in all somatic plant tissues.
AtSORORIN is needed for centromeric sister chromatid cohesion during male meiosis
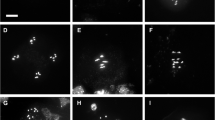
Our analysis indicated that somatic divisions in root cells and microsporogenesis are most severely affected by loss of AtSORORIN. To analyse if the underlying cause for the latter can be related to a perturbation of male meiosis we prepared chromosome spreads from meiocytes. Comparing wild-type and Atsororin meiocytes it is apparent that AtSORORIN is not an essential factor for sister chromatid cohesion in prophase I. Meiocytes from Atsororin plants show normal chromosome condensation and pairing during pachytene and also chiasmata at diakinesis. Bivalents were properly orientated at the metaphase I plate. Yet, in anaphase I sister chromatids split pre-maturely and were subsequently segregated at random in meiosis II (Fig. 5). While in anaphase I/telophase I we observed 5 DAPI-stained bodies at each pole of the dyad in wild type, in Atsoronin mutants around 10 DAPI stained bodies can be seen. In metaphase II these 10 DAPI stained bodies could not be aligned properly, were distributed at random during anaphase II and subsequently led to unbalanced tetrads. Supernumerary DAPI stained bodies, which we interpret as individual chromatids, were detected in 71% of Atsororin meiocytes during prophase II-metaphase II stages (n = 38), while this was never observed in wild type (n = 67; p < 0.0001).
Spreads of meiotic nuclei from wild-type plants and Atsororin, wapl1-1 wapl2 and Atsororin wapl1-1 wapl2 mutants. Meiotic progression until metaphase I, including homologous chromosome pairing and bivalent formation, appears normal in all genotypes. The number of DAPI-stained bodies is increased in mutants lacking AtSORORIN after metaphase I, yielding 10 chromatids in prophase II. Progression through meiosis II is therefore defective in Atsororin single mutants with the subsequent formation of unbalanced tetrads. Inactivation of WAPL does not rescue chromosome non-disjunction observed in anaphase II in the Atsororin single mutants. For each stage and genotype at least 5 pictures, all showing similar results, were acquired. Scale bar = 10 μm.
The wapl1-2 wapl2 double mutants showed strengthened cohesion, characterized by the distinct shape of bivalents at metaphase I, as previously described35, and regular distribution of chromosomes at meiosis I (n = 32) and II. Importantly, in male meiocytes of Atsororin wapl1-2 wapl2 triple mutants, premature loss of sister chromatids persists. Supernumerary chromatids were observed in 80% of all anaphase I/telophase I meiocytes in the triple mutant (n = 40; p < 0.0001 compared to wild-type or wapl1-2 wapl2). This means, that the premature loss of centromeric sister chromatid cohesion at anaphase I/telophase I in Atsororin mutants cannot be rescued by loss of WAPL.
To determine the precise timing of loss of sister chromatid cohesion during meiosis of Atsororin mutant plants we performed centromeric FISH analysis on meiotic spreads (Fig. 6a). As mentioned above, homologous chromosome pairing appeared normal in Atsororin mutants, underlined by the presence of 5 dominant CEN signals observed at pachytene stage. During late metaphase I/early anaphase I, five pairs of CEN signals were observed in wild type, with two distinct signals per bivalent (each signal representing two fused sister centromeres) that were orientated to opposite poles. In Atsororin mutants, homologous chromosomes showed proper bipolar orientation at metaphase I but the centromeric signals pointing to either pole were often split. All of the observed Atsororin metaphases had more than 10 CEN signals (n = 24), indicating that sister chromatid centromeres were not fused as in wild type (Fig. 6a, b). We quantified the number of centromeric signals observed at metaphase I (including cells from metaphase I to prophase II stages) and metaphase II stages in wild-type plants and Atsororin, wapl1-2 wapl2 double and Atsororin wapl1-2 wapl2 triple mutants (Fig. 6c, d). While meiocytes from wild-type and wapl1-2 wapl2 mutant plants did mostly not suffer from premature splitting of sister-centromeres at metaphase I (93.4% and 91.3% of cells with 10 centromere signals respectively, n = 76 in wild type, n = 23 in wapl1-2 wapl2; p = 0.6622) and had perfectly paired sister-centromeres at metaphase II (n = 17 in wild type, n = 10 in wapl1-2 wapl2), Atsororin and Atsororin wapl1-2 wapl2 mutants displayed split sister-centromere signals at metaphase I (n = 24, p < 0.0001 in Atsororin; n = 31, p < 0.0001 in the triple mutant), and non-paired sister-centromeres at metaphase II (n = 13, p < 0.0001 in Atsororin; n = 17, p < 0.0001 in Atsororin wapl1-2 wapl2 mutants).
Fluorescence in situ hybridization experiment on male meiocytes with a probe directed against the centromeric regions (green) in wild-type plants and Atsororin, wapl1-1 wapl2 and Atsororin wapl1-1 wapl2 mutants. a Inactivation of AtSORORIN leads to premature loss of centromeric cohesion at the metaphase to anaphase transition during meiosis I. Inlays show magnifications of sister centromeric signals during metaphase I. Scale bar = 10 μm. b Magnifications of images depicting metaphase I stages for wild-type plans, Atsororin single mutants and Atsororin wapl1-1 wapl2 triple mutants. Premature splitting of centromeric signals is evident in the absence of AtSORORIN. Centromeres are stained in green and DNA is stained in magenta. Scale bar = 10 μm. c Quantification of the number of centromeric signals from metaphase I to prophase II stages in wild-type plants (n = 73) and Atsororin (n = 25), wapl1-1 wapl2 (n = 22) and Atsororin wapl1-1 wapl2 (n = 33) mutants. Fischer’s exact test was performed (*p < 0.05; ****p < 0.0001; ns—difference not significant). d Quantification of the number of centromeric signals at metaphase II in wild-type plants (n = 13) and Atsororin (n = 12), wapl1-1 wapl2 (n = 12) and Atsororin wapl1-1 wapl2 (n = 15) mutants. Fischer’s exact test was performed (****p < 0.0001; ns—difference not significant). e Quantification of the number of centromeric signals in tetrads (balanced: 4 nuclei with 5 centromere signals each) in wild type plants (n = 33) and Atsororin (n = 25), wapl1-1 wapl2 (n = 28) and Atsororin wapl1-1 wapl2 (n = 18) mutants. Two-sided Fischer’s exact test was performed (**p < 0.01; ****p < 0.0001; ns—difference not significant).
As mentioned above, after loss of sister chromatid cohesion, progression through meiosis II is compromised and in Atsororin and Atsororin wapl1-2 wapl2 mutants individual chromatids segregated at random. We quantified tetrads with balanced chromosome numbers (Fig. 6e). While in wild-type plants, all meiocytes generated balanced tetrades (n = 33), none of the Atsororin mutants produced balanced tetrades (n = 25; p < 0.0001), wapl1-2 wapl2 mutants produced 75% of balanced tetrades (n = 28; p < 0.01) and Atsororin wapl1-2 wapl2 none (n = 18; p < 0.0001). These observations lend further support to the notion that the meiotic deficiencies in AtSORORIN cannot be rescued by loss of WAPL.
It Is interesting to note that while univalent chromosomes were not observed in WT or wapl1-1 wapl2 mutants, a significant fraction (13%) of Atsororin meiocytes showed presence of an extra univalent chromosome (scored at diakinesis-metaphase I stages; n = 53; p < 0.01). Presence of extra chromosomes could be the consequence of a previous non-disjunction event in the meiocyte precursor cells, or the result of fertilization between unbalanced generative cells (see also above). Interestingly, we did not observe univalents in the Atsororin wapl1-1 wapl2 triple mutants (n = 32).
AtSORORIN does not affect meiotic cohesin abundance and axis formation in meiotic prophase
We were curious to understand AtSORORIN’s impact on cohesion abundance in a severely affected tissue. We therefore performed chromosome spreads of male meiocytes and subsequent immune-staining using antibodies directed against the cohesin subunit SCC3 and the meiosis-specific kleisin subunit REC8 (Fig. 7; Supplementary Fig. 4b). We scored cells at the zygotene/pachytene transition as cohesins can still be observed well at this stage. To correctly stage progression of meiosis, we also detected the meiotic axis component ASY1 and the transverse filament protein of the synaptonemal complex (SC), ZYP1. Our analysis shows that during meiotic prophase, axis formation, as judged from the ASY1 signal, and SC formation, as judged from the ZYP1 signal, is indistinguishable from wild type in Atsororin, wapl1-2 wapl2 and Atsororin wapl1-2 wapl2 mutants. Furthermore, cohesion abundance and deposition, as judged from the SCC3 and REC8 signals, along the chromosome arms appears unaffected in Atsororin, wapl1-2 wapl2 and Atsororin wapl1-2 wapl2 mutants (Fig. 7; Supplementary Fig. 4b).
Discussion
Cohesin complexes are evolutionarily ancient inventions of nature, involved in proper chromosome disjunction in mitosis and meiosis, but also essential for chromosome organization67. In animal cells, Wapl has been recognized as a cohesin removal factor which itself is kept in check by the antagonizing protein Sororin23,40,45. While cohesion complex proteins, Wapl and Eco1-dependent acetylation of cohesin are conserved from yeast and plants to humans, Sororin was thought to be present only in metazoans5,41. A Sororin-like protein has been characterized in the fly, with a peculiar dual function; it serves as a Wapl antagonist and also as a centromeric cohesion protector40,41. It has also been suggested that in yeast the conserved acetyltransferase Eco1 takes over the function of Sororin68. In Arabidopsis, the protein SWI1 antagonizes the function of WAPL, but exclusively only during meiotic prophase I, and it shares no sequence homology with the vertebrate or fly relatives58,69. These results suggested that WAPL antagonists should also be present in the genomes of other eukaryotes, but possibly strongly diverged in sequence or occurring as functional domain in the context of larger proteins.
Applying sensitive remote homology searches, we identified putative Sororin relatives in various organisms, including S. pombe and A. thaliana, which are separated by ~1.5 billion years of independent development. Moreover, collecting sequences of Sororin-like proteins from distant lineages revealed that it is an evolutionary old cohesin regulator, but lost in some taxa.
We show that Sor1, the S. pombe Sororin-relative, physically interacts with cohesin (via SMC3/Psm3) and Pds5. However, we observed only a mild sister chromatid cohesion defect in sor1Δ cells, suggesting that there are other mechanisms that compensate for the absence of Sor1. Importantly, wpl1 deletion partially suppressed the sister chromatid cohesion defect caused by the sor1Δ mutation, suggesting that, similarly as metazoan Sororin, Sor1 antagonizes the function of Wapl. Our results are consistent with the notion that Sor1 is an ortholog of Sororin in the fission yeast S. pombe.
Conversely, the Arabidopsis Sororin relative is an important factor for plant viability and vigour. Atsororin mutant plants are underrepresented in segregating populations due to compromised male, but not female, transmission of the mutant allele. The few plants that develop with a homozygous Atsororin mutation are dwarfed, have a short and distorted root and are sterile. Interestingly, among the somatic tissues analysed, only roots show a strong chromosome mis-segregation phenotype, while other tissues are less affected. Somatic cells from inflorescences show hardly any mis-segregation but a widening of centromeric distances in prophase/pro-metaphase, compatible with AtSORORIN’s role in limiting WAPL’s activity. Atsororin plants are sterile and the main underlying cause appears to be premature loss of sister centromere cohesion at anaphase I during male meiosis. This is different from the defect observed in swi1 mutants, with premature loss of sister chromatid cohesion in early meiotic prophase I58. Importantly, the somatic defects of Atsororin mutants and the meiotic defect of swi1 mutants could be rescued in the absence of WAPL (wapl1 wapl2 double mutants), while the meiotic defects of Atsororin could not be alleviated.
We assume that only a sub-fraction of cohesin complexes is regulated by AtSORORIN during plant meiosis. Specifically in meiosis I, SWI158 antagonizes WAPL during meiotic prophase I and AtSORORIN possibly only during the metaphase I to anaphase I transition. The latter is possible not apparent in a swi1 mutant as all cohesive cohesin has been lost during prophase, leading to premature separation of sister chromatids. Importantly, in a Atsororin mutant, sister chromatid cohesion is maintained until metaphase I, yet centromeric fusion is lost at anaphase I onset leading to a peculiar co-segregation of separated sister chromatids to the same pole. As only a small fraction of centromeric cohesin complexes seem affected in the Atsororin mutant, this may not be sufficient for detecting differences in cytological preparations.
It is interesting to note, that a very similar phenotype compared to Atsororin has been observed in the acetyltransferase mutant CTF736, a relative of Eco1 and ESCO1/231,70. Eco1/CTF7 acetylates the cohesin subunit SMC3 during DNA replication, thereby promoting recruitment of SORORIN and antagonizing the function of WAPL38,39,40. In plants, inactivation of WAPL in a ctf7 mutant background restores somatic growth but fails to fully rescue the ctf7 fertility defect37. These results indicate that first, AtSORORIN and AtCTF7 may act in the same pathway to promote sister chromatid cohesion by antagonizing WAPL, and second, that the dramatic dwarf phenotype observed in the single Atsororin and ctf7 mutants is not a direct effect of the respective mutation, but an indirect, possibly mediated by altered cohesin dynamics. In the future, we will investigate the precise epistatic and functional relation of CTF7, SWI1 and AtSORORIN.
Sororin has initially been perceived as the only WAPL antagonist in vertebrates40, but later the histone kinase Haspin has also been described as a WAPL antagonist with respect to cohesive cohesin65,71. It is interesting to note that loop extruding cohesin is also protected from WAPL by CTCF72,73. Haspin has been implicated in centromeric localization of the chromosome passenger complex (CPC) which plays a crucial role in chromosome bi-orientation by correcting erroneous microtubule attachment74. Localization of the CPC relies on histone H3-T3 phosphorylation, which is mediated by the histone kinase Haspin/Hrk175,76,77. Hrk1/Haspin localization to centromeres depends on its interaction with Pds565,78,79.
In this sense, the protein PDS5 has emerged as a central regulator for the orchestration of cohesin dynamics. Via its conserved A P D/E A P motif 44,79, it can interact with diverse regulators. In human cells, PDS5 utilises this motif to interact with WAPL, HASPIN and SORORIN. Importantly, the three proteins share a common PDS5-interaction motif (PIM: K/R T/S Y S R K/L) and compete for PDS5 binding44,65,71. Furthermore, S. pombe Pds5 has been characterized to interact with Wpl1, Hrk1 and Eso1 (with the latter two inhibiting cohesin removal)79. Also these three proteins have a common Pds5-interaction motif79 and compete for the same binding domain on Pds5. Here we demonstrate that yet another protein, Sor1, can interact with Pds5, potentially also competing for the same binding platform.
Arabidopsis has five PDS5 genes34, of which three encode PDS5 variants with a perfectly conserved interaction motif. The two A. thaliana WAPL proteins have well-conserved PIMs at their N-termini (R T Y G R R) and are very likely direct interaction partners of PDS5 proteins, with experimental proof for the WAPL1-PDS5A pair58.
Common to all SORORIN proteins is the Sororin domain60. Previously it was shown to be important for interaction with cohesin complexes (SA2) and the maintenance of sister chromatid cohesion59,60. The Sororin domain is well-conserved in the A. thaliana and in S. pombe relatives, yet only one phenylalanine is present within the motif of the latter. We established in S. pombe that mutating this residue (F299) to alanine is as detrimental as a complete deletion of the sor1 gene.
Interestingly, while we could not identify a putative PIM in the A. thaliana Haspin protein we noticed a well-conserved Sororin domain (Y F R D I D A F E), which is not present in Haspin proteins from other organisms. In this sense, plant Haspin may be localised to cohesin via interacting with the SCC3 subunit and may also play a role as WAPL antagonist in plants.
Importantly, our study provides the first organismal in vivo evidence that SORORIN antagonizes WAPL. We conclude (1) that orthologs of SORORIN are wide-spread in eukaryotes including yeast and plant species; (2) that plants encode more than one WAPL antagonist, and (3) that they act in clearly defined tissue and developmental contexts; and (4) that AtSORORIN may have acquired, similar to Drosophila’s Dalmatian, additional WAPL-independent functions in sister centromere protection at the meiosis I to meiosis II transition.
Methods
Bioinformatic analyses
Sororin orthologs are characterized by a very short domain at the C-terminus, which is shared between mammals and insects. This region consists of a stretch of positively charged amino acids, a polar linker (varies in size between 10 and 20 amino acids) and a conserved motif predicted to form two alpha helices and a beta strand40. We could not expand the Sororin protein family to other taxonomic clades such as fungi or plants when we considered only statistically significant hits (e-value 1e−2). To identify candidates in other model organisms we used a hidden Markov model (HMM) of the C-terminal region (covering the Homo sapiens Sororin protein gi|18087845|ref|NP_542399.1|: 216-252) and searched specifically in the proteomes of Saccharomyces cerevisiae and Schizosaccharomyces pombe (HMMER suite version 2.3.2)80. We received 26 (S. cerevisiae) and 28 (S. pombe) hits with low significant e-values between 0.78 and 10. The hits were manually filtered according to the following criteria: location of the alignment at the C- terminus, conservation of the hydrophobic pattern (especially the phenylalanine residues), and no overlap with known functional domains. In budding yeast, no hit fulfilled all these criteria. In fission yeast, the best hit was to the protein SPAC9E9.05.1 (e-value 1, score −4.0). The protein is 313 residues long and the HMM alignment spanned from 241 to 310. SPAC9E9.05.1 is specific to the Schizosaccharomyces genus—no other orthologs could be detected with a NCBI-blastp search (version 2.2.26)81 besides in Schizosaccharomyces cryophilus, Schizosaccharomyces octosporus, and Schizosaccharomyces japonicus. The conservation within the SPAC9E9.05 protein family is very poor (overall S. pombe and S. japonicus are only 23% identical), the C-terminus being the highest conserved region (30% identical). No known functional domains could be detected in the PFAM database. We incorporated the SPAC9E9.05.1 Schizosaccharomyces sequences into the HMM model and extended the search to other fungi species. In the proteome of the ascomycete Pyrenophora tritici-repentis (strain Pt-1C-BFP), the best hit was to a predicted protein (gi|189210197|ref|XP_001941430.1|, score 13.5, e-value 0.089) belonging to an uncharacterized protein family that is conserved within the Pezizomycotina clade.
We confined the HMM-model to a region with highest conservation (S. pombe SPAC9E9.05.1: 298-311), using only fungi proteins, and searched specifically in Saccharomycetes species. In Lipomyces starkeyi, the best hit was significant (jgi|Lipst1_1|72111|Locus1483v3rpkm29.51, e-value 0.0041, score 20.9) and located at the c-terminus as well. Similarly, in the Yarrowia lipolytica proteome we selected YALI0C19756p (e-value 0.03, score 17.7). However, no candidate could be identified in Saccharomyces cerevisiae or in Candida species.
To identify plant candidates, we used the same HMM model as for the S. pombe screen before and searched within the Arabidopsis thaliana proteome. The best hit was to an unknown protein (AT3G56250.1, e-value 0.04, score 14.7), which is a member of a plant-specific protein family. Like for the Sororin family and the fungi candidates, the highest conservation lies in the C-terminal region. Except for some plant species, such as Oryza sativa Japonica, only one candidate gene was identified per genome.
The proteomes used in this study were retrieved from the NCBI-protein database (http://www.ncbi.nlm.nih.gov/protein) besides for Saccharomyces cerevisiae (http://www.yeastgenome.org/), Schizosaccharomyces pombe (http://www.pombase.org/), Lipomyces starkeyi (http://genome.jgi.doe.gov/Lipst1_1) and Arabidopsis thaliana (http://www.arabidopsis.org/).
Mulitple alignments were performed with MAFFT (version 7, L-INS-I method)82, secondary structure prediction with Jpred (v4)83; and analysed in Jalview84.
For the taxonomic tree, Wapl homologues were identified in an HMM search within the NCBI non-redundant protein database85 or UniProt reference proteomes86 using an alignment with representative sequences, including human WAPL NCBI|XP_042946.3:730-1269 and budding yeast Rad61 UniProt|Q99359: 260-633 (HMMER suite version 3.3.2, E-value cutoff 1e−7). HASPIN proteins were assigned with NCBI blast searches in the NCBI non-redundant protein database or in UniProt reference proteomes, using a representative set of homologues (covering the region corresponding to human HASPIN UniProt|Q8TF76:376-792) and applying highly restrictive E-value cutoffs (1e-10). The taxonomic tree was generated with the NCBI Taxonomy Common Tree87. The visualization was performed in iTOL v688.
S. pombe methods
The genotypes of S. pombe strains used in this study are listed in Table 1. Standard YES media were used to grow S. pombe strains strains89,90,91. Tagging and deletion of S. pombe genes was performed according to our protocols described in ref. 92 and ref. 93, respectively. The immunofluorescence and microscopy techniques used to analyse chromosome segregation were performed as described in ref. 94. Point mutations in the sor1 gene (to yield sor1-F299A, sor1-V302A, sor1-D303A and sor1-Y305A variants proteins) were introduced into the cloned sor1 gene using the QuikChangeII kit (Agilent Technologies) and inserted into the genome by transformation.
For Western blot analyses, proteins were separated by electrophoresis through 12% polyacrylamide gels containing SDS (0.1%) and transferred to a PVDF membrane (Millipore). The membrane was blocked with 2% (w/v) milk-PBS-T (phosphate buffer saline buffer with 0.1% (v/v) Tween-20) and probed with antibodies. TAP-tagged proteins were detected using rabbit antiperoxidase antibody linked to peroxidase (PAP, Dako; 1:10,000 dilution). Tubulin was detected using mouse-anti-α-tubulin antibody (Sigma-Aldrich T5168; 1:10,000 dilution) and rabbit anti-mouse IgG-HRP secondary antibody (Santa Cruz Biotechnology; 1:5000 dilution). GFP-tagged proteins were detected using mouse anti-GFP antibody (Roche 1814460, 1:1000 dilution) and anti-mouse-HRP antibody (Amersham, 1:5000). Pk-tagged proteins were detected using mouse-anti-Pk (V5) antibody (Serotec; 1:2000 dilution) and goat anti-mouse IgG-HRP secondary antibody (Santa Cruz Biotechnology; 1:5000 dilution) in 0.1% PBS-T. Myc-tagged proteins were detected using rabbit c-Myc antiserum (CM-100, Gramsch, Germany, 1:10,000 dilution) and secondary mouse anti-rabbit-IgG antibody conjugated to HRP (sc-2357, Santa Cruz Biotechnology, 1:20,000 dilution).
For coimmunoprecipitation, 10 ml of exponentially growing cells were collected, washed and lysed in 300 μL of IPP150 buffer [50 mM Tris-Cl (pH = 8.0), 150 mM NaCl, 10% glycerol, 0.1% NP-40, 1 mM PMSF and complete EDTA-free protease inhibitors] using glass beads as described in ref. 95. The lysates were centrifuged and subjected to affinity purification via binding to anti-GFP or anti-V5 agarose beads (Sigma-Aldrich) for 1 h at 4 °C. After washing with IPP150 buffer (3 × 1.5 ml), the bound proteins were released by the addition of SDS–PAGE sample buffer at 95 °C for 3 min. The presence of tagged proteins in the immunoprecipitates was detected by Western blot analysis as described above.
Nitrogen-starved cells were prepared as previously described96. Cells exponentially grown in EMM2 medium to a density of 107 cells/ml at 30 °C were harvested by centrifugation and washed twice with EMM2-N (EMM2 lacking NH4Cl) medium. Cells were resuspended in EMM2-N at a concentration of 107 cells/ml and incubated at 30 °C for 21 h. Nitrogen starved cells were centrifuged and resuspended in EMM2 medium. Cells were harvested at 1, 2 and 3 h. Proteins were extracted from 50 ml cultures by trichloroacetic acid (TCA) precipitation according to Grallert and Hagan97. 50 μg of proteins were separated by electrophoresis through 4–12% polyacrylamide gels and transferred onto a PVDF membrane (Immobilon-P with pore size 0.45 μm; from Millipore). Anti-Pk antibody (mouse-anti-Pk (V5) antibody, Invitrogen R960-25, 1:2000 dilution) and goat anti-mouse IgG-HRP secondary antibody (Jackson ImmunoResearch 115-035-033, 1:10,000 dilution) were used to detect Sor1-Pk. Anti-tubulin antibody (Sigma-Aldrich T5168; 1:10,000 dilution) and rabbit anti-mouse IgG-HRP secondary antibody (Jackson ImmunoResearch 115-035-033, 1:10,000 dilution) were used to detect alpha tubulin as a loading control. To measure the DNA content of the cells, flow cytometry was performed according to98 using Sytox Green (S7020, Invitrogen) and FACS NovoCyte Penteon (Agilent).
In vitro APC/C assay
In vitro-translation of Sor1-HA-His by using reticulocyte lysate was performed according to the manufacturer’s protocols (TNT Quick Couled Transcription/Translation System, Promega). Xenopus CSF-arrested egg extracts were prepared as described99. For interphase extracts, 0.4 mM CaCl2 was supplemented to CSF extracts at a final concentration of 0.4 mM to induce exit from meiosis.
Plant mutant lines and growth conditions
The Arabidopsis thaliana Columbia (Col-0) ecotype was used as wild-type reference. Atsororin mutant plants were generated via CRISPR-Cas9 (see below). The wapl1-1 wapl2 double mutant (SALK_108385, SALK_127445)35 was crossed with heterozygous AtSORORIN +/− mutant to obtain the Atsororin wapl1-1 wapl2 triple mutant. Plants were grown on soil or on media plates containing Murashige and Skoog agar medium100 with 2% sucrose. Long day growth conditions were applied with cycles of 16 h light and 8 h dark, at 21 °C and 60% humidity.
Leaves from rosette-stage plants grown on soil or the first true leaves from seedlings grown on plates, were collected for DNA isolation and genotyping. Mutants were confirmed by PCR using the primers listed below (Table 2). Atsororin mutants were confirmed by Sanger Sequencing of the PCR product (Supplementary Fig. 3a).
Floral dip transformation of A. thaliana
Arabidopsis was transformed via Agrobacterium tumefaciens mediated DNA transfer. In brief, an aliquot of A. tumefaciens electroporation-competent cells was thawed on ice and 100 ng of plasmid were added. After 15 min incubation on ice, the cells were transferred to electroporation cuvettes (Eppendorf, 4307-000-593). After electroporation (400 Ω, 25 μF, 2.5 kV), 900 μL of SOC media were added to the cuvettes and cells were left to rest for 1 h at RT. 300 μL of transformed cells were plated on 2xTY plates supplemented with 50 μg/ml gentamycin, 50 μg/ml rifampicin and 100 μg/ml kanamycin (plasmid selection). Plates were left at 30 °C overnight.
A single colony from transformed Agrobacterium tumefaciens was inoculated into 500 mL of 2xTY medium supplemented with antibiotics. After 2 days rotating at 30 °C, cells were centrifuged at 4500 × g for 30 min at 4 °C. The pellet was then resuspended in 200 mL infiltration buffer (5% sucrose in dH2O). Another centrifugation at 4500 × g for 30 min at 4 °C was performed and cells were now resuspended in 200 mL infiltration buffer containing 40 μL Silwet-L77. Prior to dipping the plants into the solution, their already developed siliques and open flowers were removed. Plants were then dipped into the Agrobacterium/infiltration buffer solution for 30 seconds and wrapped into plastic bags afterwards to avoid fast drying of the bacterial solution. Plants were transferred to the growth chamber and two days later the bags were removed.
Atsororin mutant generation
The Atsororin mutant was generated by using the CRISPR-CAS9 technology. The gRNA sequence 5’-CCGTCGGAGGAAGAATACAG-3’ is specific to exon 1 of the ATSORORIN gene (At3g56250) and induces cleavage a few nucleotides downstream of the ATG codon. The gRNA was cloned into pGGE000-EF_pChimera2, and together with the Cas9 promoter in pGGA000-AB_PcUbi, the Cas9 version in pGGB000-BC_PuCas9 and the Cas9 terminator in pGGC000-CD_PeaTer further subcloned into the destination vector pGGZ003 utilizing the GOLDENGATE technique. The final plasmid was used to transform Col-0 plants by using the floral dip method101. Transgenic plants grown on soil were identified and selected by their resistance to the herbicide Basta (applied by spraying 13.5 mg/l). For subsequent generations we screened for the absence/presence of the BASTA resistance gene (PAT) using the primers 35Sp_Fwd and Basta_Rev. Offspring of the initial transformants with or without the transgene were analysed for the presence of a mutation in the first exon 1 of the AtSORORIN gene. To do so, PCR amplicons were generated using the primers Sororin_geno_Fwd and Sororin_geno_Rev and subsequently sequenced with the primer Sororin_sequencing (Table 2). Plants with a mutation signature were grown for one or two more generations to identify individuals that inherited the mutation. We finally obtained a line without transgene and a stable heterozygous mutation in the AtSORORIN gene (Fig. 1). The Atsororin mutant line contains a 5 bps deletion within the first exon, 25 nucleotides down-stream of the ATG start codon (Fig. 3a; Supplementary Fig. 3a). It results in a premature TAA stop codon after generating a short peptide of 18 amino acid residues.
RNA extraction and qPCR
RNA was extracted from 30 mg of Arabidopsis thaliana wild-type Col-0 and Atsororin –/– buds, cauline leaves and rosette leaves with the SV Total RNA Isolation System (Promega, Z3100). For each condition and genotype three separate extractions were performed. RNA samples were quantified on a DS-11+ Series Spectrophotometer (DeNovix) and 100 ng of each sample was used for first-strand cDNA synthesis (iScript kit Bio-Rad, 1708890). qPCR was performed using KAPA SYBR® FAST (Sigma-Aldrich, KK4601) and a RealPlex MasterCycler (Eppendorf, 6302000.504) according to the master mix protocol. AtSORORIN expression was calculated using the ΔΔCt method102 normalized to ACTIN7 (AT5G09810) and the relative expression was related to the sample with the lowest Ct value (used primers are listed in Table 2).
Detection of mutant Atsororin mRNA
The specific AtSORORIN cDNA was generated using RNA extracted from cauline leaves of Atsororin –/– mutant plants. To this end, the primers Sor_Fwd_delATG and SOR_cDNA_Rev were used and the resulting cDNA amplicons were ligated into the pCR Blunt TOPO II cloning vector and subsequently sequenced using the primer M13_Rev_2 (all primers are listed in Table 2) (Supplementary Fig. 3a).
Complementation of Atsororin mutation
For complementing the Atsororin mutation, we first amplified the wild type AtSORORIN genomic version of the gene by PCR using Phusion DNA Polymerase. The primers specific for the amplification are listed in Table 2. The amplicon was then cloned into the pCB302 vector103, which is compatible with A. tumefaciens transformation and contains the BASTA resistance gene for future plant selection. Heterozygote AtSORORIN +/− plants were transformed with the pCB302 vector containing the AtSORORIN gene by the floral dip method to obtain the T1 generation of transformant plants. Two-week-old plants were selected for positive transformants by spraying the herbicide BASTA (150 mg/L BASTA in H2O). Heterozygote AtSORORIN +/− plants (based on sequencing) and BASTA-resistance were selected for three more generations. The offspring of several F3 plants were sown on soil to check their genotype. The analysed Atsororin complementation lines were those that only generated offspring containing the Atsororin mutant allele and the complementing transgene (parent plants were homozygous for both, the Atsororin mutant allele and the complementing transgene).
Seed counts
Mature but still green siliques originating from the fifth to the thirtieth flower per stem were harvested into fixing solution (1 part of glacial acetic acid and 3 parts of 96% EtOH) for distaining. After one day, the solution was renewed and seeds inside siliques were counted manually under a binocular microscope.
Alexander staining
For pollen viability assays, the anthers and pollens from mature flowers were dissected under the microscope. The individual anthers were placed on a slide and a few drops (~20 μl) of Alexander staining104 buffer (500 μl of water, 250 μl of 87% glycerin, 100 μl of 96% Ethanol, 50 μl of 1% acid fuchsin, 10 μl of 1% malachite green, 5 μl of 1% orange G, 50 μl of glacial acetic acid) were added. The anthers were covered with a coverslip and the microscopic slide was then incubated at 50 °C overnight. Stained pollen grains were observed with a microscope equipped with a differential contrast interference microscopy optics. Viable pollen grains appear round, filled with red-stained cytoplasm and coated with a thin green layer, while non-viable pollen appear only green, often shriveled and lack red cytoplasm.
Spreading of nuclei, fluorescence in situ hybridisation (FISH) and immunolocalization of meiotic proteins
For somatic cell preparations, the tissue of interest was fixed in Carnoy´s fixative (1 part of glacial acetic acid and 3 parts of 96% EtOH). After washings twice with TRIS buffer (10 mM TRIS pH 7.5, 10 mM EDTA, 100 mM NaCl), the plant material was disrupted with a plastic pestle in Lysis buffer (15 mM TRIS pH 7.5, 0.5 mM spermine, 2 mM EDTA, 80 mM KCl, 20 mM NaCl, 0.1% Triton X-100). The solution was then pipetted trough a 40-micron cell strainer and centrifuged at 500 × g for 3 min. The pellet was resuspended in 50 μL of lysis buffer and pipetted to a glass slide to let air-dry. In order to visualize meiotic progression, anther spreads were prepared as described in ref. 105.
Fluorescence in situ hybridization (FISH) was performed as described in ref. 106. In brief, slides with somatic or meiotic nuclei were washed twice with 2xSSC for 5 min. After 10 min in 4% paraformaldehyde, slides were quickly washed in water and transferred through an ethanol series (70%, 90% and 96% EtOH) and then left to dry. A Locked Nucleic Acid (LNA) probe was used to detect centromere regions (5’-TTGGCTACACCATGAAAGCTT-3’; Qiagen). 20 μL of probe mix (250 nM LNA probe, 10% dextran sulfate, 50% formamide in 2 x Saline Sodium Citrate) were pipetted on the slide. A coverslip was applied, and the slides were placed on a hot plate at 75 °C for 4 min. After an overnight incubation at 37 °C, the slides were washed twice in 2xSCC and 15 μL of 2 µg/ml 4’,6 diamidino-2-phenylindol (DAPI) diluted in Vectashield (Vector Laboratories) were applied.
Spreads of nuclei for the detection of meiotic chromatin and associated proteins were performed as previously described107. Primary antibodies were used as follows: 1:10,000 anti-ASY1 raised in guinea pig106, 1:500 anti-ZYP1 raised in rat108, 1:500 anti-SCC3 raised in rabbit109 and 1:250 anti-REC8 raised in rabbit110. The secondary antibodies are all commercially available and were used as follows: anti-guinea pig conjugated to Alexa Fluor 488 (1:400), anti-rabbit conjugated to Alexa Fluor 568 (1:400) and anti-rat conjugated to Alexa Fluor 647 (1:200).
Images were obtained with a Zeiss Axioplan microscope (Zeiss, Oberkochen, Germany) using a Quantix® CCD camera (Photometrics, Tucson, USA). Picture acquisition was performed with MetaMorph® Micoscopy Automation & Image Analysis software (Molecular Devices, Sunnyvale, USA). For meiotic prophase nuclei, Z-stacks with 100 nm intervals were acquired. Deconvolution was performed using AutoQuant software (Media Cybernetics Inc, Rockville, USA) and projections were done using Helicon Focus software (HeliconSoft, Kharkov, Ukraine).
DAPI immunostaining
For the detection of SCC3 in somatic cells of inflorescences of Arabidopsis thaliana wild-type and Atsororin –/– plants material was collected into Carnoy’s fixative. DAPI immunostainings were prepared according to Chelysheva et al.111. Rabbit anti-SCC3 1:500109 was incubated in humid chamber at 4 °C O/N, later the slides were washed in PBS-T and incubated with anti-rabbit conjugated to Alexa Fluor 568 (1:40) at 37 °C for 1 h. Slides were mounted with 2 μg/ml 4’,6 diamidino-2-phenylindol (DAPI) diluted in Vectashield (Vector Laboratories) and images were obtained with a Zeiss Axioplan microscope (Zeiss, Oberkochen, Germany) using a Quantix® CCD camera (Photometrics, Tucson, USA).
Root tip image processing
Whole roots from 2-week-old plants grown on plates were collected from different genotypes and immersed in a solution of 10 μg/mL DAPI with 0.1% Triton-X100. After 30 min incubation at room temperature, roots were placed on a slide.
Imaging was performed with a Zeiss LSM710 microscope equipped with an AiryScan Unit. To generate the movies, Z-stacks with 250 nm intervals were acquired. Deconvolution was performed with the Huygens Software.
Statistical analyses
All statistical analyses were performed using the GraphPad Prism 7 software. First, D’Agostino-Pearson omnibus normality test was performed to analyse if the data followed a Gaussian distribution. If yes, the two variables were compared using unpaired t-tests. When no Gaussian distribution was detected, unpaired Mann-Whitney tests were applied. Contingency tables were generated to compare expected (or wild type) data with mutant values. Fischer’s exact test was used when two variables were compared. For three or more variables, Chi-square tests were performed.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. Source data are provided with this paper.
References
Ishiguro, K. I. The cohesin complex in mammalian meiosis. Genes Cells 24, 6–30 (2019).
Oldenkamp, R. & Rowland, B. D. A walk through the SMC cycle: from catching DNAs to shaping the genome. Mol. Cell 82, 1616–1630 (2022).
Haering, C. H., Farcas, A. M., Arumugam, P., Metson, J. & Nasmyth, K. The cohesin ring concatenates sister DNA molecules. Nature 454, 297–301 (2008).
Peters, J. M., Tedeschi, A. & Schmitz, J. The cohesin complex and its roles in chromosome biology. Genes Dev. 22, 3089–3114 (2008).
Davidson, I. F. & Peters, J. M. Genome folding through loop extrusion by SMC complexes. Nat. Rev. Mol. Cell Biol. 22, 445–464 (2021).
Tomonaga, T. et al. Characterization of fission yeast cohesin: essential anaphase proteolysis of Rad21 phosphorylated in the S phase. Genes Dev. 14, 2757–2770 (2000).
Schubert, V. SMC proteins and their multiple functions in higher plants. Cytogenet. Genome Res. 124, 202–214 (2009).
Schleiffer, A. et al. Kleisins: a superfamily of bacterial and eukaryotic SMC protein partners. Mol. Cell 11, 571–575 (2003).
Gligoris, T. G. et al. Closing the cohesin ring: structure and function of its Smc3-kleisin interface. Science 346, 963–967 (2014).
Sonoda, E. et al. Scc1/Rad21/Mcd1 is required for sister chromatid cohesion and kinetochore function in vertebrate cells. Dev. Cell 1, 759–770 (2001).
Hu, B. et al. ATP hydrolysis is required for relocating cohesin from sites occupied by its Scc2/4 loading complex. Curr. Biol. 21, 12–24 (2011).
Orgil, O. et al. A conserved domain in the Scc3 subunit of cohesin mediates the interaction with both Mcd1 and the cohesin loader complex. PLoS Genet. 11, e1005036 (2015).
Hauf, S. et al. Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol. 3, e69 (2005).
Roig, M. B. et al. Structure and function of cohesin’s Scc3/SA regulatory subunit. FEBS Lett 588, 3692–3702 (2014).
Li, Y. et al. Structural basis for scc3-dependent cohesin recruitment to chromatin. Elife 7, e38356 (2018).
Murayama, Y. & Uhlmann, F. Biochemical reconstitution of topological DNA binding by the cohesin ring. Nature 505, 367–371 (2014).
Wells, J. N., Gligoris, T. G., Nasmyth, K. A. & Marsh, J. A. Evolution of condensin and cohesin complexes driven by replacement of Kite by Hawk proteins. Curr. Biol. 27, R17–R18 (2017).
Petela, N. J. et al. Scc2 is a potent activator of Cohesin’s ATPase that promotes loading by binding Scc1 without Pds5. Mol. Cell 70, 1134–1148.e7 (2018).
Kikuchi, S., Borek, D. M., Otwinowski, Z., Tomchick, D. R. & Yu, H. Crystal structure of the cohesin loader Scc2 and insight into cohesinopathy. Proc. Natl. Acad. Sci. USA. 113, 12444–12449 (2016).
Davidson, I. F. et al. DNA loop extrusion by human cohesin. Science 366, 1338–1345 (2019).
Kim, Y., Shi, Z., Zhang, H., Finkelstein, I. J. & Yu, H. Human cohesin compacts DNA by loop extrusion. Science 366, 1345–1349 (2019).
Ciosk, R. et al. Cohesin’s binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Mol. Cell 5, 243–254 (2000).
Kueng, S. et al. Wapl controls the dynamic association of cohesin with chromatin. Cell 127, 955–967 (2006).
Beckouët, F. et al. Releasing activity disengages Cohesin’s Smc3/Scc1 interface in a process blocked by acetylation. Mol. Cell 61, 563–574 (2016).
Chan, K. L. et al. Cohesin’s DNA exit gate is distinct from its entrance gate and is regulated by acetylation. Cell 150, 961–974 (2012).
Huis In’t Veld, P. J. et al. Characterization of a DNA exit gate in the human cohesin ring. Science 346, 968–972 (2014).
Gerlich, D., Koch, B., Dupeux, F., Peters, J. M. & Ellenberg, J. Live-cell imaging reveals a stable cohesin-chromatin interaction after but not before DNA replication. Curr. Biol. 16, 1571–1578 (2006).
Bernard, P. et al. Cell-cycle regulation of cohesin stability along fission yeast chromosomes. EMBO J. 27, 111–121 (2008).
Ben-Shahar, T. R. et al. Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science 321, 563–566 (2008).
Ivanov, D. et al. Eco1 is a novel acetyltransferase that can acetylate proteins involved in cohesion. Curr. Biol. 12, 323–328 (2002).
Tóth, A. et al. Yeast cohesin complex requires a conserved protein, Eco1p(Ctf7), to establish cohesion between sister chromatids during DNA replication. Genes Dev. 13, 320–333 (1999).
Ünal, E. et al. A molecular determinant for the establishment of sister chromatid cohesion. Science 321, 566–569 (2008).
Zhang, J. et al. Acetylation of Smc3 by Eco1 is required for s phase sister chromatid cohesion in both human and yeast. Mol. Cell 31, 143–151 (2008).
Pradillo, M. et al. Involvement of the cohesin cofactor PDS5 (SPO76) during meiosis and DNA repair in Arabidopsis thaliana. Front. Plant Sci. 6, 1034 (2015).
De, K., Sterle, L., Krueger, L., Yang, X. & Makaroff, C. A. Arabidopsis thaliana WAPL is essential for the prophase removal of cohesin during meiosis. PLoS Genet. 10, e1004497 (2014).
Bolaños-Villegas, P. et al. Arabidopsis CHROMOSOME TRANSMISSION FIDELITY 7 (AtCTF7/ECO1) is required for DNA repair, mitosis and meiosis. Plant J. 75, 927–940 (2013).
De, K. et al. The opposing actions of arabidopsis CHROMOSOME TRANSMISSION FIDELITY7 and WINGS APART-LIKE1 and 2 differ in mitotic and meiotic cells. Plant Cell 28, 521–536 (2015).
Lafont, A. L., Song, J. & Rankin, S. Sororin cooperates with the acetyltransferase Eco2 to ensure DNA replication-dependent sister chromatid cohesion. Proc. Natl. Acad. Sci. USA. 107, 20364–20369 (2010).
Song, J. et al. Cohesin acetylation promotes sister chromatid cohesion only in association with the replication machinery. J. Biol. Chem. 287, 34325–34336 (2012).
Nishiyama, T. et al. Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell 143, 737–749 (2010).
Yamada, T., Tahara, E., Kanke, M., Kuwata, K. & Nishiyama, T. Drosophila Dalmatian combines sororin and shugoshin roles in establishment and protection of cohesion. EMBO J. 36, 1513–1527 (2017).
Rankin, S., Ayad, N. G. & Kirschner, M. W. Sororin, a substrate of the anaphase-promoting complex, is required for sister chromatid cohesion in vertebrates. Mol. Cell 18, 185–200 (2005).
Schmitz, J., Watrin, E., Lénárt, P., Mechtler, K. & Peters, J. M. Sororin is required for stable binding of cohesin to chromatin and for sister chromatid cohesion in interphase. Curr. Biol. 17, 630–636 (2007).
Ouyang, Z., Zheng, G., Tomchick, D. R., Luo, X. & Yu, H. Structural basis and IP6 requirement for Pds5-dependent cohesin dynamics. Mol. Cell 62, 248–259 (2016).
Ladurner, R. et al. Sororin actively maintains sister chromatid cohesion. EMBO J. 35, 635–653 (2016).
Waizenegger, I. C., Hauf, S., Meinke, A. & Peters, J. M. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell 103, 399–410 (2000).
Buheitel, J. & Stemmann, O. Prophase pathway-dependent removal of cohesin from human chromosomes requires opening of the Smc3-Scc1 gate. EMBO J. 32, 666–676 (2013).
Gandhi, R., Gillespie, P. J. & Hirano, T. Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr. Biol. 16, 2406–2417 (2006).
Zhang, N., Panigrahi, A. K., Mao, Q. & Pati, D. Interaction of sororin protein with polo-like kinase 1 mediates resolution of chromosomal arm cohesion. J. Biol. Chem. 286, 41826–41837 (2011).
Dreier, M. R., Bekier, M. E. & Taylor, W. R. Regulation of sororin by cdk1-mediated phosphorylation. J. Cell Sci. 124, 2976–2987 (2011).
Nishiyama, T., Sykora, M. M., Huis, P. J., Mechtler, K. & Peters, J. M. Aurora B and Cdk1 mediate Wapl activation and release of acetylated cohesin from chromosomes by phosphorylating Sororin. Proc. Natl. Acad. Sci. USA. 110, 13404–13409 (2013).
Liu, H., Rankin, S. & Yu, H. Phosphorylation-enabled binding of SGO1-PP2A to cohesin protects sororin and centromeric cohesion during mitosis. Nat. Cell Biol. 15, 40–49 (2013).
Kitajima, T. S. et al. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 441, 46–52 (2006).
Hornig, N. C. D., Knowles, P. P., McDonald, N. Q. & Uhlmann, F. The dual mechanism of separase regulation by securin. Curr. Biol. 12, 973–982 (2002).
Hagting, A. et al. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J. Cell Biol. 157, 1125–1137 (2002).
Wirth, K. G. et al. Separase: A universal trigger for sister chromatid disjunction but not chromosome cycle progression. J. Cell Biol. 172, 847–860 (2006).
Sutani, T., Kawaguchi, T., Kanno, R., Itoh, T. & Shirahige, K. Budding yeast Wpl1(Rad61)-Pds5 complex counteracts sister chromatid cohesion-establishing reaction. Curr. Biol. 19, 492–497 (2009).
Yang, C. et al. SWITCH 1/DYAD is a WINGS APART-LIKE antagonist that maintains sister chromatid cohesion in meiosis. Nat. Commun. 10, 1755 (2019).
Zhang, N. & Pati, D. C-terminus of sororin interacts with sa2 and regulates sister chromatid cohesion. Cell Cycle 14, 820–826 (2015).
Wu, F. M., Nguyen, J. V. & Rankin, S. A conserved motif at the C terminus of sororin is required for sister chromatid cohesion. J. Biol. Chem. 286, 3579–3586 (2011).
McDowall, M. D. et al. PomBase 2015: Updates to the fission yeast database. Nucleic Acids Res. 43, D656–D661 (2015).
Feytout, A., Vaur, S., Genier, S., Vazquez, S. & Javerzat, J.-P. Psm3 acetylation on conserved lysine residues is dispensable for viability in fission yeast but contributes to Eso1-mediated sister chromatid cohesion by antagonizing Wpl1. Mol. Cell. Biol. 31, 1771–1786 (2011).
Furuya, K., Takahashi, K. & Yanagida, M. Faithful anaphase is ensured by Mis4, a sister chromatid cohesion molecule required in S phase and not destroyed in G1 phase. Genes Dev. 12, 3408–3418 (1998).
Chen, Z., McCroskey, S., Guo, W., Li, H. & Gerton, J. L. A genetic screen to discover pathways affecting cohesin function in Schizosaccharomyces pombe identifies chromatin effectors. G3 Genes. Genomes Genet. 2, 1161–1168 (2012).
Liang, C. et al. A kinase‐dependent role for Haspin in antagonizing Wapl and protecting mitotic centromere cohesion. EMBO Rep. 19, 43–56 (2018).
Matsuyama, A. et al. ORFeome cloning and global analysis of protein localization in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 24, 841–847 (2006).
Yatskevich, S., Rhodes, J. & Nasmyth, K. Organization of chromosomal DNA by SMC complexes. Ann. Rev. Genet. 53, 445–482 (2019).
Zhang, N. & Pati, D. Sororin is a master regulator of sister chromatid cohesion and separation. Cell Cycle 11, 2073–2083 (2012).
Mercier, R. et al. SWITCH1 (SWI1): A novel protein required for the establishment of sister chromatid cohesion and for bivalent formation at meiosis. Genes Dev. 15, 1859–1871 (2001).
Alomer, R. M. et al. Esco1 and Esco2 regulate distinct cohesin functions during cell cycle progression. Proc. Natl. Acad. Sci. USA. 114, 9906–9911 (2017).
Zhou, L. et al. The N-terminal non-kinase-domain-mediated binding of haspin to Pds5B protects centromeric cohesion in mitosis. Curr. Biol. 27, 992–1004 (2017).
Wutz, G. et al. ESCO1 and CTCF enable formation of long chromatin loops by protecting cohesinstag1 from WAPL. Elife 9, e52091 (2020).
Li, Y. et al. The structural basis for cohesin–CTCF-anchored loops. Nature 578, 472–476 (2020).
Haase, J., Bonner, M. K., Halas, H. & Kelly, A. E. Distinct roles of the chromosomal passenger complex in the detection of and response to errors in kinetochore-microtubule attachment. Dev. Cell 42, 640–654.e5 (2017).
Dai, J., Sultan, S., Taylor, S. S. & Higgins, J. M. G. The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev. 19, 472–488 (2005).
Kurihara, D., Matsunaga, S., Omura, T., Higashiyama, T. & Fukui, K. Identification and characterization of plant Haspin kinase as a histone H3 threonine kinase. BMC Plant Biol. 11, 73 (2011).
Wang, F. et al. Histone H3 Thr-3 phosphorylation by haspin positions Aurora B at centromeres in mitosis. Science 330, 231–235 (2010).
Yamagishi, Y., Honda, T., Tanno, Y. & Watanabe, Y. Two histone marks establish the inner centromere and chromosome bi-orientation. Science 330, 239–243 (2010).
Goto, Y. et al. Pds5 regulates sister-chromatid cohesion and chromosome bi-orientation through a conserved protein interaction module. Curr. Biol. 27, 1005–1012 (2017).
Eddy, S. R. Profile hidden Markov models. Bioinformatics 14, 755–763 (1998).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Katoh, K. & Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 9, 286–298 (2008).
Drozdetskiy, A., Cole, C., Procter, J. & Barton, G. J. JPred4: a protein secondary structure prediction server. Nucleic Acids Res. 43, W389–W394 (2015).
Waterhouse, A. M., Procter, J. B., Martin, D. M. A., Clamp, M. & Barton, G. J. Jalview Version 2-A multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009).
Agarwala, R. et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 46, D8–D13 (2018).
Bateman, A. et al. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 49, D480–D489 (2021).
Schoch, C. L. et al. NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database 2020, baaa062 (2020).
Letunic, I. & Bork, P. Interactive tree of life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296 (2021).
Cipak, L. et al. Generation of a set of conditional analog-sensitive alleles of essential protein kinases in the fission yeast Schizosaccharomyces pombe. Cell Cycle 10, 3527–3532 (2011).
Dudas, A., Polakova, S. & Gregan, J. Chromosome segregation: monopolin attracts condensin. Curr. Biol. 21, R634-6 (2011).
Sabatinos, S. A. & Forsburg, S. L. Molecular genetics of schizosaccharomyces pombe. Methods Enzymol. 470, 759–795 (2010).
Cipak, L. et al. An improved strategy for tandem affinity purification-tagging of Schizosaccharomyces pombe genes. Proteomics 9, 4825–4828 (2009).
Kovacikova, I. et al. A knockout screen for protein kinases required for the proper meiotic segregation of chromosomes in the fission yeast Schizosaccharomyces pombe. Cell Cycle 12, 618–624 (2013).
Polakova, S. et al. Dbl2 regulates Rad51 and DNA joint molecule metabolism to ensure proper meiotic chromosome segregation. PLoS Genet. 12, e1006102 (2016).
Phadnis, N. et al. Casein kinase 1 and phosphorylation of cohesin subunit Rec11 (SA3) promote meiotic recombination through linear element formation. PLoS Genet. 11, e1005225 (2015).
Cipak, L., Hyppa, R. W., Smith, G. R. & Gregan, J. ATP analog-sensitive Pat1 protein kinase for synchronous fission yeast meiosis at physiological temperature. Cell Cycle 11, 1626–1633 (2012).
Grallert, A. & Hagan, I. M. Preparation of protein extracts from Schizosaccharomyces pombe using trichloroacetic acid precipitation. Cold Spring Harb. Protoc. 2017, 139–143 (2017).
Sanyal, S. et al. Mutations that prevent methylation of cohesin render sensitivity to DNA damage in S. pombe. J. Cell Sci. 131, jcs214924 (2018).
Murray, A. W. Chapter 30 cell cycle extracts. Methods Cell Biol. 36, 581–605 (1991).
Murashige, T. & Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497 (1962).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ÄÄCT method. Methods 25, 402–408 (2001).
Xiang, C., Han, P., Lutziger, I., Wang, K. & Oliver, D. J. A mini binary vector series for plant transformation. Plant Mol. Biol. 40, 711–717 (1999).
Alexander, M. P. Differential staining of aborted and nonaborted pollen. Biotech. Histochem. 44, 117–122 (1969).
Vignard, J. et al. The interplay of RecA-related proteins and the MND1–HOP2 complex during meiosis in Arabidopsis thaliana. PLoS Genet 3, e176 (2007).
Sims, J., Copenhaver, G. P. & Schlögelhofer, P. Meiotic DNA repair in the nucleolus employs a nonhomologous end-joining mechanism. Plant Cell 31, 2259–2275 (2019).
Kurzbauer, M. T., Uanschou, C., Chen, D. & Schlögelhofer, P. The recombinases DMC1 and RAD51 are functionally and spatially separated during meiosis in Arabidopsis. Plant Cell 24, 2058–2070 (2012).
Higgins, J. D., Sanchez-Moran, E., Armstrong, S. J., Jones, G. H. & Franklin, F. C. H. The Arabidopsis synaptonemal complex protein ZYP1 is required for chromosome synapsis and normal fidelity of crossing over. Genes Dev 19, 2488–2500 (2005).
Chelysheva, L. et al. AtREC8 and AtSCC3 are essential to the monopolar orientation of the kinetochores during meiosis. J. Cell Sci. 118, 4621–4632 (2005).
Cromer, L. et al. Centromeric cohesion is protected twice at meiosis, by SHUGOSHINs at anaphase i and by PATRONUS at interkinesis. Curr. Biol. 23, 2090–2099 (2013).
Chelysheva, L. A., Grandont, L. & Grelon, M. Immunolocalization of meiotic proteins in Brassicaceae: Method 1. Methods Mol. Biol. 990, 93–101 (2013).
Ren, J. et al. DOG 1.0: Illustrator of protein domain structures. Cell Res. 19, 271–273 (2009).
Acknowledgements
J.G. was supported by the Austrian Science Fund (FWF) (grant P30516), the Slovak Grant Agency VEGA (1/0450/18 and 2/0026/18) and the Slovak Research and Development Agency (APVV-17-0130, APVV-18-0219, and APVV-16-0120). Research in the laboratory of P.S. was supported by the Austrian Science Fund (FWF) (I 1468-B16; Special Research Focus programme “Chromosome Dynamics” F3408-B19; FWF Doctoral Programme “Chromosome Dynamics” W1238-B20; Special Research Focus programme “Meiosis” F 8808-B) and the Austria’s Agency for Education and Internationalization (Ernst Mach Grant to T.T.N.). Research in the laboratory of J.-M.P. is supported by Boehringer Ingelheim, the Austrian Life Sciences Programme 2023 (LS23 IF, project FO999902549), the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme (1020558), the Human Frontier Science Programme (RGP0057/2018), and the Vienna Science and Technology Fund (LS19-029). J.-M.P. is also an adjunct professor at the Medical University of Vienna. We thank Vera Schoft and the Vienna Biocenter Core Facility (PlantS) for generating the Atsororin mutant line. We thank M. Yanagida, J.P. Javerzat and J. Gerton for sending yeast strains and Z. Benko, B. Huraiova, L. Cipak and S. Polakova for help with yeast experiments.
Author information
Authors and Affiliations
Contributions
A.S. and J.-M.P. performed the bioinformatic analyses and identified putative Sororin homologues in eukaryotes. M.G., I.K., T.N., G.L. and J.G. conceived and performed the experiments with S. pombe. I.P.M., C.F.S. and P.S. conceived and performed the experiments with A. thaliana. T.T.N. generated the A. thaliana sororin mutant. I.P.M., J.G., J.-M.P. and P.S. analysed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Christopher Makaroff and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prusén Mota, I., Galova, M., Schleiffer, A. et al. Sororin is an evolutionary conserved antagonist of WAPL. Nat Commun 15, 4729 (2024). https://doi.org/10.1038/s41467-024-49178-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-49178-0
- Springer Nature Limited