Abstract
People with lower extremity peripheral artery disease (PAD) have increased oxidative stress, impaired mitochondrial activity, and poor walking performance. NAD+ reduces oxidative stress and is an essential cofactor for mitochondrial respiration. Oral nicotinamide riboside (NR) increases bioavailability of NAD+ in humans. Among 90 people with PAD, this randomized double-blind clinical trial assessed whether 6-months of NR, with and without resveratrol, improves 6-min walk distance, compared to placebo, at 6-month follow-up. At 6-month follow-up, compared to placebo, NR significantly improved 6-min walk (+7.0 vs. −10.6 meters, between group difference: +17.6 (90% CI: + 1.8,+∞). Among participants who took at least 75% of study pills, compared to placebo, NR improved 6-min walk by 31.0 meters and NR + resveratrol improved 6-min walk by 26.9 meters. In this work, NR meaningfully improved 6-min walk, and resveratrol did not add benefit to NR alone in PAD. A larger clinical trial to confirm these findings is needed.
Similar content being viewed by others
Introduction
People with lower extremity peripheral artery disease (PAD) have severe walking disability, but few effective treatments exist1. In PAD, lower extremity ischemia causes insufficient oxygen and nutrient delivery to lower extremity skeletal muscle, which increases oxidative stress, damages skeletal muscle fibers, and impairs mitochondria function.2,3,4,5,6,7.
NAD+ is an essential coenzyme for mitochondrial respiration and a co-substrate for enzymes involved in metabolic regulation, cellular stress resistance, and DNA damage repair, such as poly (ADP-ribose) polymerase (PARP)8,9. In preclinical study, NAD+ activated endothelial nitric oxide synthase (eNOS) and reduced oxidative stress to increase nitric oxide abundance10. Greater NAD+ abundance increased sirtuin1 (SIRT1) expression and improved skeletal muscle health, mitochondrial activity, and nitric-oxide-mediated endothelial function11,12,13. Nicotinamide riboside (NR) is a precursor to NAD+ and is available as an over-the counter supplement. In 2022, sales of NR in the U.S. exceeded 60 million dollars. In mice, oral NR, a precursor to NAD+, increased skeletal muscle SIRT-1 and mitochondrial activity and improved limb strength and running endurance13,14. In humans, oral NR increased NAD+ abundance15,16.
Resveratrol, a naturally occurring polyphenol, may increase SIRT1 affinity for NAD+17. Therefore, the NICotinamidE riboside with and without resveratrol to improve functioning in PAD (NICE) clinical trial tested the hypotheses that NR alone improves 6-min walk distance, compared to placebo, and that NR + resveratrol improves 6-min walk distance, compared to placebo, in people with PAD.
Here we show that NR meaningfully improves 6-min walk, and resveratrol does not add benefit to NR alone in PAD.
Results
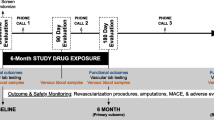
Ninety participants were randomized and 89 (98.9%) completed 6-month follow-up (Fig. 1). Of 104 people who began run-in, eight (7.7%) did not meet criteria for passing and were excluded (Supplementary Table 1). Of 90 randomized, mean age was 71.2 years (standard deviation (SD): 9.1), 47.8% were Black, and 46.7% were female (Table 1). Proportions of participants with at least 75% adherence to pills were 75% for NR, 52% for NR + resveratrol, and 76% for placebo.
Primary outcomes
Compared to placebo, NR improved 6-min walk by 17.6 meters (90% CI: + 1.77, +∞, P = 0.08) at 6-month follow-up, meeting the pre-specified criterion for statistical significance (Table 2 and Fig. 2A). Compared to placebo, NR+ resveratrol did not significantly improve 6-min walk at 6-month follow-up (+3.65 meters (90% CI:−11.2, +∞, P = 0.38)) (Table 2 and Fig. 2A).
Figure A depicts the 6-month change in 6-min walk (primary outcome) results for N = 90 participants. Mixed models for repeated measures (MMRM) were used to compare 6-month change in 6-min walk distance between the NR + resveratrol and placebo groups and between the NR alone and placebo groups using baseline, 3-month, and 6-month values for 6-min walk distance, adjusting for age, sex, race, and baseline 6-min walk. The a priori statistical analysis plan defined a one-sided P value of <0.10 to define statistical significance. The circle represents NR alone, the square represents NR + resveratrol, and the triangle represents placebo. Figure B depicts 3-month change in 6-min walk (secondary outcome) for N = 90 participants. Mixed models for repeated measures (MMRM) were used to compare change in 6-min walk distance between the NR + resveratrol and placebo groups and between the NR alone and placebo groups using baseline, 3-month, and 6-month values for 6-min walk distance, adjusting for age, sex, race, and baseline 6-min walk. The a priori statistical analysis plan defined a one-sided P value of <0.10 to define statistical significance. The circle represents NR alone, the square represents NR + resveratrol, and the triangle represents placebo. Figure C depicts 6-month change in 6-min walk among the 60 participants who took at least 75% of study pills. Mixed models for repeated measures (MMRM) were used to compare 6-month change in 6-min walk distance between the NR + resveratrol and placebo groups and between the NR alone and placebo groups using baseline, 3-month, and 6-month values for 6-min walk distance, adjusting for age, sex, race, and baseline 6-min walk. The a priori statistical analysis plan defined a one-sided P value of <0.10 to define statistical significance. The circle represents NR alone, the square represents NR + resveratrol, and the triangle represents placebo.
Secondary outcomes
At 3-month follow-up, compared to placebo, NR alone (+22.4 meters, (90% CI: +7.3,+∞), P = 0.029) and NR + resveratrol (+20.6 meters (90% CI: +6.3,+∞), P = 0.034) each significantly improved 6-min walk (Table 2, Fig. 2B). At 6-month follow-up, compared to placebo, NR significantly improved peak treadmill walking time (+2.1 min (90% CI: +0.24, +∞, P = 0.08)), while NR + resveratrol did not significantly improve treadmill walking time (+1.7 min (90% CI:−0.21, +∞, P = 0.12)) (Table 3). At 6-month follow-up, compared to placebo, NR alone did not significantly improve the WIQ distance score (−7.1 (90% CI:−15.2, +∞, P = 0.87)), physical activity total counts/day (+10,995 (90% CI:−2,078, +∞, P = 0.14)), or physical activity counts/min (+8.24 (90% CI:−23.85, +∞, P = 0.37)) (Table 3). At 6-month follow-up, compared to placebo, NR + resveratrol did not significantly improve the WIQ distance score (−5.1 (90% CI:−12.6, +∞, P = 0.80)), physical activity total counts/day (−842 (90% CI:−13,125, +∞, P = 0.54)), or physical activity counts/min (+7.47 (90% CI:−22.97, +∞, P = 0.38)) (Table 3).
Compared to placebo, the combined NR groups significantly improved 6-min walk distance at 3-month follow-up (+25.25 meters (90% CI: +10.43, +∞, P = 0.015)), maximal treadmill walking time at 6-month follow-up (+2.06 min (90% CI: + 0.24, +∞, P = 0.075)) and gastrocnemius muscle satellite cell abundance at 6-month follow-up (+11.14 (90% CI: +2.16, +∞, P = 0.060)) (Tables 4 and 5). Compared to placebo, the combined NR groups did not significantly improve 6-min walk distance (+14.05 meters, 90% CI: −1.25, +∞, P = 0.12), the WIQ distance score (−7.10 (90% CI:−15.19, +∞, P = 0.87), physical activity total activity counts/day (+10,995 (90% CI:−2,078, +∞, P = 0.14)), physical activity counts/min (+8.24 (90% CI:−23.85, +∞, P = 0.37)) or gastrocnemius muscle measures of NAD+ abundance or Percent Type I myofibers at 6-month follow-up (Tables 4 and 5).
Compared to NR alone, NR + resveratrol did not significantly improve 6-min walk at 3-month follow-up or 6-min walk, maximal treadmill walking time or the WIQ distance score at 6-month follow-up (Table 6).
Exploratory outcomes
At 6-month follow-up, compared to placebo, NR alone significantly increased gastrocnemius muscle satellite cell abundance (+11.14 satellite cells/100 fibers, 90% CI:+2.16, +∞, P = 0.06). At 6-month follow-up, compared to placebo, there was no significant effect of NR alone or NR + resveratrol on WIQ speed score, WIQ stair-climbing score, SF-36 physical functioning score, or on gastrocnemius muscle biopsy measures of NAD+ abundance, or myofiber type (Table 7). At 6-month follow-up, compared to placebo, NR + resveratrol did not significantly increase gastrocnemius muscle satellite cell abundance (Table 7). At 6-month follow-up, compared to NR alone, NR + resveratrol did not significantly improve WIQ speed score, WIQ stair climbing score, or SF-36 physical functioning score (Supplementary Table 4). At 6-month follow-up, compared to placebo, the combined groups of NR alone and NR + resveratrol did not significantly improve the WIQ speed score, the WIQ stair climbing score, or the SF-36 physical functioning score (Supplementary Table 3).
Post-hoc analyses
Among all participants with 75% or greater adherence to pills, at 6-month follow-up, compared to placebo, NR improved 6-min walk by 31.0 meters (90% CI: + 13.2, +∞, P = 0.014) and NR + resveratrol improved 6-min walk by 26.9 meters (90% CI: 9.1, +∞, P = 0.028) (Fig. 2C). Among participants randomized to NR alone and among participants randomized to NR + resveratrol, those with 75% or greater adherence to study pills improved 6-min walk at 6-month follow-up, while those with <75% adherence declined in 6-min walk (Table 8). Among participants randomized to placebo, mean 6-min walk declined at 6-month follow-up, regardless of adherence (Table 8).
Adverse events
Participants randomized to NR + resveratrol reported higher rates of diarrhea during the study (54.6%), compared to those randomized to NR alone (39.3%) and those randomized to placebo (27.6%). Participants randomized to NR + resveratrol also reported higher rates of nausea or emesis (36.4%) compared to those randomized to NR alone (14.3%) and placebo (24.1%) (Supplementary Table 5). One participant randomized to placebo experienced chest pain during the 6-min walk at three-month follow-up, was hospitalized, and later underwent coronary artery bypass grafting. No other serious adverse events were related to study participation.
Discussion
In this double-blind randomized clinical trial of 90 participants with PAD, compared to placebo, NR improved 6-min walk distance by 17.6 meters at 6-month follow-up, consistent with a clinically meaningful effect18,19. Compared to placebo, NR significantly improved 6-min walk by 22.4 meters and NR + resveratrol significantly improved 6-min walk by 20.6 meters at 3-month follow-up. In post-hoc analyses, compared to placebo, NR alone significantly improved 6-min walk by 31.0 meters and NR + resveratrol improved 6-min walk by 26.9 meters among people with at least 75% adherence to study pills. NR increased satellite cell abundance in gastrocnemius muscle, compared to placebo, meeting the pre-specified criterion for statistical significance, but did not affect muscle fiber type.
To our knowledge, no prior randomized clinical trials have demonstrated beneficial effects of NR on walking performance in any human population. A clinical trial of 40 sedentary obese men showed no effect of NR 1000 mgs twice daily on changes in skeletal muscle NAD+ metabolites, insulin sensitivity, body composition, or mitochondrial activity20,21,22,23,24. Among 30 people with heart failure and ejection fraction <40%, NR 2000 mgs daily meaningfully increased plasma whole blood NAD+, but had no effect on 6-min walk, compared to placebo24. In contrast to participants in these prior clinical trials, PAD is characterized by lower extremity ischemia and increased lower extremity skeletal muscle reactive oxygen species (ROS)2,3,4.
While the magnitude of 6-min walk improvement at 6-month follow-up was greater in the NR alone group compared to the NR + resveratrol group, the difference between these two groups was not statistically significant. Among people randomized to NR alone, 75% took 75% or more study pills, while among those randomized to NR + resveratrol, only 52% took 75% or more of study pills. The poorer adherence among those randomized to NR + resveratrol explained the smaller effect of NR + resveratrol on improved 6-min walk, compared to placebo. In analyses of participants with at least 75% adherence, NR alone and NR + resveratrol each had similarly large and clinically meaningful effects on 6-min walk. Reasons for the lower adherence rate in people randomized to NR + resveratrol are unknown, but people randomized to NR + resveratrol reported higher rates of diarrhea and higher rates of nausea or emesis, compared to those randomized to NR alone or placebo. It is possible that study intervention adherence would have been poorer and overall effect sizes from NR may have been lower if successful completion of the run-in had not been required for eligibility.
This trial has several limitations. First, the sample size was relatively small. Results require confirmation in a larger study. Second, just 17 participants had muscle biopsy at baseline and follow-up, limiting statistical power to detect NR effects on muscle. Third, due to the COVID-19 pandemic, just 26 participants had treadmill testing at baseline and follow-up. Fourth, results may not be generalizable to potential participants who would not have passed the study run-in.
Among people with PAD, NR meaningfully improved 6-min walk at 6-month follow-up, and resveratrol did not add benefit to NR alone. Among participants with at least 75% adherence, the magnitude of the effect of NR on 6-min walk was comparable to the effects of supervised exercise for PAD. Further study is needed to confirm these findings in a larger cohort of participants with PAD.
Methods
The Institutional Review Board of Northwestern University approved the protocol. Participants gave written informed consent. The study was a parallel-design, double-blinded, randomized clinical trial conducted in Chicago at Northwestern University Feinberg School of Medicine. Enrollment occurred between 12/13/2018 and 9/14/2022. The final follow-up occurred on 4/3/2023 when the last participant completed follow-up testing. Eligible participants were randomized to one of three groups: nicotinamide riboside 1,000 mg daily, nicotinamide riboside 1000 mg + 125 mgs resveratrol daily, or placebo. Enrollment was completed when the target sample size was attained. The study protocol is available in the supplementary information file as Supplementary Note 1.
Participant identification
Participants were recruited with advertisements on Chicago-area buses and trains, postcards mailed to people aged 50 and older, and by contacting people with PAD who previously participated in research with the principal investigator (MMM) and expressed interest in future research. Participants received $25.00 for completing 6-month follow-up testing.
Inclusion criteria
The inclusion criterion was presence of PAD, defined by an ankle brachial index (ABI) ≤0.90 in either leg25,26 or by medical record documentation of PAD from a vascular laboratory test obtained at a medical center or angiographic evidence of PAD (defined as 70% or greater stenosis in a lower extremity artery). Vascular laboratory testing criteria acceptable for inclusion included a toe brachial index <0.70, an ABI value ≤0.90, 70% or greater stenosis in a lower extremity artery on Duplex testing, or a post-exercise ABI decline of at least 20%.
Exclusion criteria
Exclusion criteria included above or below-knee amputation, presence of critical limb ischemia, inability to walk without a wheelchair, requiring a walker for ambulation, presence of an ulcer on the bottom of either foot, end stage kidney disease, significantly impaired liver function, and failure to pass run-in. Participants whose walking was limited by a symptom other than PAD were excluded. Potential participants who planned any major surgery or lower extremity revascularization during the next 6 months and those who completed any major surgery, coronary revascularization, or lower extremity revascularization during the previous three months were excluded. Additional exclusion criteria included supervised treadmill exercise participation during the previous 6-months, participation in a randomized clinical trial during the previous three months, unstable angina, inability to communicate in English, pregnancy or pre-menopausal state, major medical illness, life-expectancy <6 months, dementia or a mini-mental status examination score <2327, and visual impairment limiting walking ability. People taking 250 mgs or more of nicotinamide riboside per day, vitamin B3, niacin, a slow release form of niacin, or resveratrol within the past 6 months were excluded.
Run-in
The run-in was intended to identify and exclude potential participants who were unlikely to adhere to study interventions. Potential participants were asked to take five placebo pills daily (two NR placebo pills twice daily and one resveratrol placebo daily) for 14 days. Potential participants who did not take at least 70% of study pills were excluded.
Randomization
A SAS program randomized eligible participants to one of three groups with equal probability (NR 1000 mgs alone, NR 1000 mgs + 125 mgs resveratrol, or placebo) using a randomly permuted block method with block sizes of four and six.
Interventions
Interventions were administered in a double-blinded fashion for 6 months. Participants were asked to take five pills daily: Two 250 mgs of NR (or placebo) pills twice daily and one 125 mg resveratrol (or placebo) once daily. Participants were asked to record pill consumption on a paper log and return dispensed bottles at 3-month and 6-month follow-up\. Adherence was assessed using the medication log and pill counts.
Nicotinamide riboside
NR (250 mg pills) and matching placebo were manufactured by ChromaDex.
Resveratrol
Resveratrol capsules (125 mgs of 98% pure trans-resveratrol) and matching placebo were manufactured by ReserveAge.
Ankle brachial index measurement
A hand-held Doppler probe (Pocket Dop II; Nicolet Biomedical Inc, Golden, CO) was used to obtain systolic pressures twice in the right and left brachial, dorsalis pedis, and posterior tibial arteries using established methods25,26.
Medical history
Medical history was obtained using questionnaires administered by a trained and certified health interviewer28. Participant’s sex was designated based on the health interviewer’s assessment. Race was obtained from participants using an open-ended question with fixed categories.
Leg symptoms
Leg symptoms were characterized using the San Diego claudication questionnaire29. Classical intermittent claudication (IC) was defined as exertional calf pain that did not begin at rest, caused the participant to rest, and resolved within ten min of rest29,30. Most people with PAD do not have classic IC symptoms30. People with PAD who report no exertional leg symptoms (i.e. asymptomatic) and those with exertional leg symptoms other than classic IC have significantly greater functional impairment and faster functional decline than people without PAD1,30. To enhance generalizability of results, participants without classic IC symptoms were included in the trial30.
Primary outcome
The primary outcome was 6-month change in 6-min walk distance. There were two primary comparisons: Change in 6-min walk at 6-month follow-up between participants randomized to NR vs. placebo and change in 6-min walk at 6-month follow-up between participants randomized to NR + resveratrol vs. placebo.
Secondary outcomes
Secondary outcomes were 3-month change in 6-min walk and 6-month change in maximal treadmill walking time, the Walking Impairment Questionnaire (WIQ) distance score, and daily physical activity measured by ActiGraph. Comparisons of 6-month changes in gastrocnemius muscle biopsy measures of NAD+ abundance, muscle phenotype, and satellite cell abundance between participants randomized to either NR or NR + resveratrol (combined) and placebo were secondary outcomes.
Exploratory outcomes
Exploratory outcomes were 6-month change in the WIQ speed score, the WIQ stair climbing score, and the Short-Form-36 Physical Functioning score. Comparisons of 6-month change in gastrocnemius muscle biopsy measures of NAD+ abundance, muscle phenotype, and satellite cell abundance between NR and placebo and between NR + resveratrol and placebo were exploratory outcomes.
6-min walk test
Following a standardized protocol28,31, participants walked up and down a 100-foot hallway for six min after instructions, delivered by script, to cover as much distance as possible. Distance completed after six min was recorded. In PAD, a small minimum clinically important difference (MCID) was defined as ~8 meters and a large MCID as 20 meters18.
Treadmill walking performance
Maximal treadmill walking time was measured using the Gardner-Skinner protocol at baseline and 6-month follow-up32. Treadmill testing was not available during the COVID-19 pandemic even after in-person visits became possible for other outcomes. Large MCID values for maximal treadmill walking time range from 2.5 to 4.0 min19.
Walking Impairment Questionnaire Scores
The WIQ is a PAD-specific measure of self-reported limitations in walking distance, speed, and stair climbing, scored on a 0–100 scale (100 = best)33. An MCID for the WIQ was defined as approximately five points19.
Health-related quality of life
The Short-Form 36 Physical Functioning (SF-36 PF) score measured health-related quality of life (range 0–100, 100 = best). The MCID is 5–7 points34,35.
Physical activity
Free-living physical activity, measured as total counts per day, was acquired over seven days with the ActiGraph accelerometer36.
Gastrocnemius skeletal muscle biopsy
An open muscle biopsy was performed in the medial head of the gastrocnemius muscle after subcutaneous lidocaine administration at baseline and 6-month follow-up5,6,7,28. Approximately 250 mgs of muscle tissue was removed. Samples were snap frozen in liquid nitrogen and stored at −80 degrees Celsius until testing.
Gastrocnemius muscle measures
For measurement of NAD+ abundance, nucleotides were measured by High Performance Liquid Chromatography (HPLC) with a Shimadzu LC-20A pump and UV-VIS detector using a Supelco LC-18-Tcolumn, normalized to muscle weight (15 cm × 4.6 cm)37. Satellite cell abundance and fiber type were determined on frozen muscle sections using antibodies against: Pax7 (Pax7 concentrate, DSHB; 1:100), type 1 myosin (BA.D5 concentrate, DSHB; 1:100), and laminin (Millipore-Sigma, L9393; 1:100) as previously described5,6 (Supplementary Fig. 1).
Other measures
Height and weight were measured at baseline. Body mass index (BMI) was calculated as weight (kg)/[height (meters)]2.
Adverse events
Information about adverse and serious adverse events was obtained monthly with questionnaires.
Sample size calculations
Anticipating a 90% follow-up rate, 30 participants randomized per group provided 80% power to detect a difference of 0.58 standard deviation (SD) of change in 6-min walk distance at a one-sided significance level of 0.10 based on a two-sample t-test38. Ninety participants provided more than 80% power to detect a 30-meter difference between NR + resveratrol and placebo and between NR alone and placebo at follow-up38. There was similar power to detect a 30-meter difference between the NR+ resveratrol group and NR alone groups. The study had 80% power to detect a between group difference of 0.58 standard deviations for other outcomes. The study had power to detect a 1.74 min difference in maximal treadmill walking time and 14.1 points for the WIQ distance score38.
Statistical analyses
The two primary outcomes were the differences in 6-month change in 6-min walk distance between the NR and placebo groups and between the NR + resveratrol and placebo groups. Secondary analyses included the difference in 3-month change in 6-min walk distance between the NR and placebo groups, between the NR + resveratrol and placebo groups, between the NR + resveratrol and NR groups, and between the two NR groups combined and placebo. Secondary analyses included the difference in 6-month change in 6-min walk between the NR + resveratrol and NR groups and between the two NR groups combined and placebo. Secondary analyses included differences in 6-month change in maximal treadmill walking time, the WIQ distance score, and physical activity between the NR and placebo groups, between the NR + resveratrol and placebo groups, between the NR + resveratrol and NR groups, and between the combined NR + resveratrol and NR groups and placebo. Secondary analyses included differences in 6-month change in gastrocnemius biopsy measures between the two NR groups combined and placebo. Exploratory analyses compared change in exploratory outcomes at 6-month follow-up between the groups defined for secondary analyses and 6-month changes in the gastrocnemius muscle measures between the NR + resveratrol group and placebo and between the NR and placebo groups. In post-hoc analyses, differences in 6-month changes in 6-min walk distance were compared between the NR and placebo groups and between the NR + resveratrol and placebo groups among all participants with at least 75% adherence to pills.
Pre-specified analyses were performed according to each participant’s assigned group, irrespective of pill adherence. Baseline characteristics were summarized as means and standard deviations for continuous variables and frequencies and percentages for categorical variables. For the primary outcomes, mixed models for repeated measures (MMRM) were used to compare 6-month change in 6-min walk distance between the NR + resveratrol and placebo groups and between the NR alone and placebo groups using baseline, 3-month, and 6-month values for 6-min walk distance, adjusting for age, sex, race, and baseline 6-min walk. The a priori statistical analysis plan defined a one-sided P value of <0.10 to define statistical significance. One-sided 90% confidence intervals (CI) were calculated for between group differences. Because the clinical trial was a Phase II trial, to help ensure that a true beneficial effect of the interventions was not dismissed due to lack of statistical significance, the pre-specified P value for statistical significance was more liberal than a P value of <0.05. MMRM analysis included participants missing either 3-month or 6-month follow-up visits under the missing at random assumption and results were used for baseline to 3-month follow-up and baseline to 6-month follow-up. Analyses were repeated among people with at least 75% adherent. 6-month change in 6-min walk were analyzed for each group according to whether participants had 75% or greater adherence or <75% adherence, using MMRM. For outcomes other than 6-min walk, one-sided ANCOVA tests compared changes in each outcome, adjusting for age, sex, race, and the baseline value for each outcome. Analyses that compared the combined NR groups to placebo also adjusted for resveratrol randomization. There were no adjustments for multiple comparisons. Analyses were performed using SAS version 9.4.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The de-identified participant baseline characteristics, treatment assignments and outcome data generated in this study have been deposited in the American Heart Association GitHub database. Data can be accessed at https://doi.org/10.5281/zenodo.11099249. Data may be used for education and research purposes. The study protocol is available in the supplementary information file as Supplementary Note 1. Source data are provided with this paper.
References
Polonsky, T. S. & McDermott, M. M. Lower extremity peripheral artery disease without chronic limb-threatening ischemia: a review. JAMA 325, 2188–2198 (2021).
Weiss, D. J. et al. Oxidative damage and myofiber degeneration in the gastrocnemius of patients with peripheral arterial disease. J. Transl. Med. 11, 230–239 (2013).
Gillani, S., Cao, J., Suzuki, T. & Hak, D. J. The effect of ischemia reperfusion injury on skeletal muscle. Injury 12, 670–675 (2012).
McDermott, M. M. et al. Skeletal muscle pathology in peripheral artery disease: a brief review. Arterioscler. Thromb. Vasc. Biol. 40, 2577–2585 (2020).
Kosmac, K. et al. Correlations of calf muscle macrophage content with muscle properties and walking performance in peripheral artery disease. J. Am. Heart Assoc. 9, e015929 (2020).
White, S. H. et al. Walking performance is positively correlated to calf muscle fiber size in peripheral artery disease subjects, but fibers show aberrant mitophagy: an observational study. J. Transl. Med. 14, 284 (2016).
Ferrucci, L. et al. Transcriptomic and proteomic of gastrocnemius muscle in peripheral artery disease. Cir. Res. 132, 1428–1443 (2023).
Zapata-Perez, R., Wanders, R. J. A. & van Karnebeek, C. D. M. NAD+ homeostasis in human health and disease. EMBO Mol. Med. 13, e13943 (2021).
Freeberg, K. et al. Dietary supplementation with NAD+-boosting compounds in humans: current knowledge and future directions. J. Gerontol: Med. Sci. 78, 2435–2448 (2023).
Chen, Z. et al. Shear stress, SIRT1, and vascular homeostasis. Proc. Natl Acad. Sci. USA 107, 10268–10273 (2010).
Elhassan, Y. S. et al. Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 28, 1717–1728 (2019).
de Picciotto, N. E. et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15, 522–530 (2016).
Zhang, H. et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352, 1436–1443 (2016).
Khan, N. A. et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol. Med. 6, 721–731 (2014).
Martens, C. R. et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 9, 1286 (2018).
Trammell, S. A. J. et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 7, 12948 (2016).
Howitz, K. et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 (2003).
McDermott, M. M. et al. Meaningful change in 6-min walk in people with peripheral artery disease. J. Vasc. Surg. 73, 267–276 (2021).
Gardner, A. W., Montgomery, P. S. & Wang, M. Minimal clinically important differences in treadmill, 6-min walk, and patient-based outcomes following supervised and home-based exercise in peripheral artery disease. Vasc. Med. 23, 349–357 (2018).
Dollerup, O. L. et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men. Am. J. Clin. Nutr. 108, 343–353 (2018).
Dollerup, O. L. et al. Effects of nicotinamide riboside on endocrine pancreatic function and incretin hormones in nondiabetic men with obesity. J. Clin. Endocrinol. Metab. 104, 5703–5714 (2019).
Dollerup, O. L. et al. Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men. J. Physiol. 598, 731–754 (2020).
Conze, D., Brennar, C. & Kruger, C. Safety and metabolism of long-term administration of NIAGEN in a randomized, double-blinded, placebo-controlled clinical trial of healthy overweight adults. Sci. Rep. 9, 9772 (2019).
Wang, D. D. et al. Safety and tolerability of nicotinamide riboside in heart failure with reduced ejection fracture. JACC Clin. Transl. Sci. 7, 1183–1196 (2022).
McDermott, M. M. et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J. Vasc. Surg. 32, 1164–1171 (2000).
Aboyans, V. et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 126, 2890–2909 (2012).
Heun, R., Papassotiropoulos, A. & Jennssen, F. The validity of psychometric instruments for detection of dementia in the elderly general population. Int. J. Geriatr. Psychiatry 13, 368–380 (1998).
McDermott, M. M. et al. Effect of low-intensity vs high-intensity home-based walking exercise on walk distance in patients with peripheral artery disease: the LITE randomized clinical trial. JAMA 325, 1266–1276 (2021).
Criqui, M. H. et al. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc. Med. 1, 65–71 (1996).
McDermott, M. M. Lower extremity manifestations of peripheral artery disease: the pathophysiologic and functional implications of leg ischemia. Circ. Res. 116, 1540–1550 (2015).
McDermott, M. M. et al. 6-min walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 130, 61–68 (2014).
Gardner, A. W. et al. Progressive vs. single-stage treadmill tests for evaluation of claudication. Med. Sci. Sports Exerc. 23, 402–408 (1991).
Regensteiner, J. G. et al. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J. Vasc. Med. Biol. 2, 142–152 (1990).
Ware, J. E., Snow, K. K., Kosinski, M. A. & Gandek, B. G. SF-36 Health Survey: Manual And Interpretation Guide (The Health Institute, New England Medical Center, 1993).
Ward, M. M., Guthrie, L. C. & Alba, M. I. Clinically important changes in Short Form 36 Health Survey Scales for use in rheumatoid arthritis clinical trials: the impact of low responsiveness. Arthritis Care Res. 66, 1783–1789 (2014).
Kelly, L. A. et al. Validity of actigraphs uniaxial and triaxial accelerometers for assessment of physical activity in adults in laboratory conditions. BMC Med. Phys. 13, 5 (2013).
Peek, C. B. et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342, 6158 (2013).
McDermott, M. M. et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA 310, 57–65 (2013).
Acknowledgements
Funded by the American Heart Association, Grant Numbers 18SFRN33900097, 18SFRN33900136, and 18SFRN33970010. Nicotinamide riboside was provided by ChromaDex. Resveratrol was provided by ReserveAge
Author information
Authors and Affiliations
Contributions
M.M.M., C.R.M., K.J.D., M.H.C., L.F., P.G., J.M.G., M.R.K., K.K., D.L.J., C.A.P., R.S., L.T., L.Z., S.W. and C.L. designed the study. M.M.M., K.J.D., L.T. and L.Z. supervised data collection or statistical analyses. C.P., K.J.H., K.K., D.L.J., C.A.P., R.S. and P.Z. analyzed samples or collected samples, or interpreted data. M.M.M., C.R.M., D.Z., C.P., M.H.C., L.F., P.G., J.M.G., K.J.H., M.R.K., K.K., C.A.P., R.S., L.T., L.Z., P.Z. and L.C. interpreted results of data analyses. M.M.M. drafted the manuscript. M.M.M., C.R.M., K.J.D., D.Z., C.P., M.H.C., L.F., P.G., J.M.G., K.J.H., M.R.K., K.K., D.L.J., C.A.P., R.S., L.T., S.W., L.Z., P.Z. and C.L. provided critical feedback for the revising the manuscript. M.M.M., C.R.M., K.J.D., D.Z., C.P., M.H.C., L.F., P.G., J.M.G., K.J.H., M.R.K., K.K., D.L.J., C.A.P., R.S., L.T., S.W., L.Z., P.Z. and C.L. approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. McDermott reports research funding from Helixmith and other research support from ArtAssist, and Mars. The remaining authors report no competing interests.
Peer review
Peer review information
Nature Communications thanks Jacqueline Birks, and other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McDermott, M.M., Martens, C.R., Domanchuk, K.J. et al. Nicotinamide riboside for peripheral artery disease: the NICE randomized clinical trial. Nat Commun 15, 5046 (2024). https://doi.org/10.1038/s41467-024-49092-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-49092-5
- Springer Nature Limited