Abstract
Triple oxygen isotopes of Cenozoic intrusive rocks emplaced along the Ross Sea coastline in Antarctica, reveal that meteoric-hydrothermal waters imprinted their stable isotope composition on mineral phases, leaving a clear record of oxygen and hydrogen isotope variations during the establishment of the polar cap. Calculated O- and H-isotope compositions of meteoric waters vary from −9 ± 2‰ and −92 ± 5‰ at 40 ± 0.6 Ma, to −30 and −234 ± 5‰ at 34 ± 1.9 Ma, and intersect the modern Global Meteoric Water Line. These isotopic variations likely depict the combined variations in temperature, humidity, and moisture source regions, resulting from rearrangement of oceanic currents and atmospheric cooling during the onset of continental ice cap. Here, we report a paleo-climatic proxy based on triple oxygen geochemistry of crystalline rocks that reveals changes in the hydrological cycle. We discuss the magnitude of temperature changes at high latitudes during the Eocene-Oligocene climatic transition.
Similar content being viewed by others
Introduction
Variations in the continental hydrological cycle during Earth’s evolution are crucial to our understanding of atmospheric conditions in the geological past and defining boundary conditions for future temperature projections. Changes in the water cycle, such as changes to atmospheric and oceanic circulation, variations in atmospheric humidity, and changes in moisture transport, all contributed to reaching tipping points in the paleo-climate record.
During the Cenozoic, a prolonged trend of global temperature decrease brought the climatic system from Palaeocene/Eocene hyperthermals to Eocene/Oligocene glacial conditions1. This finding is based on many proxies provided by materials that equilibrated with ambient (meteoric/oceanic) waters, such as marine sediments, biological materials, and continental carbonates2,3,4. The sedimentary records from terrestrial margins of Antarctica have provided evidence for the expansion and retreat of land-terminating glaciers, which delivered eroded material to the sea, and indicate that stable, continental-scale ice sheets were established after ~32.8 Ma5. Whether the cooling that led to glacial conditions was forced by pCO2 (partial pressure of CO2 in the atmosphere) drawdown alone, or by geodynamic, tectonic, and atmospheric mechanisms acting in concert, is a matter of debate6,7,8. A strong coupling between sea surface temperature (SST), mean air temperatures (MAT), and atmospheric pCO2 has been established for the Eocene-Oligocene epochs9,10,11,12. Numerical simulations and geochemical records indicate that the drawdown of atmospheric CO2 concentration5,12 during this period led to the onset of ice caps, initially in continental interiors. Ice tongues subsequently expanded towards the continental margins, affecting continental and oceanic water balance (groundwater availability and evaporation), and ocean surface temperatures13,14. Climate models indicate that the effects of declining atmospheric pCO2 concentration grew progressively3,10,15,16,17, and co-vary with the records of cooling circum-Antarctic SSTs in the late Eocene3,18.
Reduction of atmospheric pCO2 influenced both atmospheric temperatures and precipitation, with the result of decreasing relative humidity and moisture availability19. Therefore, the stable isotope composition of Eocene meteoric waters should have varied in response to differences in the global hydrological cycle. Due to the meteoric origin of hydrothermal waters, the stable isotope compositions of rocks that interacted with hydrothermal waters can be used as a proxy for the meteoric water values responsible for the water/rock interaction20,21,22,23,24,25.
In this work, we used the triple oxygen isotope (17O/16O, 18O/16O) and hydrogen isotope geochemistry (D/H) of Cenozoic rocks belonging to local hydrothermal systems along the Ross Sea coastline in Antarctica, to estimate the initial oxygen and hydrogen isotope compositions of meteoric waters responsible for the hydrothermal alteration. We analysed alkaline intrusive rocks (diorite to qz-syenite and their country rocks) that pre-date and post-date the onset of the Antarctic glaciation, to constrain the isotopic composition of palaeo-meteoric waters and the prevailing atmospheric conditions at the Eocene-Oligocene (E-O) boundary.
Results
Interaction of meteoric waters with intrusive rocks
Alkaline intrusive rocks were emplaced along the Ross Sea Embayment in Antarctica from 51.6 ± 0.6 to 29 ± 1.7 Ma (Ar-ages)25,26. Plutonic bodies consist of amphibole (+/- biotite)-bearing syenites and monzonites, with subordinate monzogabbro/monzodioritic facies. During cooling, meteoric-hydrothermal waters circulated through the plutons and their country rocks producing low-to-intermediate temperature (250–350 °C) alteration. The lowest oxygen and hydrogen isotope values occur near the borders of the plutons due to higher water/rock (W/R) ratios where the magmatic bodies emplaced along crustal fractures25. Hydrothermal fluids are dominated by meteoric waters: their hydrogen isotopic composition is nearly identical to that of the local meteoric water, while their oxygen isotope composition is usually shifted to more positive δ-values due to water/rock interaction27. One can therefore use the stable isotope composition of hydrothermally altered rocks to estimate the meteoric water isotopic composition at the time of hydrothermal water/rock interaction.
The interaction between water and rock during hydrothermal alteration can be modelled in terms of simple mass balance mixing processes28, and triple oxygen geochemistry has expanded the possibility to reconstruct the composition of the interacting water from hydrothermally altered rocks beyond the limitation of an unknown degree of W/R equilibration21 (see Supplementary material). The variables controlling the isotopic compositions inherited from hydrothermally altered rocks are (a) hydrothermal fluid compositions; (b) initial mineral or rock composition; (c) temperature of interaction; and (d) fluid/rock ratios. When (b), (c), and (d) are constrained, the isotopic composition of hydrothermal fluid composition can be estimated by fitting mineral isotopic data with mixing trajectories calculated for variable degrees of water/rock interaction21 (see Fig. 1).
Each panel (boxes a–e) represents a time interval and the related outcrop(s), namely: (a) Oakley Glacier syenite, 40 ± 0.6 Ma; (b) Mt. McGee granite, 38 ± 0.8 Ma; (c) Styx Glacier and Cape Crossfire monzonites, 35 ± 2 Ma; (d) Cape King qz-diorite, 34 ± 1.9 Ma; (e) No Ridge monzo-sienite, 31.8 ± 0.5 Ma. Mixing curves represent hydrothermal alteration of Antarctic igneous rocks for 200 °C, 300 °C, and 400 °C. The dots on the mixing curves define the percentages of W/R (water-rock) interaction. The stars indicate rock values for infinite W/R ratios. Starting δ18Oqz values vary in the range of mantle and crustal rocks22,23,31: δ18Oqzsyenite = 12 ‰, δ18Oqzgranite = 15‰, δ18Oqzmonzonite = 9‰, δ18Oqzqz-diorite = 9‰. The initial Δ¹7O values are in the range of granitoid rocks28. The grey, dotted lines are also W/R mixing curves (same as the black lines), but they are the W/R curves that fit the varied model parameters reported in the model data (SI_Model Data). Their end also defines the infinite water value. The best-fit W/R mixing line defines the δ18Ow of Eocene waters. The grey and the pale grey areas represent the composition of modern ice and snow, and surface and subsurface waters, respectively, according to 73. Error bars report the maximum error derived from the lab internal standard, that is 0.3 ‰ for δ18O and 8 ppm for Δ¹7O.
The isotopic compositions of our samples are expressed in the conventional δ notation, where the δ17O and δ18O values are defined as follows:
A linearized form of the δ-notations is given by the \({{{{{\rm{\delta }}}}}}\)’-notation29, which is defined as follows:
The Δ′17O value is defined as30:
The Δ’17O value of meteoric-hydrothermal water interacting with rocks covers a distinct range (Fig. 1) ranging from 0 to 0.06‰, averaging 0.03‰30. The isotopic equilibrium between quartz and water at hydrothermal temperatures (200, 300, and 400 °C, respectively) is calculated using the triple oxygen equilibrium fractionation factor between silica and water29. Unaltered igneous rocks have a narrow range of Δ’17O values because the high-T θ value (where θ = ln(α17O) / ln(α18O) for minerals and melts) is close to the reference slope value of 0.52822. In contrast, hydrothermally altered rocks show significantly different values, due to the negative δ18O value of the meteoric water responsible for the alteration and the extent of the water/rock ratio21,22,23,24,31,32,33. We chose initial Δ’17Oqz values from − 0.052 ‰ to − 0.090, in the range of values for igneous rocks of continental and mantle source22,23,31, in order to have the best fit through the measured data starting from the initial δ18Oqz values of the unaltered facies of the lithotypes.
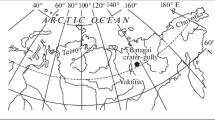
The targeted hydrothermal centres for triple oxygen isotope analysis in this study were the following: Oakley Glacier monzo-syenite (40 Ma); Mt McGee granite country rock and monzonite (38 Ma); Styx Glacier syenite (35 Ma); Cape King gabbro-diorite (34 Ma); Cape Crossfire monzo-diorite (34 Ma); No Ridge monzo-syenite (32 Ma)25. Additional details about samples are reported in Supplementary Information (SI_Samples).
Our dataset of δ17O and δ18O values (SI_Table 2) shows that the quartz of these intrusive rocks was altered from its original igneous value due to water/rock interaction. To estimate the oxygen isotope composition of primary meteoric waters, we interpolated the measured data of quartz from the hydrothermally altered rocks with mixing trajectories in the Δ’17O - δ18O space, for water-rock temperatures of 200, 300 and 400 °C. These temperatures encompass the range of temperature of hydrothermal water-rock interaction, interpreted to be ~300 ± 50 °C34. For each assumed δ18Owater value, we calculated trajectories that fit through the measured δ18O values of quartz. These trajectories intersect the triple oxygen isotope composition of rocks fully equilibrated with the assumed primary meteoric water. In the Δ’17O-δ’18O space, the data are fit by distinct curves (Fig. 1a–e), and the estimated δ18O values for Eocene waters are in the range of: − 9.0 ± 2‰ at 40 ± 0.6 Ma (Oakley Gl.); − 14.5 ± 2‰ at 38 ± 0.8 Ma (Mt McGee); − 22.0 ± 3‰ at 35 ± 1.75 Ma (Styx Gl.); − 28.0 ± 3‰ at 35 ± 2.5 Ma (Cape Crossfire); − 30.0 ± 2‰ at 34 ± 1.9 Ma (Cape King); and − 19.0 ± 1‰ at 31.8 ± 0.5 Ma (No Ridge).
When plotted as calculated δ18Owater values vs. time (Fig. 2), we see a large inflection point across the E-O boundary. Our data interpolation curve parallels the high-resolution curve of benthic forams4 and shows the minimum δ18Owater values at 34 Ma (provided the Arage error limit). A similar although broad co-variation trend is observed for the compiled lowest δD values of meteoric waters (calculated from hydrothermal phases) of Tertiary intrusives25. This likely suggests that the isotopic signal depicted from meteoric hydrothermal waters closely reflects the variations of atmospheric conditions at the time of hydrothermal alteration. It is interesting to note that our data, similar to what is seen from the benthic foraminifera δ18O curve, define a δ18Owater increase back to higher values at ~32 Ma. This period is reported to have experienced a rise in pCO2 bringing the atmospheric temperatures back to warmer conditions35.
A clear variation at the Eocene-Oligocene boundary along a prolonged decreasing trend can be envisaged. The grey line is the long terms smoothed δ18O benthic forams vs. Age4 (left δ18O scale). The dark line is the interpolated line of the data reported in this study (right scale). Error bars of Ar-ages are from 25.
Accurate evaluation of the compiled variations in MAT (mean atmospheric temperature) from south high latitude terrestrial proxies (south of 45°, most around 70°) defines atmospheric cooling to be in the range of 4 °C from 38 to 34 Ma, although offsets among temperature proxies up to 8 °C have been proposed36. Similarly, MAT proxy records from (mainly) northern mid-latitudes provide estimates of cooling from 0 °C to 8 °C37. As for global sea surface temperatures, there is increasing evidence of heterogenous temperature variations during late Eocene- early Oligocene based on oceanic proxy records, with differences in the range of 10 ± 2 °C within the 38-34 Ma interval4,38.
The stable isotope composition of meteoric waters along East Antarctica coastline constrains the hydrochemical cycle over the continental margin; thus, we can compare whether the atmospheric conditions inferred from coastal crystalline rocks are consistent with those from marine proxies. Using modern local39,40 and global41 δ18O/T(°C) lapse rates, the − 15.5‰ difference in calculated δ18Owaters of hydrothermal systems at the 38 and 34 Ma, corresponds to a surface temperature decrease of ~ 19 ± 5 °C (based on modern δ18O/T(°C) rate in Antarctica) and ~12 ± 5.4 °C (based on modern δ18O/T(°C) rate on the global scale), respectively. The values based on Antarctic δ18O/T(°C) rates39,40 exceed reconstructions based on oceanic proxies and likely indicate that modern high-latitude δ18O/T(°C) slopes do not hold for absolute paleotemperatures. This may be due to to a combined effect of atmospheric temperature drop and changes in moisture circulation42,43.
Discussion
The δD values for biotes and amphiboles of intrusive rocks from Cape King, No Ridge, Styx Glacier, and Cape Crossfire intrusions were measured and compiled along with literature data of rock samples from the same outcrops (SI_Table 4). We observe distinct compositional ranges among different hydrothermal systems, and variable H-isotope data within the same outcrop, the latter suggesting different degrees of water-rock interaction and isotopic equilibration. For δDwater recalculation we discarded a few granite country rocks in the SE region of Mt McGee that are cut by a late mafic dikes (δD = − 185 ± 5 ‰, age 34.7 ± 0.7 Ma26. Their δD values (≤ − 170‰) are the lowest among those from the Mt McGee area, and suggest they may be related to a much later water-rock interaction event. Similarly, we discarded one sample (DF8, δD = 216‰) from Oakley Gl., which displays evidence of oxidation25.
To convert the δD values of measured kaersutitic amphiboles and biotites into δD values of meteoric-hydrothermal waters assuming an equilibration temperature of 300 °C, we adopted 1000ln α biotite-water and 1000ln α amphibole-water values of + 20‰44,45. Due to the uncertainty in the hydrogen fractionation factor for Fe-rich amphibole, the calculated δD values of meteoric hydrothermal waters may slightly change, but their relative H-isotope differences between the hydrous silicates and the hydrothermal water remain constant, allowing us to relative hydrogen isotope variations of meteoric waters through the Eocene. It has been recently questioned whether δD values of hydrothermal waters may be accurately derived from the δD values of hydrosilicates, because there may be continuous H-exchange between fluid and rock during cooling from peak hydrothermal temperatures24,46. Taking this point into consideration, we obtained O-isotope and H-isotope data independently; thus, we measured quartz for triple oxygen isotope ratios and we were able to calculate O-isotope composition of hydrothermal waters (SI_Analytical Methods). Then, we used the δD values of the amphibole/biotite to derive the corresponding hydrogen isotope composition of the water. We used the calculated δDwater and δ18O water values to estimate the stable isotope compositions of Cenozoic meteoric waters. Our data show that the oldest samples have a larger range of δDwater values (− 92 > δD > −196‰ at 40 ± 0.6 Ma; − 69 > δD > −171‰ at 38 ± 0.8 Ma) than younger samples (− 154 > δD > − 194‰, at 35 ± 2.5 Ma; − 150 > δD > −234‰, at 34 ± 1.9 Ma; − 115 > δD > −165‰, at 31.8 ± 0.5 Ma), and significantly higher δ18O values (− 9 ± 2 and − 14.5 ± 2‰ at 40 and 38 Ma, respectively) than younger samples (− 22 ± 3; − 30 ± 2; − 19 ± 1‰, at 35, 34, and 32 Ma, respectively). Similar δ18O and δD values have been reported for average precipitations (δDwater values between − 113 and − 151‰) of the Antarctic Peninsula at 35.9 ± 1.1 Ma47.
Coupled δD - δ18O values define different compositional ranges for the samples at 40-38 Ma, and 34-32 Ma, representing how meteoric waters varied through the Eocene-Oligocene transition (Fig. 3). They intersect the modern GMWL, although significantly higher δ18O values are shown by Cenozoic meteoric waters compared to the modern (i.e., modern snow samples at Styx Gl. are up to 10‰ lighter40). This implies that the modern GMWL possibly holds for the geological past, at least since the middle Cenozoic, which was a warm period thought to be particularly humid48,49. Accordingly, relative humidity, air masses temperature, and temperature of the oceanic source area of the precipitation controlled the deuterium-excess parameter (d = δD – 8*δ18O)50 for this period. Modern snow precipitations in East Antarctica have a d-excess variability from − 20 to + 29‰51; thus a variability up to a ± 30‰ range in d-excess may be expected also for the geological past, as a consequence of variable moisture source and water vapour transport during changing atmospheric conditions. In fact, the d-excess in marine water vapour inversely correlates with moisture source relative humidity, due to the kinetic fractionation during evaporation from the ocean into the atmosphere52, and positively correlates with sea-surface temperature, due to the isotopic fractionation between seawater and water vapour50,53,54. During the late Eocene, moisture decrease has been inferred for the Antarctic Peninsula55 and Greenland56 based on paleo-botanical data and terrestrial biomarkers preserved in marine sediments. This is in agreement with the variations in weathering regime along the continental coastline of Antarctica, from mainly chemical, typical of warm and humid climate, to physical, characteristic of arid and cold temperature conditions, recorded in the Ross Sea and the Weddel Sea areas57,58. Our data do not depict any large d-excess variations, because short-term differences would be averaged at the My-time scale resolution of our research. However, it is reasonable to hypothesize that variations of d-excess in the order of ± 30‰ (or more) may have occurred in a geological time interval likely characterised by large differences in atmospheric moisture. This would also help to explain the low δD values slightly above or below the modern GMWL (Fig. 3).
Meteoric waters that fed hydrothermal systems in Northern Victoria Land (NVL), Antarctica at the Eocene-Oligocene transition intercept the Modern Global Meteoric Water Line (GMWL). The range of Modern Antarctic precipitation is from40. Error bars on the X-axis represent the range of δ18Ow values that fit the varied model parameters (SI_Model Data) and error bars on the Y-axis are the standard error of the mean for the measured δD values.
Theoretical estimates of moisture poleward transport suggest that the δ18O and δD values of meteoric precipitations are more influenced by atmospheric water sources rather than local conditions59, so Antarctic coastal data should reflect the combined effects of atmospheric conditions and oceanic circulations. The difference in δ18Owater values based on our data with those based on δ18O values of benthic foraminifera may indicate that Eocene/Oligocene Antarctic moisture sources were situated at lower latitudes, allowing for a larger distillation along the moisture pathways, and eventually changing the transport characteristics (i.e., eddy fluxes vs. advection)59. The onset of a “proto” Antarctic Circumpolar Current (ACC) also caused significant changes in the circulation patterns at high latitudes, implying that moisture origin, transport and supply to northern Victoria Land coastal areas might have been strongly modified60,61,62,63,64,65,66,67. Due to the ice cap extension beyond the polar circle and variations of oceanic currents, the vapour source of Antarctic precipitations may have been “pushed” towards low latitudes.
Transport modelling of modern precipitation predicts that moisture source latitude is a function of altitude and distance from the coastline. East Antarctica moisture sources are presently at latitudes between 46° and 50°_S. Interestingly, Victoria Land presently has a moisture sources at even lower latitudes (i.e., 42°_S)68. Because the water-vapour fractionation factor increases with decreasing temperatures, the residual water vapour resulting from the Rayleigh condensation processes becomes increasingly depleted in oxygen and hydrogen isotope composition with distance from the source. Moreover, the opening of the Southern Ocean gateways (i.e., the Drake Passage and the Tasmanian Gateway) produced a cooling of the Southern Ocean and a reduction of poleward heat transport60,61. Thus, a progressively increasing temperature gradient was established from the equator to the poles69, likely resulting in less evaporation at higher latitudes. It is therefore possible that Antarctic meteoric waters before the E-O transition had been more influenced by precipitation generated in proximity of the coastline.
If so, the rearrangement of oceanic currents and the shift of atmospheric temperatures along Antarctic margins induced irreversible changes in the hydrological regime of the coastal areas during the Eocene-Oligocene climatic transition. Temperature-induced isotopic variation possibly occurred in conjunction with a reduction in moisture availability and/or a variation of moisture source areas, which resulted in low δ18O values of meteoric waters after the E/O transition. This would also explain why the δ18O values that we reconstruct are even lower than what we would expect from the paleo-temperature reconstructions.
Methods
Mineral separates of quartz were obtained by crushing and sieving the rock sample, followed by standard magnetic separation and hand-picking under the binocular microscope. Oxygen isotope compositions were measured using laser fluorination70, and hydrogen isotope compositions were determined using high-T reduction71. All isotope data are reported relative to the VSMOW-SLAP2 scale using internal standards that were directly calibrated to VSMOW2 and SLAP272.
References
Zachos, J., Pagani, M., Sloan, L., Thomas, E. & Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 (2001).
Beerling, D. J. & Royer, D. L. Convergent cenozoic CO2 history. Nat. Geosci. 4, 416–420 (2011).
Anagnostou, E. et al. Changing atmospheric CO2 concentration was the primary driver of early Cenozoic climate. Nature 533, 380–384 (2016).
Westerhold, T. et al. An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science 369, 1383–1387 (2020).
Galeotti, S. et al. Antarctic Ice Sheet variability across the Eocene-Oligocene boundary climate transition. Science 352, 76–80 (2016).
Barker, P. F. & Thomas, E. Origin, signature and palaeoclimate influence of the Antarctic Circumpolar Current. Earth Sci. Rev. 66, 143–162 (2004).
Zhang, Z.-S., Yan, Q. & Wang, H.-J. Has the Drake passage played an essential role in the Cenozoic cooling? Atmos. Ocean. Lett. 3, 288–292 (2010).
Bijl, P. K. et al. Eocene cooling linked to early flow across the Tasmanian Gateway. Proc. Natl. Acad. Sci. USA 110, 9645–9650 (2013).
Pearson, P. N. & Palmer, M. R. Atmospheric carbon dioxide over the past 60 million years. Nature 406, 695–699 (2000).
DeConto, R. M. & Pollard, D. Rapid Cenozoic glaciation of Antarctica induced by declining atmospheric CO2. Nature 421, 245–249 (2003).
Zachos, J. C., Dickens, G. R. & Zeebe, R. E. An early Cenozoic perspective on greenhouse warming and carbon cycle dynamics. Nature 451, 279–283 (2008).
DeConto, R. M. et al. Thresholds for Cenozoic bipolar glaciation. Nature 455, 652–657 (2008).
Carter, A., Riley, T. R., Hillenbrand, C.-D. & Rittner, M. Widespread Antarctic glaciation during the Late Eocene. Earth Planet. Sci. Lett. 458, 49–57 (2017).
Tripati, A. & Darby, D. Evidence for ephemeral middle Eocene to early Oligocene Greenland glacial ice and pan-Arctic sea ice. Nat. Commun. 9, 1038 (2018).
Pearson, P. N., Foster, G. L. & Wade, B. S. Atmospheric carbon dioxide through the Eocene–Oligocene climate transition. Nature 461, 1110–1113 (2009).
Pagani, M. et al. The role of carbon dioxide during the onset of Antarctic glaciation. Science 334, 1261–1264 (2011).
Zhang, Y. G., Pagani, M., Liu, Z., Bohaty, S. M. & DeConto, R. M. A 40-million-year history of atmospheric CO2. Phil. Trans. R. Soc. A Math. Phys. Eng. Sci. 371, 20130096 (2013).
Duncan, B. et al. Climatic and tectonic drivers of late Oligocene Antarctic ice volume. Nat. Geosci. 15, 819–825 (2022).
Trenberth, K. E. Changes in precipitation with climate change. Clim. Res. 47, 123–138 (2011).
Sharp, Z. D., Masson, H. & Lucchini, R. Stable isotope geochemistry and formation mechanisms of quartz veins; extreme paleoaltitudes of the Central Alps in the Neogene. Am. J. Sci. 305, 187–219 (2005).
Herwartz, D. et al. Revealing the climate of snowball Earth from Δ17O systematics of hydrothermal rocks. Proc. Natl. Acad. Sci. USA 112, 5337–5341 (2015).
Sharp, Z. D., Wostbrock, J. A. G. & Pack, A. Mass-dependent triple oxygen isotope variations in terrestrial materials. Geochem. Perspect. Lett. 7, 27–31 (2018).
Zakharov, D. O. & Bindeman, I. N. Triple oxygen and hydrogen isotopic study of hydrothermally altered rocks from the 2.43–2.41 Ga Vetreny belt, Russia: an insight into the early Paleoproterozoic seawater. Geochim. Cosmochim. Acta 248, 185–209 (2019).
Chamberlain, C. P. et al. Triple oxygen isotopes of meteoric hydrothermal system–implicationsfor paleoaltimetry. Geochem. Perspect. Lett. 15, 6–9 (2020).
Dallai, L. & Burgess, R. A record of Antarctic surface temperature between 25 and 50 million years ago. Geology 39, 423–426 (2011).
Rocchi, S. et al. Cenozoic magmatism in the western Ross Embayment: Role of mantle plume versus plate dynamics in the development of the West Antarctic Rift System. J. Geophys. Res. 107, 2195–2217 (2002).
Craig, H. The isotopic geochemistry of water and carbon in geothermal areas, in: Tongiorgi, E. (Ed.), Nuclear geology in geothermal areas. Consiglio Nazionale delle Ricerche, Laboratorio di Geologia Nucleare, Pisa, 17-53 (1963).
Taylor, H. P.Jr. Water/rock interactions and the origin of H2O in granitic batholiths Journal of the Geol.J. Geol. Soc. 133, 509–558 (1977).
Miller, M. F. Isotopic fractionation and the quantification of 17O anomalies in the oxygen three-isotope system: an appraisal and geochemical significance. Geochim. Cosmochim. Acta 11, 1881–1889 (2002).
Luz, B. & Barkan, E. Variations of 17O/16O and 18O/16O in meteoric waters. Geochim. Cosmochim. Acta 74, 6276–6286 (2010).
Pack, A. & Herwartz, D. The triple oxygen isotope composition of the Earth mantle and understanding D17O variations in terrestrial rocks and minerals. Earth Planet. Sci. Lett. 390, 138–145 (2014).
Zakharov, D. O. et al. Triple oxygen isotope systematics as a tracer of fluids in the crust: A study from modern geothermal systems of Iceland. Chem. Geol. 530, 119312 (2019).
Sharp, Z. D. et al. A calibration of the triple oxygen isotope fractionation in the SiO2-H2O system and applications to natural samples. Geochim. Cosmochim. Acta 186, 105–119 (2016).
Dallai, L., Ghezzo, C. & Longinelli, A. Fossil hydrothermal systems tracking Eocene climate change in Antarctica. Geology 29, 931–934 (2001).
Pagani, M., Zachos, J. C., Freeman, K. H., Tipple, B. & Bohaty, S. Marked decline in atmospheric carbon dioxide concentrations during the Paleogene. Science 309, 600–602 (2005).
Tibbett, E. J., Burls, N. J., Hutchinson, D. K. & Feakins, S. J. Proxy-model comparison for the Eocene-Oligocene transition in southern high latitudes. Paleoceanogr. Paleoclimatology 38, e2022PA004496 (2023).
Hutchinson, D. K. et al. The Eocene–Oligocene transition: A review of marine and terrestrial proxy data, models and model–data comparisons. Climate 17, 269–315 (2021).
Liu, M. et al. Global cooling during the Eocene-Oligocene climate transition. Science 323, 1187–1190 (2009).
Masson-Delmotte, V. et al. A review of Antarctic surface snow isotopic composition: observations, atmospheric circulation, and isotopic modeling. J. Clim. 21, 3359–3387 (2008).
Stenni, B. et al. Snow accumulation rates in northern Victoria Land, Antarctica, by firn-core analysis. J. Glaciol. 46, 541–552 (2000).
Rozansky, K., Araguas Araguas, L. & Gonfiantini, R. Relation between long-term trends of oxygen-18 Isotope composition of precipitation and climate. Science 258, 981–984 (1993).
Jouzel, J. et al. Validity of the temperature reconstruction from water isotopes in ice cores. J. Geophys. Res. 102, 26471–26487 (1997).
Froehlich, K., Gibson, J. J., Aggarwal, P. K. Deuterium excess in precipitation and its climatological significance. In Proceedings of the Study of Environmental Change Using Isotope Techniques, C and S Papers Series No. 13/P, 541 Pages. Report Number: IAEA-CSP-13/P; IAEA-CN-80/104 34, 54–66 (2002).
Suzuoki, T. & Epstein, S. Hydrogen isotope fractionation between OH-bearing minerals and water. Geochimica Cosmochimica Acta 40, 1229–1240 (1976).
Marks, M., Vennemann, T., Siebel, W. & Markl, G. Nd-, O-, and H-isotopic evidence for complex, closed-system fl uid evolution of the peralkaline Ilimaussaq intrusion, South Greenland. Geochimica et. Cosmochimica Acta 68, 3379–3395 (2004).
Criss, R. E., Lanphere, M. A. & Taylor, H. P. J. R. Effects of regional uplift, deformation and meteoric-hydrothermal metamorphism on K-Ar ages of biotites in the southern half of the Idaho Batholith. J. Geophys. Res. 87, 7026–7046 (1982).
Feakins, S. J., Warny, S. & DeConto, R. M. Snapshot of cooling and drying before onset of Antarctic Glaciation. Earth Planet. Sci. Lett. 404, 154–166 (2014).
Jahren, A. H. & Sternberg, L. S. L. Humidity estimate for the middle Eocene Arctic rainforest. Geology 31, 463–466 (2003).
Bowen, J. G., Beerling, D. J., Koch, P. L., Zachos, J. C., Quattlebaum, T. A humid climate state during the Palaeocene/Eocene thermal maximum. Nature. 432, 495–499 (2004).
Merlivat, L. & Jouzel, J. Global climatic interpretation of the deuterium-oxygen 18 relationship for precipitation. J. Geophys. Res. 84, 5029–5033 (1979).
Landais, L., Ekaykin, A., Barkan, E., Winkler, R. & Luz, B. Seasonal variations of 17O-excess and d-excess in snow precipitation at Vostok station, East Antarctica. J. Glaciol. 58, 725–733 (2012).
Pfahl, S. & Sodemann, H. What controls deuterium excess in global precipitation? Clim. Past 10, 771–781 (2014).
Uemura, R. Y., Matsui, K., Yoshimura, H., Motoyama & Yoshida, N. Evidence of deuterium excess in water vapor as an indicator of ocean surface conditions. J. Geophys. Res. 113, D19114 (2008).
Rahul, P., Prasanna, K., Ghosh, P., Anilkumar, N. & Yoshimura, K. Stable isotopes in water vapor and rainwater over Indian sector of Southern Ocean and estimation of fraction of recycled moisture. Sci. Rep. 8, 7552 (2018).
Griesner, K. W., Nelson, D. M. & Warny, S. Declining moisture availability on the Antarctic Peninsula during the Late Eocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 383–384, 72–78 (2013).
Inglis, G. N., Carmichael, M., Farnsworth, A., Lunt, D. & Pancost, R. D. A long-term, high-latitude record of Eocene hydrological change in the Greenland region. Palaeogeogr. Palaeoclimatol. Palaeoecol. 537, 109378 (2020).
Ehrmann, W. U. & Mackensen, A. Sedimentological evidence for the formation of an East Antarctic ice sheet in Eocene/Oligocene time. Palaeogeogr. Palaeoclimatol. Palaeoecol. 93, 85–112 (1992).
Wellner, J. S. et al. History of an evolving ice sheet as recorded in SHALDRIL cores from the Northwestern Weddell Sea, Antarctica. In Tectonic, Climatic, and Cryospheric Evolution of the Antarctic Peninsula Vol. 63 (eds Anderson, J. B. & Wellner, J. S.) 131–152 (American Geophysical Union, Washington, D.C., 2011).
Bailey, A., Posmentier, E. & Feng, X. Patterns of evaporation and precipitation drive global isotopic changes in atmospheric moisture. Geophys. Res. Lett. 45, 7093–7101 (2018).
Sijp, W. P. et al. The role of ocean gateways on cooling climate on long time scales. Glob. Planet. Change 119, 1–22 (2014).
Bijl, et al.Eocene cooling linked to early flow across the Tasmanian Gateway. Proc. Natl. Acad. Sci. USA 110, 9645–9650 (2013).
Kennett, J. P. Cenozoic evolution of Antarctic glaciation, the Circum-Antarctic Ocean, and their impact on global paleoceanography. J. Geophys. Res. 82, 3843–3860 (1977).
Livermore, R., Hillenbrand, C.-D., Meredith, M. & Eagles, G. Drake Passage and Cenozoic climate: An open and shut case? Geochem. Geophy. Geosy. 8, Q 01005 (2007).
Toggweiler, J. R. & Bjornsson, H. Drake passage and palaeoclimate. J. Quat. Sci. 15, 319–328 (2000).
Borrelli, C., Cramer, B. S. & Katz, M. E. Bipolar Atlantic deepwater circulation in the middle-late Eocene: Effects of Southern Ocean gateway openings. Paleoceanogr. Paleoclimatology 29, 308–327 (2014).
Katz, M. E. et al. Stepwise transition from the Eocene greenhouse to the Oligocene icehouse. Nat. Geosci. 1, 329 (2008).
Evangelinos, D. et al. Late Miocene onset of the modern Antarctic Circumpolar Current. Nat. Geosci. 17, 165–179 (2024).
Sodemann, H. & Stohl, A. Asymmetries in the moisture origin of Antarctic precipitation. Geophys. Res. Lett. 36, L22803 (2009).
Bijl, P. K. et al. Early Palaeogene temperature evolution of the southwest Pacific Ocean. Nature 461, 776–779 (2009).
Sharp, Z. D. Oxygen isotope geochemistry of the Al2SiO5 polymorphs. Am. J. Sci. 295, 1058–1076 (1995).
Sharp, Z. D., Atudorei, V. & Durakiewicz, T. A rapid method for determination of hydrogen and oxygen isotope ratios from water and hydrous minerals. Chem. Geol. 178, 197–210 (2001).
Wostbrock, J. A. G., Cano, E. J. & Sharp, Z. D. An internally consistent triple oxygen isotope calibration of standards for silicates, carbonates and air relative to VSMOW2 and SLAP2. Chem. Geol. 533, 119432 (2020).
Aron, P. G. et al. Triple oxygen isotopes in the water cycle. Chem. Geol. 565, 120026 (2021).
Acknowledgements
L.D. is grateful to the PNRA for logistics support in Antarctica and to Ray Burgess for sharing fieldwork. L.D. thanks Museo Nazionale dell’Antartide for providing rocks samples. L.D. is also grateful to Viorel Atudorei, Erick Cano, and Tommaso Di Rocco for their help in analytical work. The research was supported by CNR-Short Term Mobility Programme n. 0063613/2018.
Author information
Authors and Affiliations
Contributions
L.D. conceived the research, sampled the rocks and performed the analyses; L.D. and Z.D.S. discussed the data, made the modelling, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Daniel Herwartz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dallai, L., Sharp, Z.D. A tipping point in stable isotope composition of Antarctic meteoric waters during Cenozoic glaciation. Nat Commun 15, 4509 (2024). https://doi.org/10.1038/s41467-024-48811-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-48811-2
- Springer Nature Limited