Abstract
The Omicron variant of SARS-CoV-2 became the globally dominant variant in early 2022. A sub-lineage of the Omicron variant (BA.2) was identified in England in January 2022. Here, we investigated hospitalisation and mortality risks of COVID-19 cases with the Omicron sub-lineage BA.2 (n = 258,875) compared to BA.1 (n = 984,337) in a large cohort study in England. We estimated the risk of hospital attendance, hospital admission or death using multivariable stratified proportional hazards regression models. After adjustment for confounders, BA.2 cases had lower or similar risks of death (HR = 0.80, 95% CI 0.71–0.90), hospital admission (HR = 0.88, 95% CI 0.83–0.94) and any hospital attendance (HR = 0.98, 95% CI 0.95–1.01). These findings that the risk of severe outcomes following infection with BA.2 SARS-CoV-2 was slightly lower or equivalent to the BA.1 sub-lineage can inform public health strategies in countries where BA.2 is spreading.
Similar content being viewed by others
Introduction
Omicron (B.1.1.529) is currently the globally most prevalent SARS-CoV-2 variant, accounting for >95% of reported variants since late February 20221. The BA.2 sub-lineage of Omicron was first identified in England in January 20222. BA.2 is more transmissible than the previous BA.1 sub-lineage3. Small studies have provided inconsistent evidence on the severity of BA.2 relative to BA.13,4,5.
This study aimed to determine the relative risks of hospitalisation and death for Omicron BA.2 compared to BA.1 in a large national cohort.
Results
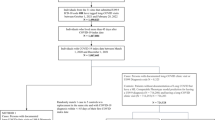
A total of 258,875 BA.2 and 984,337 BA.1 cases with positive specimens between 01 December 2021 and 18 March 2022 (Fig. 1) were included to investigate the risks of the three main outcomes: any hospital attendance within 14 days of a positive test (including admissions), hospital admission within 14 days of positive test where there were ≥2 days of stay and death within 28 days of a positive test. The median age was 38 years (inter-quartile range 25–53) for BA.2 and 35 years (inter-quartile range 22–49) for BA.1 cases; BA.2 cases were more likely to live in less deprived areas or in London, East of England or South East, and had more often received a third vaccine dose, compared to BA.1 cases (Table S1).
Descriptive frequencies of the earliest specimen date of COVID-19 cases with Omicron lineages BA.1 (top panel) and BA.2 (bottom panel), in England between 01 December 2021 and 25 March 2022 (n = 1,243,212). COVID-19: coronavirus disease 2019. Shading indicates the method of case identification: validated whole genome sequencing, or genotyping in combination with available S-gene status (SGTF or SGTP) for the episode, or from 24th January onwards S-gene status was used in absence of sequencing or genotyping for the episode.
Our findings show that the risk of death was lower for BA.2 compared to BA.1 cases (adjusted hazard ratio [HR] = 0.80, 95% CI 0.71–0.90) and the risk of hospital admission was also slightly statistically significantly lower for BA.2 (adjusted HR = 0.88, 95% CI 0.83–0.94). Risk of any hospital attendance was similar between BA.2 and BA.1 (adjusted HR = 0.98, 95% CI 0.95–1.01; Table S2). There were some indications that the risks may differ between age groups, with reduced risks of hospital admission and death for cases with BA.2 compared to BA.1 being specific to adults aged 40–79 years, whilst the risks did not significantly differ between BA.2 and BA.1 for children and for those aged ≥80 years (tests for interaction, all P values ≤0.01; Fig. 2; Table S3). However, broad CIs for the HRs in younger age groups complicates drawing conclusions that the risks are age specific.
Risk of hospitalisation and mortality, overall and by age group, for COVID-19 cases with Omicron lineage BA.2 compared to BA.1 in England, 01 December 2021–2025 March 2022. The central measures are adjusted hazard ratios and the errors bars are the corresponding 95% confidence intervals from Cox regression models stratified for exact specimen date, area of residence, age group and vaccination status, and additionally using regression adjustments for within-age-group residual differences in exact age, sex, ethnicity, index of multiple deprivation (IMD) quintile and within-IMD-quintile residual differences in exact IMD rank, and reinfection status. A shows risk of any hospital attendance, including admissions, within 14 days of the earliest specimen date in the COVID-19 episode following infection with SARS-CoV-2 lineage BA.2, compared to BA.1. B shows the risk of hospital admission within 14 days. C shows the risk of death within 28 days. For the death outcome, hazard ratios were not estimated for cases aged <20 years due to small numbers. Likelihood ratio test (LRT) P values from two-sided tests for interaction between age group and variant status for attendance, admission, and death are 0.010, 0.0003958, and 0.003 respectively. These explorative tests were not adjusted for multiple comparisons. The adjusted hazard ratio estimates and 95% confidence intervals that the figure is based on are included in Table S3.
Adjusted HRs by vaccination and reinfection status showed no significant variation in the risks for BA.2 compared to BA.1, with point estimates indicating lower or similar risks for BA.2 than BA.1 in all subgroups. The point estimate for risk of death for BA.2 compared to BA.1 was somewhat lower for unvaccinated people than those of the other subgroups, but the CIs were wide and overlapped and the corresponding tests for interaction did not indicate significant differences (tests for interaction, all P values≥0.11; Fig. 3; Table S4).
Risk of hospitalisation and mortality by vaccination status and reinfection status, for COVID-19 cases with Omicron lineage BA.2 compared to BA.1 in England, 01 December 2021–2025 March 2022. The central measures are adjusted hazard ratios and the error bars are the corresponding 95% confidence intervals (CI) from Cox regression models with an interaction term between variant (BA.2 vs BA.1), and vaccination (A total n = 1,243,212; unvaccinated or ≤28 days after vaccination = 247,748; ≥28 days after first dose = 64,694; ≥14 days after second dose = 294,071; ≥14 days after third dose = 537,822) or reinfection (B total n = 1,243,212; reinfection = 125,239; first infection = 1,117,973) status. The models were stratified for exact specimen date, area of residence, age group and vaccination status, and additionally using regression adjustments for within-age-group residual differences in exact age, sex, ethnicity, index of multiple deprivation (IMD) quintile and within-IMD-quintile residual differences in exact IMD rank, and reinfection status. LRT P values from two-sided tests for interaction between vaccination status and variant status for attendance, admission, and death are 0.65, 0.87, and 0.11 respectively. LRT P values from two-sided tests for interaction between reinfection status and variant status for attendance, admission, and death are 0.34, 0.70, and 0.16, respectively. These explorative tests were not adjusted for multiple comparisons. The adjusted hazard ratio estimates and 95% confidence intervals that the figure is based on are included in Table S4.
Sensitivity analyses examining the effect of differing variant classification methods showed HR estimates were generally higher for the subgroups with non-sequencing-classified variants (Table S5). These were however based on smaller numbers and had much wider CIs than the estimates for the subgroup with sequencing-classified variants, which were consistent with the primary analysis. Alternative definitions of hospitalisation and death endpoints had no substantial effects on the results (Table S6).
We explored the impact of epidemic phase bias (see Methods)6. This sensitivity analysis indicated that the risks might be somewhat lower for BA.2 than BA.1 than found in the main analysis (Table S7).
When restricted to different calendar periods based on changes in testing advice (Methods, Table S8), or to a shorter calendar period when the incidence of BA.1/BA.2 was similar (Table S9), the resulting HRs were consistent with and had 95% CIs that overlapped with the main HR estimates.
Discussion
In this large national study, we found lower or similar risks of severe outcomes for cases with the BA.2 variant compared to BA.1. These risks might vary by age group, with possibly lower risks of mortality and hospital admission for adult BA.2 compared to BA.1 cases aged 40–79, but insignificant differences in the risks for children and for those aged ≥80 years (Fig. 2). We found no significant differences between BA.2 and BA.1 by vaccination or reinfection status, but the precision of the estimates was low in these subgroups.
Results have been mixed in previous reports on BA.2 versus BA.1 severity: a small cohort in France found evidence of a 3.5-fold risk of hospitalisation but based on only 207 BA.2 cases5; studies in Denmark3; and South Africa4 found no significant differences in risk of hospitalisation or death, with relative risks ranging between 0.91 and 1.20, consistent with our findings of at most minor differences in risk between BA.2 and BA.1.
Several SARS-CoV-2 variants have evolved during the pandemic, with inconsistent transmissibility and severity patterns. Omicron BA.1 replaced Delta as the globally dominant variant in early 20221, likely due to greater transmissibility7, but is associated with substantially lower severity8,9,10. Both Delta and the previous Alpha (B.1.1.7) variant had higher transmissibility and severity compared to the previous variants11,12,13. It is therefore reassuring that the BA.2 sub-lineage, despite being more transmissible than BA.14, may be associated with no greater risk of severe outcomes than Omicron BA.1. These patterns are analogous with previous findings for the AY.4.2 sub-lineage of the Delta variant, which had higher transmissibility but lower or similar severity risks compared to other Delta sub-lineages14.
To prioritise high specificity in the context of lower risks with Omicron compared to previous variants and high vaccine coverage, which might result in higher proportions of incidental admissions, the primary hospital admission definition included inpatient stays where the duration of stay was ≥2 days. This differs from the definitions used in previous variant severity analyses in England, which considered admissions with ≥1 day of inpatient stay7, 9; however, sensitivity analyses indicated consistent results between alternative outcome definitions.
This is the largest nationwide study to date to report on BA.2 severity. It includes sufficient numbers to estimate risks by age group, which is important because BA.2 severity has not previously been well described in older age groups15. Limitations of the routine surveillance data include reporting lags for hospitalisations and deaths. However, by stratifying on specimen date and region, differential misclassification by variant should not be expected. Cases’ variant status was ascertained based on several different methods; by using a combination of S-gene status, genotyping and sequencing, we could maximise numbers. Sensitivity analyses restricted to cases with variants called by relatively less specific genotyping or S-gene status did not detect reductions in severity between the two lineages, but the primary results were consistent with those for cases with whole-genome sequenced specimens, the most specific method.
There were no data available on some factors that may have affected the risk of hospitalisation or death, such as comorbidities and the use of novel antivirals or monoclonal antibodies. However, there is little reason to expect that these factors are differentially associated with infecting Omicron sub-lineage and they are therefore unlikely to confound the association between sub-lineage and the outcomes. Furthermore, the adjustments for age, sex and deprivation may have partially adjusted for these factors indirectly. The analysis was based on data for test-positive cases whose infecting Omicron sub-lineage was determined. This might lead to selection bias if detection rates or variant calling rates differ by Omicron sub-lineage. However, we are not aware of any data to suggest this.
In conclusion, our study indicates no increased severity of the BA.2 sub-lineage of the SARS-CoV-2 Omicron variant, compared to the previously dominant Omicron BA.1 sub-lineage in England. These findings will be useful in informing public health strategies in countries where BA.2 is spreading.
Methods
Case ascertainment
Laboratory-confirmed COVID-19 cases in England with specimen dates between 01 December 2021 and 25 March 2022 were extracted from UKHSA’s Second Generation Surveillance System (SGSS)16, and linked to validated whole genome sequencing results available on the Cloud Infrastructure for Big Data Microbial Bioinformatics database17 using a unique identifier. PCR genotyping results and S-gene target data were obtained from SGSS.
BA.1 is associated with S-gene target failure (SGTF) and BA.2 with S-gene target positivity (SGTP), but other variants such as Delta (B.1.617.2) are also associated with SGTP. Genotyping could call Omicron variants but not distinguish Omicron sub-lineages. Therefore, the study included: (1) all sequencing-confirmed BA.1/BA.2 cases; (2) before 24th January when Delta variants were still prevalent, cases with SGTF/SGTP status only if genotyping results indicated Omicron; and (3) from 24th January onwards, when the predictive values of SGTF/SGTP to call BA.1/BA.2 were ≥95%18, all cases with SGTF/SGTP data that did not already have sequencing/genotyping data (S-gene information available up to 15 March 2022) (Fig. 1).
The cases were further linked to the National Immunisation Management Service for vaccination status; UKHSA’s COVID-19 mortality dataset19,20; and NHS Digital’s Emergency Care Data Set and Secondary Uses Service datasets21, including data submitted by NHS Trusts up to 8th May 2022, for hospitalisation data.
Statistical methods
HRs of the outcomes for BA.2 versus BA.1 cases were estimated using Cox regression models. Separate models were fitted for each of the three outcomes. To adjust for confounders, the models were stratified for exact specimen date, area of residence, age group and vaccination status, and additionally used regression adjustments for within-age-group exact age (linear terms), sex, ethnicity, index of multiple deprivation (IMD) quintile and within-IMD-quintile exact IMD rank (linear terms), and reinfection status. We estimated the corresponding HRs by age group, vaccination or reinfection status by fitting secondary models that additionally included interaction terms between variant sub-lineage and each of these potential modifiers. The interactions were tested for significance using likelihood ratio tests.
Sensitivity analyses
In sensitivity analyses, we examined the effect of differing variant classification methods (Table S5), or alternative definitions of the hospitalisation and death endpoints (Table S6). We explored the impact of epidemic phase bias, which can occur when comparing two virus variants in different phases of growth if infection severity is correlated with time from infection to test. The relative severity between variants of cases that test positive within the same calendar period can then be biased towards overestimated relative risks for a new, more rapidly spreading, variant compared to a previous variant (Table S7)6. To assess whether changes in testing guidelines over time affected the association between sub-lineage and severe outcomes we undertook severity analyses, repeating the main analysis limiting cases to three periods: 01 December 2021 to 10 January 2022 (the date at which PCR confirmation of asymptomatic LFT positives stopped), 11 January to 20 February 2022 (when testing in schools ceased) and 21 February to 31 March 202222 (Table S8). A further sensitivity analysis considered a period when the prevalence of the two sub-lineages was similar (14 February 2022 to 27 February 202223, Table S9).
Software and databases
Data were analysed using R version 4.2.1. SGSS runs SQL Server 2019 (version 15).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data used in this analysis were anonymised, the individual-level nature of the data used risks individuals being identified, or being able to self-identify if the data are released publicly. Requests for access to the underlying source data should be directed to the Office for Data Release (odr@ukhsa.gov.uk) at UKHSA. Details of applying for access to data held by UKHSA can be found at https://www.gov.uk/government/publications/accessing-ukhsa-protected-data/accessing-ukhsa-protected-data and criteria against which applications will be judged can be found at https://www.gov.uk/government/publications/accessing-ukhsa-protected-data/approval-standards. Accessible links for the databases used in this study can be found at: https://sgss.phe.org.uk/Security/Login?ReturnUrl=%2f for SGSS, https://www.climb.ac.uk/ for CLIMB, https://www.scwcsu.nhs.uk/services/nhs-immunisation-management-service/ for NIMS, https://digital.nhs.uk/services/secondary-uses-service-sus for SUS for SUS, https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-sets/emergency-care-data-set-ecds for ECDS.
Code availability
The code used in this analysis is available on GitHub. H.H. Webster, T. Nyberg, M.A. Sinnathamby, N. Abdul Aziz, N. Ferguson, G. Seghezzo, P.B. Blomquist, J. Bridgen, M. Chand, N. Groves, R. Myers, R. Hope, E. Ashano, J. Lopez-Bernal, D. De Angelis, G. Dabrera, A. Presanis, S. Thelwall. Hospitalisation and mortality risk of SARS-CoV-2 variant Omicron sub-lineage BA.2 compared to BA.1, England. Ba2_severity, DOI: 10.5281/zenodo.7043668, 2022.
References
GISAID. Tracking of variants. Available from: https://www.gisaid.org/hcov19-variants/.
UK Health Security Agency (UKHSA). SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 35. London: UKHSA; 2021. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050999/Technical-Briefing-35-28January2022.pdf.
Fonager, J. et al. Molecular epidemiology of the SARS-CoV-2 variant Omicron BA.2 sub-lineage in Denmark, 29 November 2021 to 2 January 2022. Eur. Surveill. 27, pii=2200181 (2022).
Wolter, N., Jassat, W., DATCOV-Gen author group, von Gottberg, A., Cohen, C. Clinical severity of Omicron sub-lineage BA.2 compared to BA.1 in South Africa. medRxiv. Available from: https://www.medrxiv.org/content/10.1101/2022.02.17.22271030v1.
Gautret, P. et al. The severity of the first 207 infections with the SARS-CoV-2 Omicron BA.2 variant, in Marseille, France, December 2021–February 2022. J. Med. Virol. 2022. https://doi.org/10.1002/jmv.27760.
Seaman, S. et al. Adjusting for time of infection or positive test when estimating the risk of a post-infection outcome in an epidemic. medRxiv https://doi.org/10.1101/2021.08.13.21262014.
Lyngse, F. et al. SARS-CoV-2 Omicron VOC Transmission in Danish Households. medRxiv https://doi.org/10.1101/2021.12.27.21268278.
Nyberg, T. et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B. 1.1. 529) and delta (B. 1.617. 2) variants in England: a cohort study. Lancet 399, 1303–1312 (2022).
Veneti, L. et al. Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 Omicron BA.1 variant compared with the Delta variant, Norway, December 2021 to January 2022. Euro Surveill. 27, 2200077 (2022).
Auvigne, V. et al. Severe hospital events following symptomatic infection with Sars-CoV-2 Omicron and Delta variants in France, December 2021–January 2022: a retrospective, population-based, matched cohort study. eClinicalMedicine https://www.medrxiv.org/content/10.1101/2022.02.02.22269952v3 (2022).
Twohig, K. et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect. Dis. 22, 35–42 (2022).
Volz, E. et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature 593, 266–269 (2021).
Nyberg, T. et al. Risk of hospital admission for patients with SARS-CoV-2 variant B.1.1.7: cohort analysis. BMJ. 373, n1412 (2021).
Nyberg, T. et al. Hospitalisation and mortality risk for COVID-19 cases with SARS-CoV-2 AY.4.2 (VUI-21OCT-01) compared to non-AY.4.2 Delta variant sub-lineages. J. Infect. Dis. 226, 808–811 (2022).
Huang, J. & Zeng, G. Letter to the editor: epidemiology of the SARS-CoV-2 variant Omicron BA.2 – vigilance needed. Eur. Surveill. 27, pii=22-00254 (2022).
Clare, T., Twohig, K., O’Connell, A.-M. & Dabrera, G. Timeliness and completeness of laboratory-based surveillance of COVID-19 cases in England. Public Health 194, 163–166 (2021).
Connor, T. et al. CLIMB (the Cloud Infrastructure for Microbial Bioinformatics): an online resource for the medical microbiology community. Microb. Genom. 2, e000086 (2016).
UK Health Security Agency (UKHSA) SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 36. (UKHSA, London, 2021.) https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1056487/Technical-Briefing-36-22.02.22.pdf.
Graphnet. National Immunisation management system. Available from: https://www.graphnethealth.com/solutions/immunisation-systems.
Brown, A. et al. Epidemiology of confirmed COVID-19 deaths in adults, England, March–December 2020. Emerg. Infect. Dis. 27, 1468–1471 (2021).
Bhattacharya, A. et al. Healthcare-associated COVID-19 in England: a national data linkage study. J. Infect. 83, 565–572 (2021).
Halford, F., Nash, S., Tessier, E., Kall, M., Dabrera, G. Understanding reported COVID-19 cases in England following changes to testing, between November 2021 and April 2022. medRxiv https://doi.org/10.1101/2022.06.28.22276549 (2022).
UK Health Security Agency (UKHSA). SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 44. (UKHSA; London, 2022.) https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1093275/covid-technical-briefing-44-22-july-2022.pdf.
Acknowledgements
We would like to acknowledge the efforts of multiple teams in enabling the severity assessment of this SARS-CoV-2 variant. We would like to thank the UKHSA Second Generation Surveillance System, DataLake and Datastore teams for maintaining the databases in which the data are stored. We thank NHS Digital and Hospital-onset COVID team at UKHSA for enabling surveillance of hospitalisation. We also thank colleagues for maintaining COVID-19 vaccination surveillance at the National Immunisation Management service, Julia Stowe and Freya Kirsebom (UKHSA, Immunisation and Vaccine Preventable Diseases Division) and colleagues for helpful discussions surrounding hospitalisation surveillance, and Andre Charlett (UKHSA Statistics, Modelling and Economics division) for his ongoing work overseeing the UKHSA COVID-19 data streams. This work was supported by UK Research and Innovation (UKRI) Medical Research Council (MRC) (NMF: Centre for Global Infectious Disease Analysis [MR/R015600/1]; DDA, AMP: [Unit Programme number MC/UU/00002/11]); UKRI MRC/Department of Health and Social Care (DHSC) National Institute for Health and Care Research (NIHR) COVID-19 rapid response call (TN, DDA, AMP: [MC/PC/19074]; NMF: [MR/V038109/1]); NIHR Health Protection Units in: Modelling and Health Economics (NMF), and Behavioural Science and Evaluation (DDA); philanthropic funding from Community Jameel (NMF); and the WHO Regional Office for Europe (TN, DDA, AMP). The funders played no direct role in the study. The views expressed are those of the authors and not necessarily those of the NIHR or the DHSC.
Author information
Authors and Affiliations
Contributions
T.N., N.M.F., D.D.A., G.D., A.M.P., and S.T. conceived and designed the study. P.B.B., J.B., E.A., G.S., R.H., and J.L.B. lead the collection of and advised on the use and implementation of the data on S-gene target, hospitalisation, COVID-19 mortality and vaccination. M.C. led the genomic sequencing surveillance for UKHSA and the creation of this data resource. N.G. and R.M. developed case definitions by which variant status is assigned. T.N. designed the statistical analysis, with support from N.M.F., D.D.A. and A.M.P. H.H.W. linked the datasets and performed the statistical analysis, supported by T.N. and N.A.A. H.H.W. and M.A.S. drafted the first version of the manuscript. T.N. and S.T. contributed to a revised draft. All authors read, revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
N.M.F. sits on a number of UK government and World Health Organization groups providing scientific advice relating to SARS-CoV-2. G.D. declares that his employer UK Health Security Agency (previously operating as Public Health England) received funding from GlaxoSmithKline for a research project related to influenza antiviral treatment. This preceded and had no relation to COVID-19, and G.D. had no role in and received no funding from the project. D.D.A. sits on two UK government groups providing scientific advice relating to SARS-CoV-2 (Scientific Pandemic Influenza Group on Modelling [SPI-M] and UKHSA’s Variant Technical Group [VTG]). The remaining authors declare no competing interests.
Ethical statement
This surveillance was performed as part of the UK Health Security Agency’s (UKHSA) responsibility to monitor COVID-19 during the current pandemic. UKHSA has legal permission, provided by Regulation 3 of The Health Service (Control of Patient Information) Regulations 2002 to process confidential patient information under Sections 3(i) (a) to (c), 3(i)(d) (i) and (ii) and 3(iii) as part of its outbreak response activities. This study falls within the research activities approved by the UKHSA Research Ethics and Governance Group.
Peer review
Peer review information
Nature Communications thanks Vincent Auvigne and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Webster, H.H., Nyberg, T., Sinnathamby, M.A. et al. Hospitalisation and mortality risk of SARS-COV-2 variant omicron sub-lineage BA.2 compared to BA.1 in England. Nat Commun 13, 6053 (2022). https://doi.org/10.1038/s41467-022-33740-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-33740-9
- Springer Nature Limited