Abstract
The counterregulatory response to hypoglycemia that restores normal blood glucose levels is an essential physiological function. It is initiated, in large part, by incompletely characterized brain hypoglycemia sensing neurons that trigger the secretion of counterregulatory hormones, in particular glucagon, to stimulate hepatic glucose production. In a genetic screen of recombinant inbred BXD mice we previously identified Agpat5 as a candidate regulator of hypoglycemia-induced glucagon secretion. Here, using genetic mouse models, we demonstrate that Agpat5 expressed in agouti-related peptide neurons is required for their activation by hypoglycemia, for hypoglycemia-induced vagal nerve activity, and glucagon secretion. We find that inactivation of Agpat5 leads to increased fatty acid oxidation and ATP production and that suppressing Cpt1a-dependent fatty acid import into mitochondria restores hypoglycemia sensing. Collectively, our data show that AgRP neurons are involved in the control of glucagon secretion and that Agpat5, by partitioning fatty acyl-CoAs away from mitochondrial fatty acid oxidation and ATP generation, ensures that the fall in intracellular ATP, which triggers neuronal firing, faithfully reflects changes in glycemia.
Similar content being viewed by others
Introduction
The defense against hypoglycemia is a major survival function. It is coordinated by a distributed glucose sensing system which comprises hypoglycemia-activated (glucose-inhibited or GI) neurons located in different brain nuclei and which control autonomic nervous activity, the hypothalamic-pituitary-adrenal axis, and, eventually, the secretion of the counterregulatory hormones, glucagon, epinephrine, norepinephrine, corticosterone and growth hormones1,2,3. These hormones reduce insulin secretion, decrease insulin-stimulated glucose uptake, and trigger hepatic glucose production to restore normoglycemia. In addition to this hormonal counterregulatory response (CRR), hypoglycemia also triggers search for, and ingestion of sugar-containing food in order to replenish the body glucose stores1.
Hypoglycemia-activated GI neurons are present in the brainstem, including the dorsal vagal complex (DVC) and the parabrachial nucleus, and in several hypothalamic nuclei, including the paraventricular (PVN), ventromedial (VMN), dorsomedial, and arcuate (ARC) nuclei1,4. In the hypothalamus, insulin-induced hypoglycemia (IIH) strongly activates neurons of the PVN and ARC5,6 suggesting an important role of these nuclei in initiating the CRR. These nuclei are connected to the DVC, to control autonomic nervous system activation in response to IIH7.
The mechanisms of hypoglycemia sensing that induce neuron firing are not completely characterized and appear to rely on different processes. For instance, in the VMN, GI neuron activation depends on the energy sensor AMP-activated protein kinase (AMPK)8, which is activated by a fall in intracellular energy level, and which regulates the activity of an ion channel, possibly the cystic fibrosis transmembrane conductance regulator (CFTR)9; AMPK also preserves GI neurons function by upregulating the expression of anti-oxidant proteins, in particular thioredoxin 28. In agouti-related peptide (AgRP) neurons of the ARC, an alternate mechanism for hypoglycemia sensing is active10,11. This depends on the fall in intracellular ATP that accompanies developing hypoglycemia leading to reduced Na+/K+ATPase activity, plasma membrane depolarization and neuronal firing. In the ARC, but not in the VMN or lateral hypothalamus, the ATP level is indeed reduced during fasting and there is a linear relationship between extracellular glucose concentrations and intracellular ATP levels. Furthermore, intra-ARC injection of the Na+/K+/ATPase inhibitor ouabain, strongly increases food intake as a result of AgRP neuron activation11. Thus, fluctuations in intracellular ATP levels critically control AgRP neurons activity.
The secretion of counterregulatory hormones is mainly triggered by a fall in blood glucose concentration but is also under the regulatory influences of other metabolic signals. For instance, activation by hypoglycemia of parabrachial GI neurons, which stimulate glucagon secretion through their projections onto VMN neurons, is markedly decreased by leptin acting on the parabrachial neurons12. Thus, there is evidence that the overall CRR, although directed by glucose levels, is also under the control of signals of whole-body energy status. How hypoglycemia sensing is influenced by other metabolic signaling pathways is only poorly understood.
In a preceding study, we performed a genetic screen using a panel of recombinant inbred BXD mice to identify novel hypothalamic regulators of insulin-induced glucagon secretion5. We identified two QTLs, one on chromosome 8 and one on chromosome 15. Combined with transcriptomic analysis of the hypothalamus of the BXD mice, we identified Irak4, located in the QTL of chromosome 15, as a candidate gene whose expression negatively correlated with the glucagon trait. We confirmed that Irak4, by modulating hypothalamic Il-1β signaling, indeed controls hypoglycemia-induced glucagon secretion5.
Here, we characterized the role of Agpat5, the candidate gene of the chromosome 8 QTL, in glucagon secretion. Agpat5 (1-acylglycerol-3-phosphate-O-acyltransferase 5), one of five Agpat isoforms, is associated with the mitochondrial membrane and catalyzes the production of phosphatidic acid (PA) from lysophosphatidic acid (LPA) and acyl-CoAs13. We demonstrated that Agpat5 inactivation in AgRP neurons led to reduced hypoglycemia-induced glucagon secretion. This was associated with reduced activation by hypoglycemia of AgRP neurons as assessed by c-Fos immunostaining, patch clamp analysis, and in vivo fiber photometry; and with defective activation of the vagal nerve. We further demonstrate that Agpat5 inactivation increased Cpt1a-dependent mitochondrial fatty acid β-oxidation (FAO), oxygen consumption rate (OCR), and ATP production. These data show that Agpat5 is required to divert fatty acyl-CoAs from entering the mitochondria to prevent FAO-dependent ATP production, thereby ensuring proper hypoglycemia sensing.
Results
Agpat5 inactivation in AgRP neurons impairs glucagon secretion
A previous genetic screen for hypoglycemia-induced glucagon secretion using a panel of 36 recombinant inbred BXD mouse lines identified a QTL on chromosome 85. Analysis of RNASeq data from the hypothalamus of the same panel of BXD mice, revealed that expression of only three genes located in this QTL displayed significant correlation with the glucagon trait, Agpat5 and two genes of unknown function (261005L07Rik and LOC547150). Here, we further analyzed the hypothalamic RNASeq data from the BXD mice and identified a cis-eQTL for Agpat5 expression that was located in the same genomic interval of chromosome 8 that controls glucagon secretion, between markers rs13479628 and rs33450716 (Fig. 1A, B). We then determined that the plasma glucagon levels correlated, across the BXD lines, with Agpat5 mRNA expression in the hypothalamus (p = 0.0022, r = 0.493) (Fig. 1C), but not in the liver (p = 0.1192, r = 0.2644) (Fig. 1D) nor in the subcutaneous adipose tissue (p = 0.5495, r = −0.1080) (Fig. 1E). Thus, these genetic and transcriptomic data indicated a role for hypothalamic Agpat5 expression in the regulation of glucagon secretion.
Hypothalamic Agpat5 on chromosome 8 is a candidate regulator of insulin-induced hypoglycemia. A QTL mapping of hypothalamic Agpat5 expression reveals a cis-eQTL on chromosome 8. B The cis-eQTL for Agpat5 (black) overlaps with the cQTL for glucagon secretion (blue). C Correlation between Agpat5 mRNA expression in the hypothalamus of the BXD mice and the glucagon trait. D, E No correlation was found between Agpat5 mRNA expression in the liver (C) or adipose tissue (D) of the BXD mice and the glucagon trait. F Representative image of in situ hybridization detection of Agpat5 mRNA in the PVN (top panel) and ARC (bottom panel) hypothalamic nuclei. Scale bar, 200 μm. G Structure of the wild-type (WT) Agpat5 allele around exons 3 and 4 (top), of the flox allele (middle) and of the recombined allele (bottom). The primers used for genotyping (P1, P2, P3, P4, P5 in green), their location and size of the amplified fragments are indicated. H Genotyping of WT and flox alleles using ear DNA. I PCR analysis of the recombined allele in DNA from microdissected ARC, PVN and paraventricular thalamus (PVT) of Agpat5flox/flox (Ctrl) and KO AgRPAgpat5KO (KO) mice. H, I Representative images are presented. J tdTomato expression in the ARC of AgRPCre/+;Rosa26tdTom and AgRPAgpat5KO;Rosa26tdTom mice. Scale bars, 200 μm. K Quantification of AgRP neurons in the ARC of AgRPCre/+;Rosa26tdTom and AgRPAgpat5KO;Rosa26tdTom mice. n = 3 mice per genotype, three different sections/mouse. Data are mean ± SEM. Two-tailed, unpaired Student’s test.
Agpat5 is ubiquitously expressed14 and in situ hybridization confirmed that it was present in all hypothalamic nuclei, including in the ARC and PVN (Fig. 1F). As insulin-induced hypoglycemia strongly activates PVN neurons and AgRP neurons of the ARC5,6 (see below) we decided to assess the impact of inactivating Agpat5 in the PVN and in AgRP neurons on hypoglycemia-induced glucagon secretion. We generated Agpat5flox/flox mice (Fig. 1G, H) and crossed them first with Sim1-Cre mice to inactivate Agpat5 in the PVN (Sim1Agpat5KO mice) (Supplementary Fig. 1A). Male and female Agpat5flox/flox and Sim1Agpat5KO mice displayed identical body weight (Supplementary Fig. 1B), glucose tolerance (Supplementary Fig. 1C, D), insulin sensitivity (Supplementary Fig. 1E, F), and hypoglycemia-stimulated glucagon secretion (Supplementary Fig. 1G–J).
We next inactivated Agpat5 in AgRP neurons by crossing Agpat5flox/flox mice with AgRP-Cre mice (AgRPAgpat5KO mice). We confirmed that recombination took place only in the ARC (Fig. 1I) and this did not change the number of AgRP neurons, as determined by fluorescence microscopy quantification of tdTomato-positive cells in the hypothalamus of AgRPCre/+;Rosa26tdTom and AgRPAgpat5KO;Rosa26tdTom mice (Fig. 1J, K). Agpat5flox/flox and AgRPAgpat5KO mice of both sexes showed no difference in body weight gain, in glucose tolerance tests nor in insulin tolerance tests, except for a significant delay in the glycemia recovery rate in female mice (Supplementary Fig. 2A–F). The identical rates of hypoglycemia development after insulin injection in control and mutated mice (Supplementary Fig. 2E, F) also indicated that insulin sensitivity was not modified by Agpat5 inactivation in AgRP neurons. Male AgRPAgpat5KO mice had normal feeding patterns as measured over a 24 h ad libitum feeding period (Supplementary Fig. 2G). Moreover, there was no significant difference in food consumption over a 4 h refeeding period following a 16 h fast (Supplementary Fig. 2H).
We then tested the glucagon response to IIH. Mice were first injected intraperitoneally (i.p.) with saline and plasma glucagon was measured one hour after the injection. The experiment was repeated two weeks later with i.p. injection of insulin. Insulin induced the same level of hypoglycemia in Agpat5flox/flox and AgRPAgpat5KO mice of both sexes (Fig. 2A, B). However, plasma glucagon levels were significantly less increased in male and female AgRPAgpat5KO mice as compared to Agpat5flox/flox mice (Fig. 2C, D). We then performed hyperinsulinemic-hypoglycemic clamps to precisely control the level of hypoglycemia and measured the rate of glucose infusion required to maintain hypoglycemia, and the plasma glucagon concentrations at the end of the clamps. Agpat5flox/flox and AgRPAgpat5KO mice reached the exact same hypoglycemic levels (Fig. 2E) but the glucose infusion rate was higher in the mutated mice (Fig. 2F, G) and these mice secreted less glucagon (Fig. 2H). Thus, Agpat5 in AgRP neurons was required for normal hypoglycemia-induced glucagon secretion.
A, B Glycemia in Agpat5flox/flox mice and AgRPAgpat5KO mice one hour after NaCl or insulin injection; A male mice, B female mice. Plasma glucagon levels one hour after NaCl or insulin injection in Agpat5flox/flox and AgRPAgpat5KO mice C male mice, D female mice. A–D Two independent cohorts, n = 18–22 mice per genotype. Data are mean ± SEM, two-way ANOVA, *** p < 0.001 and ****p < 0.0001, two-way ANOVA with Tukey’s multiple comparisons correction. E–H Hyperinsulinemic-hypoglycemic clamps performed in Agpat5flox/flox mice and AgRPAgpat5KO male mice: E Glycemic levels. F Glucose infusion rates. Data are mean ± SEM, (repeated measurements) with Sidak’s multiple comparisons correction. G Plasma glucagon levels at the end of the clamp. Data are mean ± SEM, n = 8 mice. *p < 0.05, two-tailed, unpaired Student’s t-test.
Agpat5 inactivation reduces the number of glucose responsive AgRP neurons and vagal activity
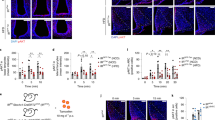
To determine whether Agpat5 was affecting the glucose sensitivity of AgRP neurons, we first quantitated c-Fos expression by immunofluorescence microscopy after saline or insulin injections. Insulin-induced hypoglycemia reached the same level in control and mutated mice (Fig. 3A). The number of c-Fos positive cells markedly increased after insulin injection in the ARC of Agpat5flox/flox mice, but not in AgRPAgpat5KO mice (Fig. 3B, C).
A–C Agpat5flox/flox and AgRPAgpat5KO male mice were injected i.p. with NaCl or insulin and their brains were collected one hour later for c-Fos immunodetection. A Glycemic levels after NaCl or insulin injections. B c-Fos immunostaining. Scale bar, 200 μm. C Quantification of c-Fos staining in the ARC. Data are mean ± SEM. c-Fos positive nuclei have been counted at three different bregmas/mouse. Two independent mouse cohorts, n = 6–7 mice per group. Data are mean ± SEM, ***p < 0.001 and ****p < 0.0001, two-way ANOVA with Tukey’s multiple comparisons correction. The glucose responsiveness of AgRP neurons of mice of the indicated genotypes was analyzed by patch clamp analysis performed on acute brain slices. AgRP neurons were identified by the expression of tdTomato. D Proportions of GI and NR neurons in mice from each genotype (AgRPCre/o+;Rosa26tdTom vs AgRPAgpat5KO;Rosa26tdTom). *p < 0.05, two-sided Fisher’s exact test. E Example of GI neurons recorded in the three indicated mouse lines. The concentration of glucose in the superfusates is indicated at the top of each recording. F Change in membrane potential induced by 0.1 mM glucose in AgRP GI neurons from each mouse genotype. G Change in membrane resistance. Data from n = 18 neurons/n = 8–10 mice per genotype. Before-after graphs display individual values. ***p < 0.001, two-tailed, paired Student’s t-test.
Next, we assessed the glucose responsiveness of AgRP neurons by patch clamp analysis. Acute brain slices were prepared from AgRPCre/+;Rosa26tdTom mice and AgRPAgpat5KO;Rosa26tdTom mice and patch clamp recordings of AgRP neurons were performed while switching the extracellular glucose concentrations from 5 mM to 0.1 mM. In AgRPCre/+;Rosa26tdTom mice 72 percent of AgRP neurons were GI neurons and the others 28 percent were glucose non-responder (NR). In AgRPAgpat5KO;Rosa26tdTom mice, the percentage of AgRP GI neurons was reduced to 33 percent of the recorded neurons and NR neurons increased to 67 percent (Fig. 3D, E). The membrane potential of the GI neurons measured in both groups of mice were similarly increased by lowering the glucose concentration from 5 to 0.1 mM glucose (Fig. 3F). Although the GI neurons membrane resistance increased upon exposure to low glucose concentrations in AgRPCre/+;Rosa26tdTom mice, no statistically significant difference was observed in AgRPAgpat5KO;Rosa26tdTom mice, possibly due to the small number of GI neurons detected in this genotype (Fig. 2G). Thus, inactivation of Agpat5 in AgRP neurons suppressed the GI response of a subset of these neurons but left unaffected the response of the remaining GI neurons.
To determine whether the glucose responsiveness of AgRP neurons was affected by Agpat5 inactivation in living mice, we performed fiber photometry recordings. AgRPCre/+ mice and AgRPAgpat5KO littermates received stereotactic injection of an AAV9-syn-FLEX-jGCamp7s-WPRE in the ARC and were implanted at the same site with an optic fiber. GCaMP7 expression in the ARC was verified by fluorescence microscopy at the end of the experiments (Fig. 4A). On the day of the experiments, mice were fasted for 5 h before initiating GCaMP7 fluorescence recording for a 30 min basal period followed by an i.p. injection of saline or insulin and a further 60 min recording period (Fig. 4B). Insulin induced the same level of hypoglycemia in both groups of mice (Fig. 4C). The GCaMP7 fluorescence signal after saline injection remained the same during the recording period in AgRPCre/+ and AgRPAgpat5KO mice (Fig. 4D), as quantitated over 10 min periods (Fig. 4E). After insulin injection the GCaMP7 signal increased in AgRPCre/+ mice with a lag period of ~40 min; this increase was, however, not observed in AgRPAgpat5KO mice (Fig. 4F, G). Thus, inactivation of Agpat5 led to a reduction in the number of AgRP neurons that can be activated by hypoglycemia as detected by c-Fos staining, patch clamp analysis, and in vivo intracellular Ca2+ recordings.
A Expression of GCaMP7 in the ARC of AgRPCre/+ mice following the stereotactic injection of AAV-FLEX-GCaMP7. Scale bar, 200 μm. B Scheme of the fiber photometry experiment. C Glycemia following NaCl or insulin injection in AgRPCre/+ and AgRPAgpat5KO mice, n = 6. Data are mean ± SEM, ****p < 0.0001, two-way ANOVA with Tukey’s multiple comparisons correction. D Recordings of the GCaMP7 signal in AgRP neurons of AgRPCre/+ and AgRPAgpatKO mice following NaCl injection. Data are mean ± SEM. E Quantification over 10 min periods of the GCaMP7 signal of (D). Data are mean ± SEM, two-way ANOVA with Sidak’s multiple comparisons correction. F Recordings of the GCaMP7 signal following insulin injection. Data are mean ± SEM. G Quantification over 10 min periods of the GCaMP7 signal of (F). Data are mean ± SEM, *p < 0.05, two-way ANOVA with Sidak’s multiple comparisons correction. Data are from three independent experiments, n = 6 mice. H Representative traces of parasympathetic nerve firing rate in Agpat5flox/flox (top) and AgRPAgpat5KO (bottom) mice in the basal state (left) and following insulin injection (right). I Glycemic levels of Agpat5flox/flox mice and AgRPAgpat5KO mice in the basal state and 1 h following insulin injection. J Quantification of vagal nerve firing in the basal state and following insulin-induced hypoglycemia, n = 6–8 mice. I, J Data are mean ± SEM, **p < 0.01, ***p < 0.001 and ****p < 0.0001, two-way ANOVA with Tukey’s multiple comparisons correction.
As vagal nerve activation during hypoglycemia stimulates glucagon secretion15, we recorded vagal nerve activity in Agpat5flox/flox and AgRPAgpat5KO littermates under basal conditions for 30 min and for one hour after i.p. injection of insulin (Fig. 4H). The glycemic levels upon insulin injection were similar between the two groups (Fig. 4I). Vagal activity increased 2-fold in Agpat5flox/flox mice ~40–50 min after insulin injection, but not in AgRPAgpat5KO animals (Fig. 4J). Thus, reduced AgRP activation by hypoglycemia in AgRPAgpat5KO mice led to impaired vagal nerve firing and reduced glucagon secretion.
Agpat5 controls FAO and ATP production
We next investigated how Agpat5 could interfere with glucose sensing by AgRP neurons. Agpat5 is associated with mitochondria and catalyzes the production of PA from LPA and fatty acyl-CoAs13. PA has been described as a competitive inhibitor of dynamin-related protein-1 (Drp1), the main regulator of mitochondrial fission, preventing mitochondrial network fragmentation16,17. As mitochondrial dynamics in AgRP and POMC neurons has previously been reported to control their glucose responsiveness18,19,20 we first assessed whether Agpat5 inactivation could lead to changes in mitochondrial morphology. Electron microscopy analysis of mitochondria in AgRP neurons of AgRPCre/o+;Rosa26tdTom and AgRPAgpat5KO;Rosa26tdTom male mice (Fig. 5A) revealed that mitochondrial area and perimeter cumulative distributions were identical in both groups of mice (Fig. 5A–C). Thus, a role for Agpat5 in controlling mitochondrial dynamics was not supported.
Mitochondria morphology was analyzed in AgRP neurons of AgRPCre/+;Rosa26tdTom mice and of AgRPAgpatKO;Rosa26tdTom littermate mice in ad libitum fed state. A Electron microscopy pictures of AgRP neurons of AgRPCre/+;Rosa26tdTom mice and AgRPAgpatKO;Rosa26tdTom mice. AgRP neurons were identified by correlative light and electron microscopy. B Cumulative distribution of mitochondria areas. C Cumulative distribution of mitochondria perimeters,3 mice per genotype; 90 AgRP neurons; 2424–2491 mitochondria. Scale bar, 500 nm. Two-tailed, paired Student’s test. Western blot analysis of Agpat5 expression in GT1-7 cells transfected with a scrambled or two different Agpat5-specific siRNAs. D Detection of Agpat5 by immunoprecipitation followed by immunoblotting with the Agpat5 antibody. Lysates of HEK293T cells transfected with a GFP or an Agpat5GFP expression plasmid served as negative and positive controls for Agpat5, respectively. E Quantification of Agpat5 expression. n = 3 replicates. Data are mean ± SEM, *p < 0.05, **p < 0.01, one-way ANOVA with Dunnett’s multiple comparisons correction. Oxygen consumption rates (OCR) and ATP production (defined by difference between basal OCR and OCR after blocking ATP synthase with oligomycin) were measured in GT1-7 cells transfected with scrambled or Agpat5-specific siRNAs. The role of β-oxidation in ATP production was verified using the CPT1a inhibitor etomoxir or by siRNA-mediated Cpt1a silencing, n = 9–10 biological replicates. Data are mean ± SEM, *p < 0.05, one-way ANOVA with Tukey’s post hoc test. F OCR in GT1-7 cells in the absence of etomoxir and quantification of ATP production by cells. G OCR in GT1-7 cells in the presence of etomoxir and quantification of ATP production by cells. H PCR quantification of Agpat5 expression, n = 5 replicates. Data are mean ± SEM, ****p < 0.0001, one-way ANOVA with Dunnett’s multiple comparisons correction. I OCR in GT1-7 cells transfected with the scrambled siRNA, an Agpat5 siRNA, and/or a Cpt1a siRNA. J Quantification of ATP production by cells in (I), n = 5 biological replicates, n = 5 technical replicates per assay. K PCR quantification of Agpat5 and Cpt1a expression in cells used in (J), n = 3 biological replicates. J, K Data are mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001, two-way ANOVA with Tukey’s multiple comparisons correction.
Activation of AgRP neuron firing by hypoglycemia is triggered by a fall in intracellular ATP content that reduces the activity of the Na+/K+ATPase, leading to membrane depolarization10,11. Thus, in conditions of hypoglycemia, the role of Agpat5 may be to prevent fatty acyl-CoAs from entering the mitochondria for FAO ensuring that reduced ATP production reflects the decrease in blood glucose concentrations. To test this hypothesis, we evaluated whether silencing Agpat5 expression in the GT1-7 neuronal cell line would regulate mitochondrial oxygen consumption rate and ATP production. GT1-7 cells were transfected with two different Agpat5-specific siRNAs or a control siRNA. The efficacy of Agpat5 silencing was tested by western blot analysis using a newly produced and validated Agpat5 polyclonal antibody (Supplementary Fig. 3). Agpat5 was detected in GT1-7 cells using an immunoprecipitation/immunoblotting protocol (Fig. 5D); Gfp- and Agpat5Flag-transfected HEK293T cells served as negative and positive controls, respectively (Fig. 5D). Silencing Agpat5 expression by both siRNAs led to a 40–50 percent reduction in Agpat5 protein expression (Fig. 5E).
Oxygen consumption rates (OCR) measured by Seahorse analysis showed that in GT1-7 cells, mitochondrial respiration did not decrease with glucose supplementation (Fig. 5F), indicating that GT1-7 cells preferentially use mitochondrial respiration and not glycolysis for ATP generation. Silencing Agpat5 expression increased basal mitochondrial respiration and ATP production in GT1-7 cells (Fig. 5F,G). The results were consistent for both specific siRNAs, minimizing the possibility of an off-target effect (Fig. 5H).
We next tested the impact on OCR of inhibiting Cpt1 with etomoxir. Etomoxir had no effect on OCR in control siRNA-transfected cells, indicating that endogenous FAO rates are low in control GT1-7 cells (Fig. 5F, G). In contrast, etomoxir suppressed the increase in OCR and ATP production in Agpat5-silenced cells (Fig. 5F, G), indicating that Agpat5 normally prevents increased FAO. To confirm this observation, we transfected GT1-7 cells with a control, an Agpat5-specific or a Cpt1a-specific siRNA. Agpat5 and Cpt1a mRNAs expression were decreased by ≥50 percent by their respective siRNAs (Fig. 5K). Reducing Cpt1a expression had no effect on GT1-7 cells OCR. However, reducing Cpt1a expression abolished the increase in OCR and ATP production induced by Agpat5 silencing (Fig. 5I, J). These results support the hypothesis that the role of Agpat5 is to divert fatty-acyl-CoAs from Cpt1-mediated entry into mitochondria.
Inactivation of Cpt1a in AgRP Agpat5KO mice restores glucose sensing
To determine whether the reduced glucose responsiveness of AgRP neurons from AgRPAgpat5KO mice could be restored by blocking FAO, we generated AgRPAgpat5KO;Cpt1aKO mice by crossing the AgRPAgpat5KO mice with Cpt1aflox/flox mice21. We first analyzed c-Fos expression in AgRP neurons of Agpat5flox/flox;Cpt1aflox/flox mice, AgRPAgpat5KO mice, and AgRPAgpat5KO;Cpt1aKO mice one hour after insulin injection. Mice from the three genotypes showed similar glycemia before and after the insulin injections (Fig. 6B). Quantification of the number of c-Fos positive cells indicated that hypoglycemia induced a lower number of c-Fos positive cells in AgRPAgpat5KO mice than in Agpat5flox/flox;Cpt1aflox/flox mice (Fig. 6A, and compared with Fig. 3B). Importantly, AgRPAgpat5KO;Cpt1aKO mice displayed the same number of c-Fos positive AgRP neurons as Agpat5flox/flox;Cpt1aflox/flox mice (Fig. 6 A, C).
Agpat5flox/flox;Cpt1aflox/flox mice, AgRPAgpat5KO mice and AgRPAgpat5KO/Cpt1aKO mice were injected with insulin and c-Fos expression in the ARC nucleus was quantitated 1 h later. A c-Fos staining in the ARC of the three mouse lines. Scale bar, 200 μm. B Glycemic levels. C Quantification of c-Fos staining in the ARC of the three mouse lines, n = 4–5 mice, three different bregmas/animal. Mice from the three genotypes were injected with NaCl or insulin and plasma glucagon levels were measured one hour later. D Body weight. E Glycemia 1 h after i.p. NaCl or insulin injection. F Plasma glucagon levels. B–F Data are mean ± SEM, two independent cohorts, n = 10–15 mice. *p < 0.05, **p < 0.01 and ***p < 0.001, one-way (C) or two-way ANOVA with Tukey’s multiple comparisons correction (B, D–F). 5 h-fasted Agpat5flox/flox mice and AgRPAgpat5KO mice received an i.p. injection of NaCl or nicotinic acid (NA) to reduce plasma free fatty acids levels. 30 min later they received an i.p. insulin injection and plasma glucagon was measured one hour later. G Plasma free fatty acid levels before and 30 min after i.p. NA injection, n = 5 mice. H Glycemia 30 min after NaCl or NA injection and 1 h following insulin injection in NaCl or NA injected mice. I Plasma glucagon levels in the conditions described in (H). NA-induced decrease in free fatty acids restored glucagon levels in AgRPAgpat5KO mice to those of Agpat5flox/flox mice, two independent cohorts, n = 10–11 mice. G–I Data are mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001, two-way ANOVA with Tukey’s multiple comparisons correction.
We next tested whether Cpt1a inactivation in AgRP neurons affected their glucose responsiveness and glucose homeostasis. We first performed patch clamp analysis of AgRP neurons in AgRPCre/+;Rosa26tdTom mice and AgRPCpt1aKO;Rosa26tdTom male littermates. In both groups of mice the percentage of AgRP GI neurons was the same (~70 percent) (Supplementary Fig. 4A–C), and the same as observed in non-littermate AgRPCre/+;Rosa26tdTom mice (see Fig. 3D). Changes in membrane potential and membrane resistance in response to switching glucose concentrations from 5 to 0.1 mM were also identical between both groups of mice (Supplementary Fig. 4D, E). Furthermore, Cpt1aflox/flox mice and AgRPCpt1aKO littermates displayed identical glucose tolerance, insulin sensitivity, and glucagon response to insulin-induced hypoglycemia (Supplementary Fig. 4F–I). Thus, inactivating Cpt1a in AgRP neurons did not impact their glucose responsiveness nor any of the measured glucose homeostasis parameters. Importantly, however, inactivation of Cpt1a in AgRP neurons lacking Agpat5 (AgRPAgpat5KO;Cpt1aKO;Rosa26tdTom mice) restored the number of GI neurons found in control mice (Fig. 3D), which displayed similar changes in membrane potential and membrane resistance induced by low glucose concentrations.
We next assessed insulin-induced glucagon secretion. Mice from the three genotypes had the same body weight, the same basal glycemia and insulin-induced hypoglycemia (Fig. 6D, E). Glucagon secretion was significantly lower in AgRPAgpat5KO mice than in Agpat5flox/flox;Cpt1aflox/flox mice. In mice with inactivation of both Agpat5 and Cpt1a, hypoglycemia did not induce a significant reduction in glucagon secretion as compared to Agpat5flox/flox;Cpt1aflox/flox mice (Fig. 6F).
Together the above data support the hypothesis that the role of Agpat5 is to divert fatty-acyl-CoAs from entering the mitochondria to prevent an increase in ATP production that would impede neuronal firing upon hypoglycemia. A corollary of this hypothesis is that insulin-induced glucagon secretion in AgRPAgpat5KO mice should be increased if circulating free fatty acids (FFAs) could be lowered. To verify this hypothesis, we treated Agpat5flox/flox and AgRPAgpat5KO mice with nicotinic acid (NA) to block lipolysis and reduce circulating FFAs. Plasma FFAs in 6 h fasted Agpat5flox/flox and AgRPAgpat5KO male mice were the same (224.8 ± 16.5 and 228.9 ± 23.2 μM, respectively) and were similarly reduced 30 min after NA injection (133.6 ± 5.3 and 138.4 ± 16.6 μM, respectively) (Fig. 6G). Thirty minutes after saline or NA injection, the mice received an i.p. injection of insulin. The hypoglycemic levels reached one hour later did not differ between of Agpat5flox/flox and AgRPAgpat5KO mice (Fig. 6H). Plasma glucagon levels in Agpat5flox/flox mice and AgRPAgpat5KO mice were identical after saline or NA injections (Fig. 6I). After insulin injection plasma glucagon was less increased in AgRPAgpat5KO mice as compared to Agpat5flox/flox mice, as expected. However, insulin-induced hypoglycemia after NA pre-treatment led to the same plasma glucagon levels in Agpat5flox/flox mice and AgRPAgpat5KO mice (Fig. 6I). Thus, lowering circulating FFA levels corrected the glucagon secretion defect of AgRPAgpat5KO mice, in agreement with the hypothesis that reducing FAO and ATP production is required for normal hypoglycemia-induced activation of AgRP neurons and glucagon secretion.
Discussion
Here, we investigated the role of Agpat5 as a regulator of hypoglycemia-induced glucagon secretion. Our physiological studies show that inactivation of Agpat5 in AgRP neurons suppresses their activation by hypoglycemia, impairs hypoglycemia-induced vagal nerve activity, and reduces glucagon secretion. Experiments performed in a hypothalamic cell line and in mice led to the conclusion that the role of Agpat5 is to divert free fatty acids away from Cpt1a-dependent entry in the mitochondria, FAO, and ATP production. This ensures that a fall in glycemia leads to a commensurate decrease in intracellular ATP levels and AgRP neurons firing. The role of Agpat5 is, thus, to ensure that AgRP neurons respond faithfully to developing hypoglycemia.
In a previous screen of recombinant inbred BXD mice we identified two QTLs for insulin-induced glucagon secretion, one on chromosome 8 and the other on chromosome 155. Irak4, located in the QTL of chromosome 15 has been confirmed as a genetically controlled, hypothalamic regulator of glucagon secretion. We found that increased Irak4 expression, leading to increased Il-1ß signaling, reduced hypoglycemia-induced glucagon secretion5.
Agpat5 appeared as the most likely candidate regulator of hypoglycemia-induced glucagon secretion of the chromosome 8 QTL5. Interestingly, Agpat5 has also been identified in another genetic screen of BXD mice as a determinant of basal insulinemia and whole-body insulin resistance22 and cis-eQTLs were identified for liver and fat Agpat5 expression. In addition, Agpat5 silencing in these two tissues by antisense oligonucleotide treatment reduced basal insulinemia and improved glucose tolerance22. As our own screen was based on insulin-induced hypoglycemia Agpat5 could, in principle, mediate its effect on glucagon secretion by controlling insulin sensitivity and hypoglycemia development and/or on hypoglycemia sensing. A correlation between the glucagon trait and Agpat5 levels was, however, found only for Agpat5 expression in the hypothalamus and not in the liver or fat. In addition, inactivation of Agpat5 in AgRP neurons had no impact on basal insulinemia nor on insulin sensitivity as assessed in insulin tolerance tests. Furthermore, hypoglycemia induced by i.p. insulin injection or during a hypoglycemic clamp led to lower glucagon secretion in AgRPAgpat5KO than in control mice. Also, c-Fos immunolabelling and electrophysiological recordings both showed that hypoglycemia activated a lower number of AgRP neurons in AgRPAgpat5KO than in control mice. Thus, Agpat5 in AgRP neurons plays a role in hypoglycemia sensing but not in whole body insulin sensitivity. The effect of inactivating Agpat5 on glucagon secretion was seen in both male and female mice. Insulin-induced glucagon secretion shows a sexual dimorphism23,24 with a stronger response in female mice, but Agpat5 inactivation does not seem to affect differentially the glucagon secretion between sexes.
To determine the mechanisms by which Agpat5 could modify glucose sensing by AgRP neurons, we first investigated the possibility that it regulates mitochondrial dynamics. Indeed, Agpat5 is present at the surface of mitochondria and its enzymatic activity leads to the production of PA, an inhibitor of Drp-1, the main regulator of mitochondrial fission16,17. Several reports have shown that mitochondrial dynamics has an important role in controlling AgRP and POMC neurons function and physiological role18,19,25,26. For instance, inactivating Drp1 in AgRP neurons reduces body weight gain as a result of lower food intake and increases energy expenditure19. Similarly, inactivation of Mfn1 or Mfn2, which control mitochondria fusion, reduces the electrical activity of AgRP neurons of mice fed a high fat diet and leads to lower body weight gain and adiposity18. However, electron microscopy analysis of mitochondrial shape and surface area in AgRP neurons showed no impact of Agpat5 inactivation, indicating that this gene is unlikely to control mitochondrial dynamics.
We, thus, tested the possibility that Agpat5 inactivation would lead to a change in FAO. Agpat5 catalyzes the production of PA from lyso-PA and fatty acyl-CoAs; lyso-PA being itself produced from glycerol-phosphate and fatty acyl-CoAs, a reaction catalyzed by a family of glycerol-phosphate-acyltransferases (Gpat1-4)27. Previous studies in mouse liver have shown that inactivation of the mitochondria-associated Gpat1 partitions fatty acyl-CoAs towards triglyceride synthesis and away from FAO28,29 but had no effect on mitochondrial morphology28,30. Here, we found that Agpat5 silencing in GT1-7 cells increased OCR and ATP production, an effect that could be blocked by pharmacological inhibition of Cpt1a, or by silencing Cpt1a. In mice, inactivation of Cpt1a in AgRP neurons of AgRPAgpat5KO mice restored their normal activation by hypoglycemia as determined by c-Fos immunostaining and patch clamp analysis. Thus, our data show that the role of Agpat5 is to limit the entry of fatty acids into mitochondria and prevent the FAO-dependent production of ATP. This role is important as AgRP neurons firing is triggered by a fall in intracellular ATP, which reduces the activity of the Na+/K+ATPase leading to membrane depolarization10,11. It is therefore essential for these neurons to respond to hypoglycemia that circulating FFAs, which increase during fasting, do not prevent this response by inducing more ATP production. That circulating FFAs indeed blunt glucagon secretion through an Agpat5-dependent mechanism was confirmed by showing that the impaired glucagon response to hypoglycemia of AgRPAgpat5KO mice could be largely reverted when circulating free fatty acids were lowered by nicotinic acid treatment. A role for Agpat5 in adaptation to fasting is also congruent with the observation that its expression in AgRP neurons is increased two-fold by fasting31. It is interesting to note that in the experiment aimed at testing the effect of inactivating Cpt1a in AgRPAgpat5KO mice the glucagon response to hypoglycemia was only mildly reduced in the AgRPAgpat5KO mice (Fig. 6). This could be explained by the fact that the Cpt1alox/lox mice came from a different facility and were on a C57BL6/J and not C57BL6/N background. As all the mice used for this experiment were littermates their genetic backgrounds may have been slightly different from the Control and AgRPAgpat5KO mice used in the rest of the study. This indicates that the role of Agpat5 in AgRP neurons on glucagon secretion depends on epistatic interactions, in line with the results of the genetic screen performed in BXD mice.
As inactivating Agpat5 in AgRP neurons impairs hypoglycemia sensing and reduces glucagon secretion, this indicates that the AgRP GI neurons act as first order neurons. This can be contrasted to the GI neurons of the VMN. Indeed, inactivation of both α1 and α2 subunits of AMPK in VMN neurons suppresses GI neuron activity in this nucleus but does not impair hypoglycemia-induced glucagon secretion8. This can be explained by the fact that VMN GI neurons are part of a neuronal circuit that contains afferent hypoglycemia sensing neurons located in peripheral locations such as the portal vein or the parabrachial nucleus32,33. Thus, as long as first order glucose sensing neurons function normally in this circuit, the VMN neurons glucose sensing properties are dispensable for the normal counterregulatory response.
An intriguing observation is that inactivation of Agpat5 reduces the number of AgRP neurons activated by hypoglycemia without changing the electrophysiological properties of the remaining GI neurons and without reducing the total number of AgRP neurons. This suggests that AgRP GI neurons consist of different subpopulations, which differ in their requirement for Agpat5 expression for hypoglycemia sensing. These different subpopulations may project to different second order neurons as the Agpat5-dependent GI neurons are required for the normal glucagon response but not the Agpat5-independent ones. It is known that separate AgRP neuron populations project to different brain regions and have different regulatory functions. For instance, only those AgRP neurons that project to the PVN, lateral hypothalamus, bed nucleus of the stria terminalis, and paraventricular thalamus (PVT) stimulate food intake whereas those projecting to the central amygdala, and periaqueductal gray do not34. In addition, AgRPAgpat5KO mice had normal feeding patterns as measured over a 24 h ad libitum feeding period or over a 4 h refeeding period following a 16 h fast. Thus, the Agpat5-dependent GI neurons may project to nuclei that control autonomic nervous activity, such as the PVN or bed nucleus of the stria terminalis4,35 and may not be involved in feeding control. Uncovering the molecular basis for the functional diversity of AgRP neurons and identifying their projections to pre-autonomic regions will require further investigations.
In summary, our study identified Agpat5 expressed in AgRP neurons as required for hypoglycemia-sensing by a population of AgRP neurons and for efficient triggering of glucagon secretion. Agpat5 role is to divert fatty-acyl CoA away from Cpt1a-dependent entry into mitochondria to prevent FAO and ATP generation. This is necessary for intracellular ATP levels to reflect the fall in extracellular glucose concentrations since AgRP neuron firing is triggered by a decrease in Na+/K+-ATPase activity. Thus, Agpat5 serves a protective function that ensures that AgRP neurons firing reflects extracellular glucose concentrations, independently of changes in circulating free fatty acids. This is especially important during the fasting period when glucagon secretion needs to prevent hypoglycemia development but when circulating free fatty acids increase. Finally, the results of this and our previous genetic studies5,36,37,38 have led to the identification of several genes, acting in different hypothalamic and brainstem neuronal populations, which coordinate the CRR. This enhanced description of the complexity of hypothalamic hypoglycemia sensing will pave the way for new studies aimed at understanding the molecular basis of the impaired CRR of insulin-treated diabetic patients.
Methods
Ethical compliance statement
All procedures were conducted in accordance to the Swiss National Institutional Guidelines of Animal Experimentation (OExA; 455.163) with licenses approval (VD3363 and VD3686) issued by the Veterinary Office of Canton de Vaud (Vaud, Switzerland).
Mice
Experiments were performed with 5- to 20-weeks old male and female mice. Agpat5flox/flox mice were generated by genOway (genOway, Lyon, France) by homologous recombination in C57BL6/N embryonic stem cells. A conditional knockout was created by flanking exon 3 of Agpat5 with loxP sites, allowing for Cre-dependent recombination. Exon 3 contains a catalytic motif I sequence of Agpat5 protein (HXXXXD) and its deletion results in out of frame splice and introduction of premature STOP codon in exon 4. The resulting truncated mRNA product is unstable and no functional Agpat5 protein is produced. Chimeric males were crossed with C57BL6/N females to establish Agpat5flox/flox mouse line. Sim1-Cretg/+ mice were a kind gift from Dr. Daniela Cota (University of Bordeaux, France). CPT1aflox/flox mice were generated by Prof. Peter Carmeliet (Katholieke Universiteit Leuven, Belgium) and kindly donated by Dr. Marlen Knobloch (University of Lausanne, Switzerland). Agpat5flox/flox mice were crossed with AgRP-Cretg/+ or Sim1-Cretg/+ mice to generate AgRPAgpat5KO or Sim1Agpat5KO mice and control littermates. For the generation of double Agpat5 and Cpt1a knockout mice (AgRPAgpat5KO;CPT1aKO), AgRP-Cretg/+;Agpat5flox/flox;Cpt1aflox/+ male mice were bred with AgRP-Cre+/+;Agpat5flox/flox;Cpt1aflox/+ females. For electrophysiological and electron microscopy studies mice were also crossed with Rosa26-tdTomato mice in order to identify AgRP-positive neurons (AgRPCre/+;Rosa26tdTom and AgRPAgpat5KO;Rosa26tdTom littermates). All mice were back-crossed on a C57BL/6N background for at least 6 generations. All studies used age-matched littermates, randomly assigned to experimental groups. Animals were housed on a 12 h light/dark cycle, at a temperature range of 22–24 °C with the relative humidity kept at 45–55%, with ad libitum access to water and food (standard chow SP-150; SAFE, Germany). Mice animal welfare was regularly monitored. For euthanasia, mice were first anaesthetized with isoflurane before by cervical dislocation.
Mouse genotyping
Mouse genotyping was performed by PCR analysis (Agpat5 wild-type allele forward- CTACTTTGCTCAGGTAAACTTTGTCTTTGCCC, reverse-CTGAAAATTGTCCTTCGACCTCACATGC; Agpat5 lox knock-in allele forward- CTGAAAATTGTCCTTCGACCTCACATGC, reverse-TCCTACATAGTTGGCAGTGTTTGGGGC; AgRP-Cre forward-CCGCAGGTGTAGAGAAGGCA, reverse-CATCCTTAGCGCCGTAAATCAATC; Agpat5 3exon excision forward-GGTCTCCAACTCCTAATCTCAGGTGATCTACC, reverse-CTGCTACTGGACTTAAATATACGGACAACCAGC sequence primers. Analysis of genetic recombination in brain regions was performed with DNA extracted (DNeasy Kit, cat: 69504; Qiagen) from micro-dissected 1 mm-thick tissue sections.
eQTL mapping
eQTL mapping for Agpat5 was performed using GeneNetwork (mm9 genome) using hypothalamic RNASeq data obtained from the 36 BXD mouse lines used for QTLs mapping of the glucagon secretion trait5. The RNASeq data from subcutaneous white fat and liver from BXD mice have been previously published39,40.
Immunofluorescence microscopy and in situ hybridization
Mice were deeply anesthetized with isoflurane and thensubjected to a trans-cardiac perfusion of 0.9% NaCl solution followed by 4% cold paraformaldehyde (PFA) in sodium phosphate (PB) buffer (0.1 M, pH 7.4). Brains were isolated and kept for 3 h in 4% PFA at 4 °C followed by overnight incubation in sucrose 20% at 4 °C and freezing in dry-ice. Samples were stored at −80 °C until 25 μm cryo-sections were prepared and placed on glass slides. Slides were washed three times with PBS and incubated in a blocking buffer solution (4% (v/v) of normal goat serum (NGS) and 0.3% Triton X-100 in PBS) for 1 h. Then, slides were incubated with a monoclonal rabbit anti-cFos antibody (#2250, 1/7000, Cell Signaling, Danvers, USA) diluted 1:1000 in blocking solution for 16 h at room-temperature, washed three times with PBS and incubated in AlexaFluor568-conjugated goat anti-rabbit IgG antibodies (#A-11011, Invitrogen) diluted 1:500 in blocking solution for 3 h. Nuclei were stained using DAPI nuclear stain (D1306, Invitrogen) diluted 1:10,000 in PBS for 10 min in the dark. Slides were mounted in Mowiol for fluorescence imaging and imaged using Zeiss Axio Imager M2 microscope, equipped with ApoTome.2 and a Camera Axiocam 702 mono (Zeiss, Oberkochen, Germany). For each mouse, images of the ARC at 3 bregmas were taken and c-Fos-positive cells were counted using ImageJ.
For stereological analysis of AgRP/tdTomato-positive cell numbers in AgRPCre/+;Rosa26tdTom and AgRPAgpat5KO;Rosa26tdTom, three 70 μm-thick ARC sections at three different bregmas were used. Slides were imaged using z-stack with 40× objective and cell number was quantified along the z-stack using ImageJ.
For in situ hybridization analysis, the hypothalamus was dissected and fixed for 28 h in 10% formalin before embedding in paraffin. Five μm sections were prepared and in situ hybridization for Agpat5 mRNA (#514011, bio-techne, UK) was processed using the RNAscope and RNAscope 2.0 HD Red Detection Kit Assay (Advanced Cell Diagnostics) and counterstained with Mayer’s hematoxylin. Sections were mounted using Aquatex mounting medium (# 363123S, VWR), and imaged using an Axio Imager D1 (Zeiss) microscope interfaced with AxioVision software (Zeiss).
Transmission electron microscopy analysis
Male AgRPCre/+;Rosa26tdTom and AgRPAgpat5KO;Rosa26tdTom (12 weeks old, ad libitum fed) were subjected to a trans-cardiac perfusion of fresh cold fixative solution (1% glutaraldehyde/2% cold PFA in 0.1 M phosphate buffer, pH 7.4). Brains were isolated and kept overnight in the fixative solution. After two washes with PB solution, 70 μm coronal sections containing ARC were prepared using a vibratome (VT1000S, Leica) and tdTomato-positive fluorescent cells were imaged using a ZEISS Axio Imager.M2 microscope, equipped with ApoTome.2 and a Camera Axiocam 702 mono using the AxioVision software (Zeiss, Oberkochen, Germany). Sections were osmicated (1.5% osmium tetroxide) for 1 h followed by 1 h incubation in 1% (wt/vol) tannic acid in 0.1 M PB buffer. Samples were then dehydrated with ethanol solutions (1% uranyl acetate in 70% ethanol for 30 min) and flat-embedded in Epon-araldite. Trimmed blocks were used to cut ultrathin sections that were examined using a Philips CM-10 electron microscope41. Electron microscopy images were cross-referenced with immunofluorescent images of tdTomato-positive AgRP neurons to identify the cell population of interest. Mitochondrial morphology in AgRP neurons was analyzed using ImageJ software, quantifying mitochondrial area and perimeter in tdTomato-positive AgRP neurons42.
Stereotaxic surgery and fiber photometry
Stereotaxic surgeries were performed under ketamine/xylazine anaesthesia. 8 week-old male mice were placed on a stereotaxic frame (Stoelting, Wood Dale, IL, USA) and the surface of the skulls was visualized with a digital microscope (DMS 300, Leica Microsystems GmbH, Wetzlar, Germany). Recombinant adeno associated virus (AAV) were infused unilaterally in ARC (coordinates relative to Bregma: −1.7 mm anteroposterior, −0.30 mm mediolateral, and −5.8 mm dorsoventral) at a rate of 100 nl/min using a 33-gauge stainless steel injector (Hamilton Company, Reno, NV, USA) and a microsyringe pump controller (Micro4, World Precision Instruments, Sarasota, FL, USA). AAV9-syn-FLEX-jGCamp7s-WPRE was purchased from Addgene (Cat#104491-AAV9). Optical fiber cannulas (CFML12U, Thorlabs Inc. Newton, NJ, USA) were positioned unilaterally in ARC (coordinates relative to Bregma: −1.7 mm anteroposterior, −0.30 mm mediolateral, and −5.8 mm dorsoventral) and fixed to the skull with surgical screws (P1 Technologies, Roanoke, VA, USA), tissue adhesive (VetBondTM; 3MTM, Saint Paul, MN, USA) and dental cement (Paladur; Kulzer GmbH, Hanau, Germany). Mice were placed in individual cages on heating pads (at 37 °C) for 1–2 h after surgery in order to prevent hypothermia. Animals were allowed to recover for 2 weeks in individual cages with daily handling and body weight monitoring.
The inhibition of GCaMP7 fluorescence upon refeeding was used as a positive control for the responsiveness of AgRP neurons and validation of cannula implantation. For this, naïve mice were fasted for 16 h (overnight) and presented with Chow pellets, and GCaMP7 signal reduction was assessed. For i.p. 0.9% NaCl and insulin (0.8 U/kg) injections, mice were fasted for 5 h, GCaMP7 baseline activity was recorded for 30 min before and for 1 h after the injection. Glycemia was measured at the beginning of recording and 1 h after the injection. Animals were allowed to recover for 5–7 days between recordings.
Fiber photometric recordings were performed using optical components from Doric lenses controlled by Tucker Davis Technologies fiber photometry processor RZ5P, with Doric software used for data acquisition. Emission in 405 nm channel was used as an isosbestic control and GCaMP7-specific emission was recorded in 465 nm channel. The standard fiber photometry analysis pipeline was automated using a Python code and Jupyter interface. Briefly, raw traces were uploaded and the isosbestic channel was subtracted from the GCaMP7 channel, photo-bleaching was removed by subtracting an exponential fit curve, and data was presented as ΔF/F and Z-score. Recordings were normalized to 10 min baseline before the i.p. NaCl/insulin injection, butterworth low-pass filter at 1 Hz was applied to remove high-frequency noise and 500-step decimation was applied to the original signal (recorded at 123 Hz) to facilitate statistical analysis. The code is available at https://gitlab.com/pcanilho/fiber-photometry-analysis.
Electrophysiology
Glucose responsiveness of ARC AgRP neurons was assessed by electrophysiology. Five to 7 weeks old ad libitum fed male mice were deeply anesthetized with isoflurane before decapitation, and 250 μm coronal sections containing ARC were sliced using a vibratome (VT1000S, Leica). Electrophysiological recordings were conducted in slices were placed in a submerged-type recording chamber and continuously superfused with extracellular solution at room temperature38. tdTomato-positive neurons were identified by fluorescence microscopy. Whole-cell recordings were performed in current-clamp mode using a MultiClamp 700B amplifier coupled to a 1440A Digidata digitizer (Molecular Devices). Neurons exhibiting an access resistance over 25 MΩ or changed by more than 20% during the recording were discarded from analysis. A hyperpolarization step (−20 pA, 500 ms) was applied every 30 s to assess membrane resistance. Membrane potential and resistance were monitored over time under 5 mM (normoglycemia) or 0.1 mM (hypoglycemia) extracellular glucose concentration after a 10–15 min baseline. Signals were digitized at 10 kHz, collected and analyzed using the pClamp 10 data acquisition system (Molecular Devices). Membrane potential and resistance values during the last 2.5–3 min (5–6 data points) of the low glucose application (0.1 mM) were compared to 5–6 data points monitored during baseline condition (5 mM glucose). Using a two-tailed paired t-test, a neuron was considered as GI if it exhibited a significant increase of its membrane potential (depolarization) after switching to 0.1 mM glucose and if it recovered its initial membrane potential when glucose was switched back to 5 mM. Significance was assessed using a paired t-test.
Autonomous nervous system activity recording
Unipolar parasympathetic activity was recorded in 5 h-fasted 10–13 weeks old mice, measuring the firing rate activities of the thoracic branch of the vagus nerve along carotid artery43. Recordings were performed for one and half hour under isoflurane anesthesia (for 30 min during basal condition then for 1 h following i.p. insulin (0.8 U/kg) injection) using the LabChart 8 software (AD Instrument, Oxford, UK). Data were digitized with PowerLab 16/35 (AD Instrument, Oxford, UK). Signals were amplified 105 times and filtered using 100/1000 Hz band pass filter. Firing rate analysis was performed using LabChart 8.
Glucose and insulin tolerance tests
Glucose tolerance test was performed in 8 weeks old male and female mice injected with i.p. glucose (2 g/kg) after 16 h fasting. Insulin tolerance was assessed in 12–13 weeks old male and female mice injected with i.p. insulin (0.8U/kg) after 5 h fasting, and glycemia was measured from tail blood taken at the indicated times after injection44.
Glucagon secretion and hyperinsulinemic-hypoglycemic clamp
Glucagon secretion was assessed in 10 weeks old male and female mice subjected to insulin-induced hypolgycemia (IIH). Mice were fasted for 6 h and blood was collected 1 h after i.p. saline or insulin (0.8 U/kg) injection into micro-centrifuge tubes containing aprotinin-A/EDTA. Inadequate i.p. injection or health problem were used as exclusion criteria. Plasma glucagon concentrations were assessed by ELISA (Mercodia, Uppsala, Sweden). Nicotinic acid (NA, 32 mg/kg, i.p.) injection was performed in 5 h-fasted males between 11 and 15 weeks of age. Free fatty acids (FFA) concentration in plasma was assessed using FFA Assay kit (abcam, #ab65341). Hyperinsulinemic-hypoglycemic clamps were performed in 15–16 weeks-old mice, with a 33% glucose solution being infused for 150 min through either the portal or femoral vein at an insulin injection rate of 18 mU/kg/min45, after 5 h of fasting.
Cell culture
GT1-7 cells were a kind donation from Dr. Pamela L. Mellon’s laboratory. GT1-7 cells were cultured (37 °C, 5% CO2) in DMEMGlutaMax (25 mM glucose, 1 mM sodium pyruvate, 2 mM L-glutamate) supplemented with FBS 10% (v/v) and horse serum 5% (v/v). HEK293T cells were purchased from ATCC (Manassas, USA). For mitochondrial function experiments, confluent GT1-7 cells were cultured for 16 h in 0.1 mM glucose, serum-free DMEM supplemented with 25 mM mannitol to control for osmolality.
Cells transfection
GT1-7 cells (∼70% confluence) or HEK293T cells (∼80% confluence) in six-well plates were incubated in 0.9 ml/well Opti-MEM dishes. Plasmid DNA–Lipofectamine 3000 complexes (150 µl of 2 µg plasmid DNA, 2.5% (v/v) Lipofectamine 3000, 1% P300 (v/v)) for HEK293T cells and/or siRNA-Lipofectamine RNAiMAX (150 µl of 200 nM siRNA, 2.5% (v/v) Lipofectamine RNAiMAX) for HEK293T and GT1-7 cells were added dropwise to each well. Cells were incubated at 37 °C for 3 h before the transfection media were supplemented with complete culture media and cultured for 48 h.
Preparation of cell lysates
Cells were washed with phosphate buffered saline (PBS, Gibco, 10010023) prior to the addition of ice-cold lysis buffer (50 mM Tris-HCl, pH 7.4 at 4 °C, 150 mM NaCl, 50 mM NaF, 1 mM Na4P2O7, 1 mM EDTA, 1 mM EGTA, 1% (v/v) Triton-X-100, 250 mM mannitol, 1 mM DTT, 1 mM Na3VO4, 0.1 mM benzamidine, 0.1 mM phenylmethylsulphonyl fluoride). Cell lysates were scraped into microcentrifuge tubes, incubated on ice for 20 min, centrifuged (5 min, 21,910 × g, 4 °C) and the subsequent supernatants stored at −20 °C. Lysate protein concentrations were determined using bicinchoninic acid (BCA) method.
Immunoprecipitation
Agpat5 and GFP were immunoprecipitated from 0.5 mg of cell lysate using 2 μl of anti-Agpat5 or anti-GFP antiserum, or pre-immunization rabbit serum in 500 μl IP (immunoprecipitation) buffer (50 mM Tris-HCl, pH 7.4 at 4 °C, 150 mM NaCl, 50 mM NaF, 1 mM Na4P2O7, 1 mM EDTA, 1 mM EGTA, 0.1% (v/v) Triton-X-100, 1% (v/v) glycerol, 1 mM DTT, 1 mM Na3VO4, 0.1 mM benzamidine, 0.1 mM phenylmethylsulphonyl fluoride) in a 1.5 ml microcentrifuge tube. After overnight agitation at 4 °C, Protein A agarose beads were added and incubated for 1 h with agitation. Samples were centrifuged for 30 s in a bench top centrifuge and the supernatants were collected. Beads were washed four times with 1 ml IP buffer. After the final wash, excess IP buffer was removed and 2 μl of 4X SDS-PAGE sample buffer added to each sample.
Western blot analysis
Proteins were resolved by SDS-PAGE, transferred to nitrocellulose membranes and immunodetected with antibodies diluted in TBS (Tris-Buffered saline, 20 mM Tris-HCl, 150 mM NaCl, pH 7.4) containing 0.1% (v/v) Tween-20 and 5% (w/v) (TBSt) BSA46. Briefly, nitrocellulose membranes were incubated for 16 h at 4 °C with a custom-generated, affinity-purified rabbit anti-Agpat5 antibody (1/1000 in 3% BSA/PBS (w/v), BioTem, France) and a mouse anti-α-tubulin (1/5000 in 3% BSA/PBS (w/v), T5168, Sigma-Aldrich). Then, membranes were washed and incubated with secondary antibodies for 1 h at room temperature, using infrared dye-labeled (donkey anti-rabbit #926-32213 or donkey anti-mouse #926-48072, LI-COR, Cambridge, UK) secondary antibodies (in 3% BSA, TBSt, for 1 h). Proteins were then revealed using a FusionXF (Vilber, France) infrared imaging system and densitometric analysis of band intensity was performed with ImageJ.
Mitochondrial respiration and assessment mitochondrial of fatty acid β-oxidation
Oxygen consumption rate (OCR) in GT1-7 cells was measured using a Seahorse XFe24 or a Seahorse XFe96 instrument. GT1-7 cells were seeded into 96-well plates at a density of 5 × 104 cells/well or 24-well plates at a density of 2 × 105 cells/well. When 70% confluent, cells were transfected with siRNAs, as described above. After 48 h, the medium was changed to 0.1 mM glucose DMEM and then incubated in phenol-free DMEM medium (0.1 mM glucose, 1 mM pyruvate, 1.5 mM L-carnitine, 2 mM L-glutamine, pH 7.4, HEPES-free), with vehicle or etomoxir (50 μM) for 1 h at 37 °C prior to the assay. Basal respiration was recorded for 30 min, and following the sequential addition of glucose (5 mM), oligomycin (1 µM), FCCP (1 µM), rotenone (0.5 µM) and antimycin A (1 µM) to assess maximal and non-mitochondrial respiration47. Oxygen consumption was normalized to total cellular protein levels. Analysis was performed using Wave Softwarev2.4.2 (Seahorse, Agilent).
RNA extraction and gene expression analysis
RNA was extracted from GT1-7 cells or micro-dissected brain tissues using a RNeasy kit (Qiagen, Hombrechtikon, Switzerland) and reverse-transcribed (1 µg RNA) using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems). qPCR was performed with an Applied Biosystems ABI-PRISM 7500Fast Sequence Detection System. Agpat5 gene expression was normalized to β-actin using PowerUp Sybr green master mix (Applied Biosystems) and custom oligonucleotide primers (Agpat5 forward- GCTTCCGCTGTATGGGTTCT, reverse-CATCGGTGTTCCTGCGTTCA; Cpt1a forward- ACGTTGGACGAATCGGAACA, reverse-GGTGGCCATGACATACTCCC; and β-actin forward-CTAAGGCCAACCGTGAAAAGAT, reverse-CACAGCCTGGATGGCTACGT). Relative levels of mRNA were assessed using the ΔΔCt method.
Statistical analysis
All data is expressed as mean ± SEM. Prism software 9.0.1 (GraphPad Software, San Diego, CA) was used to perform student t-tests, one or two-way ANOVA followed by an appropriate post hoc test, where appropriate, with p < 0.05 considered significant. For comparison between proportions of different groups of glucose-sensing neurons in electrophysiological experiments Fisher’s exact test was used, with p < 0.05 considered significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The raw data (Fastq files) for hypothalamus RNAseq data used in this study are available in the Gene Expression Omnibus (GEO) database under accession code GSE87586. The data sets for liver40 and white adipose tissue39 RNAseq used in this study are available in the Gene Expression Omnibus (GEO) database under accession code GEO: GSE114845 and GEO: GSE79016, respectively. The mouse metabolic phenotyping and electrophysiology data generated in this study are provided in the Source Data file. The raw data for each individual experiment are available upon request to the corresponding author. Source data are provided with this paper.
Code availability
The code for fiber photometric data analysis is available at https://gitlab.com/pcanilho/fiber-photometry-analysis.
References
Steinbusch, L., Labouebe, G. & Thorens, B. Brain glucose sensing in homeostatic and hedonic regulation. Trends Endocrinol. Metab. 26, 455–466 (2015).
Verberne, A. J., Sabetghadam, A. & Korim, W. S. Neural pathways that control the glucose counterregulatory response. Front. Neurosci. 8, 38 (2014).
Thorens, B. Neuronal regulation of glucagon secretion and gluconeogenesis. J. Diabetes Investig. 13, 599–607 (2022).
Stanley, S., Moheet, A. & Seaquist, E. R. Central mechanisms of glucose sensing and counterregulation in defense of hypoglycemia. Endocr. Rev. 40, 768–788 (2019).
Picard, A. et al. Hypothalamic Irak4 is a genetically-controlled regulator of hypoglycemia-induced glucagon secretion. Mol Metab. 61, 101479 (2022).
Winkler, Z. et al. Hypoglycemia-activated hypothalamic microglia impairs glucose counterregulatory responses. Sci. Rep. 9, 6224 (2019).
Lieu, L. et al. Effects of metabolic state on the regulation of melanocortin circuits. Physiol. Behav. 224, 113039 (2020).
Quenneville, S. et al. Hypoglycemia-sensing neurons of the ventromedial hypothalamus require AMPK-induced Txn2 expression but are dispensable for physiological counterregulation. Diabetes 69, 2253–2266 (2020).
Hirschberg, P. R., Sarkar, P., Teegala, S. B. & Routh, V. H. Ventromedial hypothalamus glucose-inhibited neurones: A role in glucose and energy homeostasis? J. Neuroendocrinol. 32, e12773 (2020).
Silver, I. A. & Erecinska, M. Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J. Neurophysiol. 79, 1733–1745 (1998).
Kurita, H. et al. Arcuate Na+,K+-ATPase senses systemic energy states and regulates feeding behavior through glucose-inhibited neurons. Am. J. Physiol. Endocrinol. Metab. 309, E320–E333 (2015).
Flak, J. N. et al. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat. Neurosci. 17, 1744–1750 (2014).
Prasad, S. S., Garg, A. & Agarwal, A. K. Enzymatic activities of the human AGPAT isoform 3 and isoform 5: Localization of AGPAT5 to mitochondria. J. Lipid Res. 52, 451–462 (2011).
Chen, R., Wu, X., Jiang, L. & Zhang, Y. Single-cell RNA-Seq reveals hypothalamic cell diversity. Cell Rep. 18, 3227–3241 (2017).
Taborsky, G. J. Jr. & Mundinger, T. O. Minireview: The role of the autonomic nervous system in mediating the glucagon response to hypoglycemia. Endocrinology 153, 1055–1062 (2012).
Adachi, Y. et al. Coincident phosphatidic acid interaction restrains Drp1 in mitochondrial division. Mol. Cell 63, 1034–1043 (2016).
Adachi, Y., Iijima, M. & Sesaki, H. An unstructured loop that is critical for interactions of the stalk domain of Drp1 with saturated phosphatidic acid. Small GTPases 9, 472–479 (2018).
Dietrich, M. O., Liu, Z. W. & Horvath, T. L. Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell 155, 188–199 (2013).
Jin, S. et al. Drp1 is required for AgRP neuronal activity and feeding. Elife 10, e64351 (2021).
Ramirez, S. et al. Mitochondrial dynamics mediated by mitofusin 1 is required for POMC neuron glucose-sensing and insulin release control. Cell Metab. 25, 1390–1399.e6 (2017).
Schoors, S. et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 520, 192–197 (2015).
Parks, B. W. et al. Genetic architecture of insulin resistance in the mouse. Cell Metab. 21, 334–346 (2015).
Steinbusch, L. K. et al. Sex-specific control of fat mass and counterregulation by hypothalamic glucokinase. Diabetes 65, 2920–2931 (2016).
Mahmood, A. S. M. H. et al. Sex differences in forebrain estrogen receptor regulation of hypoglycemic patterns of counter-regulatory hormone secretion and ventromedial hypothalamic nucleus glucoregulatory neurotransmitter and astrocyte glycogen metabolic enzyme expression. Neuropeptides 72, 65–74 (2018).
Santoro, A. et al. DRP1 suppresses leptin and glucose sensing of POMC neurons. Cell Metab. 25, 647–660 (2017).
Schneeberger, M. et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 155, 172–187 (2013).
Wendel, A. A., Lewin, T. M. & Coleman, R. A. Glycerol-3-phosphate acyltransferases: Rate limiting enzymes of triacylglycerol biosynthesis. Biochim. Biophys. Acta 1791, 501–506 (2009).
Hammond, L. E. et al. Mitochondrial glycerol-3-phosphate acyltransferase-1 is essential in liver for the metabolism of excess acyl-CoAs. J. Biol. Chem. 280, 25629–25636 (2005).
Wendel, A. A., Cooper, D. E., Ilkayeva, O. R., Muoio, D. M. & Coleman, R. A. Glycerol-3-phosphate acyltransferase (GPAT)−1, but not GPAT4, incorporates newly synthesized fatty acids into triacylglycerol and diminishes fatty acid oxidation. J. Biol. Chem. 288, 27299–27306 (2013).
Hammond, L. E. et al. Increased oxidative stress is associated with balanced increases in hepatocyte apoptosis and proliferation in glycerol-3-phosphate acyltransferase-1 deficient mice. Exp. Mol. Pathol. 82, 210–219 (2007).
Cedernaes, J. et al. Transcriptional basis for rhythmic control of hunger and metabolism within the AgRP neuron. Cell Metab. 29, 1078–1091 (2019). e5.
Garfield, A. S. et al. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metab. 20, 1030–1037 (2014).
Matveyenko, A. V. & Donovan, C. M. Metabolic sensors mediate hypoglycemic detection at the portal vein. Diabetes 55, 1276–1282 (2006).
Betley, J. N., Cao, Z. F., Ritola, K. D. & Sternson, S. M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350 (2013).
Meek, T. H. et al. Functional identification of a neurocircuit regulating blood glucose. Proc. Natl Acad. Sci. USA 113, E2073–E2082 (2016).
Picard, A. et al. Fgf15 neurons of the dorsomedial hypothalamus control glucagon secretion and hepatic gluconeogenesis. Diabetes 70, 1443–1457 (2021).
Picard, A. et al. A genetic screen identifies hypothalamic Fgf15 as a regulator of glucagon secretion. Cell Rep. 17, 1795–1806 (2016).
Lamy, C. M. et al. Hypoglycemia-activated GLUT2 neurons of the nucleus tractus solitarius stimulate vagal activity and glucagon secretion. Cell Metab. 19, 527–538 (2014).
Jha, P. et al. Genetic regulation of plasma lipid species and their association with metabolic phenotypes. Cell Syst. 6, 709–721 (2018). e6.
Diessler, S. et al. A systems genetics resource and analysis of sleep regulation in the mouse. PLoS Biol. 16, e2005750 (2018).
Kolotuev, I. Positional correlative anatomy of invertebrate model organisms increases efficiency of TEM data production. Microsc. Microanal. 20, 1392–1403 (2014).
Toda, C. et al. UCP2 regulates mitochondrial fission and ventromedial nucleus control of glucose responsiveness. Cell 164, 872–883 (2016).
Magnan, C. et al. Lipid infusion lowers sympathetic nervous activity and leads to increased beta-cell responsiveness to glucose. J. Clin. Invest. 103, 413–419 (1999).
Tarussio, D. et al. Nervous glucose sensing regulates postnatal beta cell proliferation and glucose homeostasis. J. Clin. Invest. 124, 413–424 (2014).
Burcelin, R., Da Costa, A., Drucker, D. & Thorens, B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes 50, 1720–1728 (2001).
Modi, H. et al. Autocrine secretion of IGF2 regulates adult β cell functional plasticity. Diabetes, 64, 4148–4157 (2014).
Doddaballapur, A. et al. Laminar shear stress inhibits endothelial cell metabolism via KLF2-mediated repression of PFKFB3. Arterioscler. Thromb. Vasc. Biol. 35, 137–145 (2015).
Acknowledgements
We thank Prof. Paul Franken (UNIL, Lausanne) for liver RNAseq data. We are grateful to the Mouse Metabolic Analysis facility (MEF) of CIG, University of Lausanne for hyperinsulinemic-hypoglycemic clamp experiments and to the Electron Microscopy Facility (EMF) of University of Lausanne for transmission electron microscopy sample processing and imaging. We thank Prof. Peter Carmeliet (Katholieke Universiteit Leuven, Belgium), and Prof. Marlen Knobloch (University of Lausanne) for Cpt1aflox/flox mice. We are grateful to Dr. Sevasti Gaspari, Dr. Sophie Croizier, Dr. Simon Quenneville, Dr. Isabel C. Lopez-Mejia, Paulo Canilho and Manon Gervais for useful discussions and technical guidance for this study. We are also grateful to Dr. Quenneville for cloning of murine Agpat5 cDNA. This work was supported by a European Research Council Advanced Grant (INTEGRATE, No. 694798) and a Swiss National Science Foundation grant (310030-182496) to B.T., and has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 777460 (HypoRESOLVE). The JU receives support from the European Union’s Horizon 2020 research and innovation program and EFPIA and T1D Exchange, JDRF, International Diabetes Federation (IDF), The Leona M. and Harry B. Helmsley Charitable Trust.
Author information
Authors and Affiliations
Contributions
B.T. conceived the project. B.T. and A.S. designed and performed the experiments and interpreted the data. A.S., G.L., A.P., X.P.B., and D.T. performed experiments and analyzed data. M.J. performed bioinformatic analysis. B.T. and A.S. co-wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Tamas Horvath and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Strembitska, A., Labouèbe, G., Picard, A. et al. Lipid biosynthesis enzyme Agpat5 in AgRP-neurons is required for insulin-induced hypoglycemia sensing and glucagon secretion. Nat Commun 13, 5761 (2022). https://doi.org/10.1038/s41467-022-33484-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-33484-6
- Springer Nature Limited
This article is cited by
-
Disrupted hypothalamic transcriptomics and proteomics in a mouse model of type 2 diabetes exposed to recurrent hypoglycaemia
Diabetologia (2024)
-
Metabolic Messengers: glucagon
Nature Metabolism (2023)
-
Inhibition of c-Jun in AgRP neurons increases stress-induced anxiety and colitis susceptibility
Communications Biology (2023)