Abstract
Cyclobutanols are privileged cyclic skeletons in natural products and synthetic building blocks. C(sp3)−H functionalization is a prolonged challenge in organic synthesis. The synthesis of cyclobutanols through double C(sp3)-H bond functionalization remains elusive. Here we report the efficient synthesis of cyclobutanols through intermolecular radical [3 + 1] cascade cyclization, involving the functionalization of two C − H bonds through sequential hydrogen atom transfer. The copper complex reduces the iodomethylsilyl alcohols efficiently under blue-light irradiation to initiate the tandem transformation. The mild reaction tolerates a broad range of functional groups and allows for the facile generation of elaborate polycyclic structures.
Similar content being viewed by others
Introduction
Cyclic structures are fundamental in organic chemistry. Among these structures, cyclobutanols are useful cyclic skeletons frequently observed in natural products1 and widely serve as the targets for carbon−carbon bond cleavage chemistry because of the ring strain2. New methods for the expedient synthesis of diversified cyclobutanols would offer opportunities to related fields.
Tandem radical cyclizations have become a powerful synthetic method to generate complicated and useful carbon skeletons, featuring the formation of one or more consecutive rings in a single step3,4. The utinity of this strategy has been demonstrated in numerous synthesis of complex natural products, including linear- and angular-fused triquinianes5, steroids6, milbemycin7, prostaglandins8, (-)-Maoecrystal Z9, scholarisine A10, barbiturates11, and ophiobolin sesterterpene12. Visible-light photoredox chemistry is an area seeing tremendous growth as it notably provides mild reaction conditions for the generation of radicals13,14,15,16, rendering many elegant intramolecular17,18 and intermolecular [2 + 2]-19,20, [3 + 2]-21,22, [4 + 2]-23,24, and [2 + 2 + 2]-cyclization25 reactions possible using iridium (Ir)- or ruthenium (Ru)-based visible-light sensitizers.
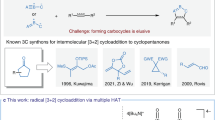
Vinyl radical initiated 1,n-hydrogen atom transfer (HAT) and cyclization is another powerful way to construct a wide range of five-membered rings, pioneered by the works of Heiba and Dessau26, Curran27, Parsons28, Renaud29, and others (Fig. 1a, b)30,31,32,33,34. In most cases, however, intramolecular versions have dominated and have necessitated that vinyl halides or pseudohalides usually require multistep synthesis. The generation of cyclic skeletons other than five-membered rings is rare. We envisioned the possibility of the in situ generation of vinyl radicals in an intermolecular manner, and if followed by HAT and cyclization, a different ring structure could potentially be furnished. The success of this hypothesis relied on a new combination of radical precursor and reactant.
a Vinyl radical triggered by activation of C(sp2)-X initiated HAT and cyclization. b Vinyl radical triggered by radical addition to alkyne initiated HAT and cyclization. c Remote C − H functionalization via HAT enabled by an iodomethylsilyl auxiliary. d Photoinduced copper-catalyzed sequential 1,n-HAT enabling the formation of cyclobutanols.
For activated C − X bonds, such as polyhalogenated alkanes35, α-halocarbonyl compounds36, geminal halogenated sugars37, and benzyl bromides bearing strong electron-withdrawing groups38 have been used to generate radical intermediates under photoredox conditions. The use of substrates containing unactivated halogen−carbon bonds is less studied because of the relatively high reduction potential and putative reactivity of the resulting unstabilized sp3 carbon radical. We have been interested in copper (Cu)-photocatalysis using aliphatic halide as the radical precursor39,40,41. Cu complexes have displayed highly tunable redox properties in their excited states, diverse ligand types, and the free conversion of multiple oxidation states42,43,44,45. Taking advantage of the silicon effect for the generation and translocation of α-carbon radicals27,46, Gevorgyan recently advanced a series of works on the direct γ-amination and γ-vinylation of alcohol derivatives through 1,6-HAT using (halomethyl)silyl auxiliaries (Fig. 1c)47,48,49,50. Despite this progress, as far as we know, examples of the use of readily available aliphatic halides as the radical precursor under visible-light photocatalysis for intermolecular cascade cyclization en route to four-membered rings are lacking. During our investigation on photoinduced Cu-catalyzed remote C − H alkynlylation with iodomethylsilyl alcohols39, we isolated an unexpected cyclized compound 3a as a minor product when we employed an electron-deficient aryl alkyne. Notably, 3a is a product of a HAT/vinyl radical formation/cyclization cascade reaction that involves the functionalization of two C(sp3)−H bonds.
Herein, we report our preliminary results on the photoinduced Cu-catalyzed intermolecular radical cascade cyclization of iodomethylsilyl alcohols with alkynes leading to synthetically useful cyclobutanol skeletons (Fig. 1d). This method not only constitutes a valuable complement to Norrish Yang–type cyclobutanol formation, which features intramolecular reaction and usually requires an arylketone or diketone for better light absorption51,52, but it also contributes an example of double C(sp3)−H functionalization chemistry53,54,55,56,57.
Results
Reaction optimization
We optimized this interesting reaction and also, more importantly, explored the reaction mechanism and its scope. The key to improving the reaction was to identify the best photocatalyst and reductant. The complexes of CuI with simple bipyridine, tripyridine, or phenanthroline ligands were less effective for this reaction (Fig. 2, entries 1–4). Photocatalysis using homoleptic or heteroleptic Cu complexes experienced significant growth42,43,44,45. We thus prepared a series of homoleptic Cu complexes; however, the [(dmp)2Cu]PF6 complex with different substitutions provided less of the product (Fig. 2, entry 5). We then turned our attention to heteroleptic diamine/bisphosphine Cu ligands with notable features including an easily tunable chromophore and bite angle to influence the photophysical properties and catalytic activity42,43. Among all of the heteroleptic complexes evaluated (Fig. 2, entry 6–8), [(DPEphos)(bcp)Cu]PF6, originally a photosensitizer for the photocatalytic reduction of water58 and carbon dioxide59 and was recently applied in carbohalide photoactivation by Evano60, proved to be superior. We examined Ru-based photocatalysts for comparison; however, the cyclized product was formed in a relatively lower yield (Fig. 2, entry 9). Palladium was not a suitable catalyst for this cyclization reaction (Fig. 2, entry 10). Further optimization of the sacrificial reductant revealed that Hünig’s base was the best candidate (Fig. 2, entry 13). Lowering the temperature did not improve the product yield (Fig. 2, entry 14), while control experiments demonstrated the strict requirement of both catalyst and light for the reaction to occur (Fig. 2, entries 15 and 16).
a 1a (0.1 mmol), 2a (0.15 mmol), PS (10 mol %), N, N-Diisopropylethylamine(DIPEA) (4 equiv) in MeCN, under N2, rt, B-LED, 24 h. The relative configuration was assigned by NOE analysis. b Determined by 1H NMR analysis with an internal standard (diethyl phthalate). The value in parentheses is the deprotected isolated yield. c CuI (10 mol%), ligand (20 mol %). d Pd(OAc)2 (10 mol %), Xantphos (20 mol %) and Cs2CO3 (2 equiv) in PhH, under N2, rt, B-LED, 24 h. e No light.
Substrate scope
With these optimized conditions in mind (Fig. 2, entry 6), we next considered the scope and limitations of the light-mediated, [(DPEphos)(bcp)Cu]PF6-catalyzed radical cascade cyclization of aliphatic silyl iodide and alkyne-possessing representative carbon skeletons and substitution patterns (Fig. 3). Silyl alcohols with various linear or branched carbon chains were efficiently converted into the corresponding products in moderate to good yields (3b–3d); a phenyl group could be tolerated (3e). The radical nature reaction conditions were compatible with a range of functional groups, including sulfonamide (3f) with an acidic proton. Unsaturated C − C bonds, such as triple bonds and double bonds, were suitable at distant positions (3g–3h). Substrates with heteroatoms, such as oxygen, sulfur, and chloride, reacted well to provide the cyclized product in moderate yields (3i–3k). We also briefly explored the substrate scope with respect to substituents on phenylacetylene. Neutral or electron-donating substituents did not provide the cyclized product. Various electronic-withdrawing groups, including CN, actinium, amide, and sulfonamide, benefited the reaction, leading to the desired products in fare to moderate yields (3l–3o). Then, we turned our attention to another challenging aspect of this reaction—that is, the abstraction of a hydrogen atom at less-reactive secondary C(sp3)−H sites. Gratifyingly, under identical reaction conditions, cyclobutanols with three stereogenic centers formed (3p). Silicon-protected primary alcohol was engaged in this tandem radical cyclization, furnishing 3q in moderate yield, suggesting that the secondary C(sp3)-H ether site could be functionalized as well. Silicon-tethered alcohols bearing a cyclic ring at the γ position reacted well, providing spiro[3.5]nonan-2-ols in moderate yields (3r and 3s). Moreover, we successfully obtained 3-phenylspiro[4.4]nonan-2-ol and 3-phenylspiro[4.5]decan-2-ol with varied ring structures (3t and 3u). The two substitutions at the γ position could be different (3v and 3w), notably, a free hydroxyl group was compatible with the reaction conditions. We also examined the feasibility of this transformation in a more complex setting. Substrates derived from natural (-)-menthol and trans-2-isopropyl-cyclopentanol all furnished the radical relay [3 + 1] cyclized products (3x) and (3y). Through ingenious substrate design, we obtained more complex ring structures, such as the introduction of an extra spiral ring (3z) or different substitution patterns on the 4,6-fused ring system (3aa). Unfortunately, neither arylacetylenes with neutral or electron-donating substituents (3ab and 3ac) nor alkyl alkyne (3ad) provided the cyclized product under standard conditions; hydrodehalogenation or the remote desaturation side products were observed instead.
Mechanistic studies
To further investigate the reaction mechanism, we conducted a series of mechanistic experiments (Fig. 4). First, the addition of the radical scavenger TEMPO completely suppressed the reaction (Fig. 4a). Second, a deuterium-labeling experiment profile also supported the radical cascade cyclization hypothesis (Fig. 4b–d).
Thus, we observed 7 + 30% and 19 + 26% deuterium incorporation at the benzylic position of 3a1-d3 and 3a2-d3 when we added D2O instead of CD3CN in the reaction, suggesting that water provided the proton needed to quench the reaction. The observed 45 and 43% deuterium enrichment at the 1-positions of 3a1-d3 and 3a2-d3, respectively, was possibly due to the H/D exchange of the acetylene–hydrogen under basic conditions. Then, 1c-d1 with deuterium-labeled at the ether carbon provided products with 33 + 43% and 18 + 44% deuterium enrichment only at the benzylic position of 3c1-d2 and 3c2-d2, respectively, indicating the intramolecular vinyl radical (1,5-HAT). Photophysical studies have revealed that the Cu complex is a single photo-absorbing species27, Stern–Volmer studies demonstrated that both iodide and DIPEA could quench the excited state of the Cu complex, and the latter had a much higher efficiency (see Supplementary Information for details). According to Evano’s study, a DIPEA-intercepted Cu(I)/Cu(I)*/Cu(0) catalytic cycle60 might account for the reduction in organic halides to the carbon radical. Together, these results provided evidence for the photoinduced Cu-catalyzed, radical HAT/vinyl radical formation/cyclization cascade reaction process.
According to the literature49,60,61,62,63 and on the basis of those findings, we proposed the following mechanism (Fig. 5). The active photoexcited [(DPEphos)(bcp)Cu]PF6 was reduced by DIPEA to provide a Cu(0) species, which served as the true reductant to induce the homolysis of the C − I bond of 1a. The resulting carbon radial (A), upon the addition of 1,6-HAT, generated the new alkyl radical (B). The addition of a radical to alkyne gave rise to the vinyl radical C. The resulting vinyl radical E would go through an intramolecular reaction with 1,5-HAT to selectively activate the C (sp3)−H of ether and to afford a nucleophilic, secondary alkyl radical species D. A 4-exo-trig-type cyclization took place to afford the cyclized radical species (E), which was quenched to deliver the final product 3a. Moreover, quantum yield (Φ = 0.44%) suggested that a radical-chain process might not be involved (see Supplementary Information for details).
To demonstrate the synthetic potential of this methodology and identify the chemical structure of the product, we conducted two derivatizations (Fig. 6). 1a underwent two types of ring-opening functionalization processes. We generated γ-fluoroketone through a radical pathway with a ring opening on the substituted side (Fig. 6a). In contrast, we isolated γ-arylketone under palladium catalysis with a ring opening on the nonsubstituted side (Fig. 6b).
In summary, we have shown that the exposure of silyl ether iodides to a heteroleptic Cu catalyst and visible light in the absence of exogenous photosensitizers at room temperature leads to the formation of a carbon radical species. This intermediate triggers a 1,6-HAT/vinyl radical formation/cyclization cascade reaction involving the functionalization of two C − H bonds to form valuable cyclobutanols. The radical nature reaction tolerates a broad range of functional groups and could proceed under complex settings, providing elaborate polycyclic molecules. It is expected that the discovered reactivity of intermolecular radical [3 + 1] cascade cyclization under visible-light/copper-catalyzed conditions would support the further study of new methods, and these developed methods would find applications in synthesis.
Methods
In a dried sealed vial, [(DPEphos)(bcp)Cu]PF6 (0.010 mmol, 10 mol %), DIPEA (0.4 mmol, 4.0 equiv), and terminal alkyne (0.15 mmol, 1.5 equiv) were dissolved in anhydrous CH3CN (1.0 mL) under N2 atmosphere. Then, tethered alcohol (0.1 mmol, 1.0 equiv) was added. The reaction mixture was stirred at room temperature under B-LED for 24 h. The distance between the vial and the lamp was about 1–3 cm. The resulting mixture was filtered and concentrated. After that, the residual oil was dissolved in THF (1.0 mL), followed by the addition of TBAF (2 mL, 1.0 M in THF). The reaction mixture was stirred at room temperature until the reaction was completed, as monitored by TLC analysis. Then the mixture was diluted with EtOAc, washed with NaCl (aq.), and dried over with anhydrous Na2SO4. After filtration and concentration, the residue was purified by silica gel chromatography with petroleum ether and ethyl acetate to afford the deprotected products.
Data availability
Supplementary Information is available in the online version of the paper. The experimental procedures and characterization of all new compounds are provided in Supplementary Information and also from the corresponding author upon reasonable request.
References
Li, J., Gao, K., Bian, M. & Ding, H. Recent advances in the total synthesis of cyclobutane-containing natural products. Org. Chem. Front. 7, 136–154 (2020).
Murakami, M. & Ishida, N. Cleavage of carbon–carbon σ-bonds of four-membered rings. Chem. Rev. 121, 264–299 (2021).
Yan, M., Lo, J. C., Edwards, J. T. & Baran, P. S. Radicals: reactive intermediates with translational potential. J. Am. Chem. Soc. 138, 12692–12714 (2016).
Nagatomo, M. & Inoue, M. Convergent assembly of highly oxygenated natural products enabled by intermolecular radical reactions. Acc. Chem. Res. 54, 595–604 (2021).
Curran, D. P. & Rakiewicz, D. M. Tandem Radical approach to linear condensed cyclopentanoids. Total synthesis of (.+-.)-Hirsutene. J. Am. Chem. Soc. 107, 1448–1449 (1985).
Stork, G. & Mook, R. Vinyl radical cyclization. 2. Dicyclization via selective formation of unsaturated vinyl radicals by intramolecular addition to triple bonds. Applications to the synthesis of butenolides and furans. J. Am. Chem. Soc. 105, 3720–3722 (1983).
Parsons, P. J., Willis, P. A. & Eyley, S. C. A tandem radical cyclisation approach to the hexahydrobenzofuran skeleton for avermectin synthesis. J. Chem. Soc. Chem. Commun. 4, 283-285 (1988).
Stork, G., Sher, P. M. & Chen, H. L. Radical cyclization-trapping in the synthesis of natural products. A simple, stereocontrolled route to prostaglandin F2.alpha. J. Am. Chem. Soc. 108, 6384–6385 (1986).
Cha, J. Y., Yeoman, J. T. S. & Reisman, S. E. A concise total synthesis of (−)-maoecrystal Z. J. Am. Chem. Soc. 133, 14964–14967 (2011).
Smith, M. W. & Snyder, S. A. A concise total synthesis of (+)-scholarisine A empowered by a unique C–H arylation. J. Am. Chem. Soc. 135, 12964–12967 (2013).
Huang, H.-M. & Procter, D. J. Radical–radical cyclization cascades of barbiturates triggered by electron-transfer reduction of amide-type carbonyls. J. Am. Chem. Soc. 138, 7770–7775 (2016).
Brill, Z. G., Grover, H. K. & Maimone, T. J. Enantioselective synthesis of anoph iobolin sesterterpene via a programmed radical cascade. Science 352, 1078–1082 (2016).
Narayanam, J. M. R. & Stephenson, C. R. J. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 40, 102–113 (2011).
Shi, L. & Xia, W. Photoredox functionalization of C–H bonds adjacent to a nitro gen atom. Chem. Soc. Rev. 41, 7687–7697 (2012).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox ca talysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Chen, J.-R., Hu, X.-Q., Lu, L.-Q. & Xiao, W.-J. Visible light photoredox-con trolled reactions of N-radicals and radical ions. Chem. Soc. Rev. 45, 2044–2056 (2016).
Guo, H., Herdtweck, E. & Bach, T. Enantioselective lewis acid catalysis in intra molecular [2+2] photocycloaddition reactions of coumarins. Angew. Chem. Int. Ed. 49, 7782–7785 (2010).
Müller, C. et al. Enantioselective intramolecular [2 + 2]-photocycloaddition reactions of 4-substituted quinolones catalyzed by a chiral sensitizer with a hydrogen-bonding motif. J. Am. Chem. Soc. 133, 16689–16697 (2011).
Du, J. & Yoon, T. P. Crossed intermolecular [2+2] cycloadditions of acyclic enones via visible light photocatalysis. J. Am. Chem. Soc. 131, 14604–14605 (2009).
Lu, Z. & Yoon, T. P. Visible light photocatalysis of [2+2] styrene cycloadditions by energy transfer. Angew. Chem. Int. Ed. 51, 10329–10332 (2012).
Lu, Z., Shen, M. & Yoon, T. P. [3+2] Cycloadditions of aryl cyclopropyl ketones by visible light photocatalysis. J. Am. Chem. Soc. 133, 1162–1164 (2011).
Zou, Y.-Q. et al. Visible-light-induced oxidation/[3+2] cycloaddition/oxidative aromatization sequence: A photocatalytic strategy to construct pyrrolo[2,1-a]isoquinolines. Angew. Chem. Int. Ed. 50, 7171–7175 (2011).
Hurtley, A. E., Cismesia, M. A., Ischay, M. A. & Yoon, T. P. Visible light photocatalysis of radical anion hetero-Diels–Alder cycloadditions. Tetrahedron 67, 4442–4448 (2011).
Lin, S., Ischay, M. A., Fry, C. G. & Yoon, T. P. Radical cation Diels–Alder cycloadditions by visible light photocatalysis. J. Am. Chem. Soc. 133, 19350–19353 (2011).
Parrish, J. D. et al. Endoperoxide synthesis by photocatalytic aerobic [2 + 2 + 2] cycloadditions. Org. Lett. 14, 1640–1643 (2012).
Heiba, E.-A. I. & Dessau, R. M. Free-radical isomerization. I. Novel rearrangement of vinyl radicals. J. Am. Chem. Soc. 89, 3772–3777 (1967).
Curran, D. P., Kim, D., Liu, H. T. & Shen, W. Translocation of radical sites by intramolecular 1,5-hydrogen atom transfer. J. Am. Chem. Soc. 110, 5900–5902 (1988).
Lathbury, D. C., Parsons, P. J. & Pinto, I. A route to the pyrrolizidine ring system using a novel radical cyclisation. J. Chem. Soc. Chem. Commun. 2, 81-82 (1988).
Beaufils, F., Dénès, F. & Renaud, P. Thiophenol-mediated hydrogen atom abstracttion: an efficient Tin-free procedure for the preparation of cyclopentane derivatives. Org. Lett. 6, 2563–2566 (2004).
Hu, X.-Q., Chen, J.-R. & Xiao, W.-J. Controllable remote C−H bond functionali zation by visible-light photocatalysis. Angew. Chem. Int. Ed. 56, 1960–1962 (2017).
Stateman, L. M., Nakafuku, K. M. & Nagib, D. A. Remote C–H functionalization via selective hydrogen atom transfer. Synthesis 50, 1569–1586 (2018).
Chu, J. C. K. & Rovis, T. Complementary strategies for directed C(sp3)−H functionalization: a comparison of transition-metal-catalyzed activation, hydrogen atom transfer, and carbene/nitrene transfer. Angew. Chem. Int. Ed. 57, 62–101 (2018).
Wu, X. & Zhu, C. Radical functionalization of remote C(sp3)–H bonds mediated by unprotected alcohols and amides. CCS Chem. 2, 813–828 (2020).
Sarkar, S., Cheung, K. P. S. & Gevorgyan, V. C–H functionalization reactions enabled by hydrogen atom transfer to carbon-centered radicals. Chem. Sci. 11, 12974–12993 (2020).
Freeman, D. B., Furst, L., Condie, A. G. & Stephenson, C. R. J. Functionally diverse nucleophilic trapping of iminium intermediates generated utilizing visible light. Org. Lett. 14, 94–97 (2012).
Nicewicz, D. A. & MacMillan, D. W. C. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science 322, 77–80 (2008).
Andrews, R. S., Becker, J. J. & Gagné, M. R. Intermolecular addition of glycosyl halides to alkenes mediated by visible light. Angew. Chem. Int. Ed. 49, 7274–7276 (2010).
Shih, H.-W., Vander Wal, M. N., Grange, R. L. & MacMillan, D. W. C. Enantioselective α-benzylation of aldehydes via photoredox organocatalysis. J. Am. Chem. Soc. 132, 13600–13603 (2010).
Cao, Z. et al. Photo-induced Copper-catalyzed alkynylation and amination of remote unactivated C(sp3)-H bonds. Chem. Sci. 12, 4836–4840 (2021).
Zhang, Y. et al. Copper-catalyzed photoinduced enantioselective dual carbofunctionalization of alkenes. Org. Lett. 22, 1490–1494 (2020).
Xiong, Y., Ma, X. & Zhang, G. Copper-catalyzed intermolecular carboamination of alkenes induced by visible light. Org. Lett. 21, 1699–1703 (2019).
Hernandez-Perez, A. C. & Collins, S. K. Heteroleptic Cu-based sensitizers in photoredox catalysis. Acc. Chem. Res. 49, 1557–1565 (2016).
Reiser, O. Shining light on copper: unique opportunities for visible-light-catalyzed atom transfer radical addition reactions and related processes. Acc. Chem. Res. 49, 1990–1996 (2016).
Minozzi, C., Caron, A., Grenier-Petel, J.-C., Santandrea, J. & Collins, S. K. Heteroleptic copper(I)-based complexes for photocatalysis: combinatorial assembly, discovery, and optimization. Angew. Chem. Int. Ed. 57, 5477–5481 (2018).
Hossain, A., Bhattacharyya, A. & Reiser, O. Copper’s rapid ascent in visible-light photoredox catalysis. Science 364, eaav9713 (2019).
Brunckova, J., Crich, D. & Yao, Q. Intramolecular hydrogen atom abstraction in carbohydrates and nucleosides: inversion of an α- to β-mannopyranoside and generation of thymidine C-4′ radicals. Tetrahedron Lett. 35, 6619–6622 (1994).
Parasram, M., Chuentragool, P., Sarkar, D. & Gevorgyan, V. Photoinduced formation of hybrid aryl Pd-radical species capable of 1,5-HAT: selective catalytic oxidation of silyl ethers into silyl enol ethers. J. Am. Chem. Soc. 138, 6340–6343 (2016).
Parasram, M., Chuentragool, P., Wang, Y., Shi, Y. & Gevorgyan, V. General, auxiliary-enabled photoinduced Pd-catalyzed remote desaturation of aliphatic alcohols. J. Am. Chem. Soc. 139, 14857–14860 (2017).
Chuentragool, P. et al. Aliphatic radical relay Heck reaction at unactivated C(sp3)−H sites of alcohols. Angew. Chem. Int. Ed. 58, 1794–1798 (2019).
Kurandina, D. et al. Transition-metal- and light-free directed amination of remote unactivated C(sp3)–H bonds of alcohols. J. Am. Chem. Soc. 141, 8104–8109 (2019).
Yang, N. C. & Yang, D.-D. H. Photochemical reactions of ketones in solution. J. Am. Chem. Soc. 80, 2913–2914 (1958).
Zheng, J., Dong, X. & Yoon, T. P. Divergent photocatalytic reactions of α-ketoesters under triplet sensitization and photoredox conditions. Org. Lett. 22, 6520–6525 (2020).
Stang, E. M. & White, M. C. Molecular complexity via C–H activation: a dehydrogenative Diels–Alder reaction. J. Am. Chem. Soc. 133, 14892–14895 (2011).
Zhou, L., Xu, B. & Zhang, J. Metal-free dehydrogenative Diels–Alder reactions of 2-Methyl-3-alkylindoles with dienophiles: rapid access to tetrahydrocarbazoles, carbazoles, and heteroacenes. Angew. Chem. Int. Ed. 54, 9092–9096 (2015).
Xu, G.-Q. et al. Dual C(sp3)−H bond functionalization of N-heterocycles through sequential visible-light photocatalyzed dehydrogenation/[2+2] cycloaddition reactions. Angew. Chem. Int. Ed. 57, 5110–5114 (2018).
Mori, K., Isogai, R., Kamei, Y., Yamanaka, M. & Akiyama, T. Chiral magnesium bisphosphate-catalyzed asymmetric double C(sp3)–H bond functionalization based on sequential hydride shift/cyclization process. J. Am. Chem. Soc. 140, 6203–6207 (2018).
Zhuang, Z., Herron, A. N., Liu, S. & Yu, J.-Q. Rapid construction of tetralin, chromane, and indane motifs via cyclative C–H/C–H coupling: four-step total synthesis of (±)-Russujaponol F. J. Am. Chem. Soc. 143, 687–692 (2021).
Luo, S.-P. et al. Photocatalytic water reduction with copper-based photosensitizers: a noble-metal-free system. Angew. Chem. Int. Ed. 52, 419–423 (2013).
Takeda, H., Ohashi, K., Sekine, A. & Ishitani, O. Photocatalytic CO2 reduction using Cu(I) photosensitizers with a Fe(II) catalyst. J. Am. Chem. Soc. 138, 4354–4357 (2016).
Michelet, B., Deldaele, C., Kajouj, S., Moucheron, C. & Evano, G. A general copper catalyst for photoredox transformations of organic halides. Org. Lett. 19, 3576–3579 (2017).
Huang, L. et al. Stereoselective radical cyclization cascades triggered by addition of diverse radicals to alkynes to construct 6(5)–6–5 fused rings. Org. Lett. 18, 5284–5287 (2016).
Li, H. et al. Sequential C–O decarboxylative vinylation/C–H arylation of cyclicoxalates via a Nickel-catalyzed multicomponent radical cascade. Chem. Sci. 11, 4904–4910 (2020).
Zhong, L.-J., Li, Y., An, D.-L. & Li, J.-H. Heteroannulation of N-Fluoro-N-alkylsulfonamides with terminal alkynes via remote C(sp3)–H Functionalization. ACS Catal. 11, 383–389 (2021).
Acknowledgements
We are grateful to the National Natural Science Foundation of China (22071073, 21772218, and 21821002), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000), State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and Central China Normal University (CCNU).
Author information
Authors and Affiliations
Contributions
Z.C. discovered the reaction, J.L. identified the optimal reaction conditions. J.L. and Z.C. performed the experiments. G.Z. directed the project and all the authors wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Xin-Hua Duan and the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cao, Z., Li, J. & Zhang, G. Photo-induced copper-catalyzed sequential 1,n-HAT enabling the formation of cyclobutanols. Nat Commun 12, 6404 (2021). https://doi.org/10.1038/s41467-021-26670-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-26670-5
- Springer Nature Limited
This article is cited by
-
Anti-Markovnikov ring-opening of sulfonium salts with alkynes by visible light/copper catalysis
Science China Chemistry (2022)