Abstract
Click reactions have become powerful synthetic tools with unique applications in the health and materials sciences. Despite the progress with optical sensors that exploit the principles of dynamic covalent chemistry, metal coordination or supramolecular assemblies, quantitative analysis of complex mixtures remains challenging. Herein, we report the use of a readily available coumarin conjugate acceptor for chiroptical click chirality sensing of the absolute configuration, concentration and enantiomeric excess of several compound classes. This method has several attractive features, including wide scope, fast substrate fixation without by-product formation or complicate equilibria often encountered in reversible substrate binding, excellent solvent compatibility, and tolerance of air and water. The ruggedness and practicality of this approach are demonstrated by comprehensive analysis of nonracemic monoamine samples and crude asymmetric imine hydrogenation mixtures without work-up. Click chemosensing addresses increasingly important time efficiency, cost, labor and chemical sustainability aspects and streamlines asymmetric reaction development at the mg scale.
Similar content being viewed by others
Introduction
Chirality plays an essential role across the chemical and pharmaceutical sciences, and the development of new methods for the synthesis and analysis of chiral compounds are frequently required tasks in academic and industrial laboratories. To accelerate the discovery process, it has become routine to perform hundreds of small-scale reactions in parallel using widely available high-throughput experimentation equipment (HTE)1,2. With regard to asymmetric reaction development, many combinations of different chiral catalysts, solvents, additives, and other parameters typically need to be evaluated. In the search for an optimized procedure, a chemist can easily alter a large set of reaction parameters and produce hundreds of chiral samples in a very short time using multiwell plate technology. In stark contrast with automated synthesis capabilities, the determination of the absolute configuration, yield and enantiomeric excess of asymmetric reactions with traditional chromatographic methods that are serial in nature and incompatible with HTE remains slow, and this has shifted increasing attention to contemporary screening techniques3. Optical methods are compatible with parallel data acquisition, miniaturization, and multiwell plate formats and offer a new path to real high-throughput analysis of chiral samples4,5,6,7. Few examples of asymmetric reaction analysis with sensors operating on the principles of dynamic covalent chemistry8,9,10,11, metal complex coordination12, and supramolecular chemistry13,14,15 to recognize a chiral target compound and to generate quantifiable ultraviolet (UV), fluorescence, and circular dichroism (CD) signals have been reported16,17,18. Comprehensive chirality sensing (CCS), i.e., determination of the absolute configuration, yield, and enantiomeric excess (ee), of crude asymmetric reaction mixtures via irreversible covalent product fixation has been largely neglected to date19.
More than 10 years ago, Sharpless coined the term “click chemistry” for reactions that are high yielding, practical and operationally safe, avoid by-product formation, proceed in environmentally benign solvents at room temperature and generally under mild conditions, and eliminate chromatographic work-up20. Since then, a variety of reaction strategies and applications that exploit this concept, in particular in the biomedical domain, have been introduced21,22,23,24,25,26,27. The inherent robustness and practicality of click chemistry encouraged us to explore probe designs that overcome drawbacks of currently used chiroptical sensing methods such as limited substrate scope (amine sensors often utilize reversible Schiff base formation and are restricted to primary substrates), competing chiral recognition processes and equilibria that complicate the analysis and diminish CD readouts, and sensitivity to moisture, air, and other chemical interferences, which limits the usefulness when complex mixtures need to be examined. We now introduce a rugged, readily available, easy to use, inexpensive click sensor that is expected to increase the speed of scientific discoveries in countless laboratories through streamlined enantioselective analysis of scalemic samples and accelerated asymmetric reaction development.
Results and discussion
Sensor development studies
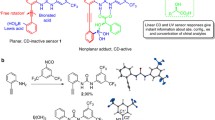
Our search for a suitable chromophoric probe that can quickly and quantitatively capture a variety of chiral target compounds through irreversible covalent bond formation at room temperature led us to prepare and investigate the coumarin-derived Michael acceptors 1–5 (Fig. 1, Supplementary Methods). Initial screening with chiral amines revealed that these 4-halocoumarins undergo smooth Michael addition and subsequent halide elimination toward 6 and 7, respectively. Further analysis of the chiroptical properties showed that the covalent attachment of 1-phenylethylamine, 8, which was one of the initially tested amines, to the coumarin scaffold results in a strong CD signal and a distinct UV change at high wavelengths at micromolar concentrations. Importantly, the Michael addition/elimination substrate binding strategy does not generate a new chirality center. In contrast to other sensor designs, this feature avoids complications arising from the formation of diastereomeric mixtures which simplifies the chirality sensing protocol described below. Comparison of the reactivity and chiroptical responses of the five probes revealed superior properties of 4-chloro-3-nitrocoumarin, 3 (see Supplementary Figures 8–13). When this sensor is employed, the reaction proceeds quantitatively at room temperature without by-product formation in various solvents ranging from chloroform to aqueous acetonitrile. The presence of the auxochromic nitro group is important for two reasons: it significantly accelerates the covalent substrate fixation and it affords a stronger Cotton effect at higher wavelengths (Supplementary Figures 8 and 9).
The scope of this sensor turned out to be very large. Encouraged by the practicality of the sensing assay and the large Cotton effects observed upon binding of 8, we decided to apply coumarin 3 to a variety of amines, amino alcohols, alcohols and amino acids, including aspartic acid, threonine, cysteine, and others with functionalized sidechains. We obtained distinct chiroptical responses in all cases (Supplementary Figures 29–69). Representative CD spectra are shown in Fig. 1. The binding of the S-enantiomers of the amines, amino alcohols and amino acids 8, 16, 27, and 35 yields a positive Cotton effect above 300 nm whereas the R-substrates induce the opposite CD response. The reverse relationship between the absolute configuration and the sign of the induced Cotton effects was observed using the alcohol 33 as the sensing target. It is noteworthy that among the 39 sensing targets are several with a secondary amino group, i.e., 16, 17, 24, 27, 28, 37, and 46, that cannot be sensed via the commonly used Schiff base formation approach9,28,29,30,31,32,33,34. The successful sensing of 32, 33, and 46 exhibiting low nucleophilicity further underscores the wide utility of our 4-halocoumarin probes.
Comparison of the chiroptical signals observed with the 4-halocoumarins 1 and 2 versus the nitro analogues 3–5 upon binding of 8 shows a strong contribution of the nitro dipole in the sensor scaffold to the CD intensity of the Michael addition/elimination product (see Supplementary Figures 8–13). The nitro group contribution results in a stronger and remarkably red-shifted CD signal which is advantageous for direct asymmetric reaction analysis because it eliminates interference from CD-active catalysts with the chiroptical measurements, vide infra. Although intramolecular hydrogen bonding (–NH···O2N–) is likely to occur with 7 in aprotic solvents it is not a prerequisite for this sensor to function. We obtained strong albeit quite different CD signatures using chloroform, dichloromethane, toluene, acetonitrile, and methanol, which is expected as the solvent choice can disturb the hydrogen bonding motif and alter conformational equilibria (Fig. 2, Supplementary Figures 14–16 and 28)35. In fact, intramolecular hydrogen bonding interactions are absent in the crystal structure of the primary amine addition product 7 and we observed very strong CD effects upon binding of substrates with secondary amino groups, for example 16, which affords a product devoid of an NH donor site (Figs. 1 and SI). The sensing does also not require CD coupling events and can therefore be applied to monofunctional aliphatic substrates such as 14–16. Altogether, these features result in a large scope which is highlighted by chirality sensing of 39 compounds. The inherently wide solvent compatibility is very attractive from an operational perspective because it simplifies adaption to asymmetric reaction conditions, for example when alcoholic co-solvents are used in catalytic enantioselective imine hydrogenations as described below. The sensing reaction and the corresponding CD effects were further investigated by UV, CD, and nuclear magnetic resonance (NMR) spectroscopy. We separately prepared the products of the reactions of 3 with 8, 17 and cis-22, respectively. The CDs of these isolated compounds match those generated in the sensing assays (Supplementary Figures 7–27). The reaction between 1-phenylethylamine and probe 3 in the presence of Et3N was closely monitored by 1H NMR spectroscopy (Fig. 2). The spectra collected after 5, 10, and 15 min show the clean transformation of 3 and 8 into 7 which is complete after approximately 15 min at room temperature. For example, the signals at 1.39 ppm (Hj) and 4.12 ppm (Hh) of 8 undergo a downfield shift to 1.78 and 5.38 ppm, respectively, as 7 is formed. Accordingly, the doublet at 8.00 ppm (Ha) of probe 3 shows an upfield shift to 7.78 ppm in the reaction mixture.
Analysis of the sensing chemistry. a The CD spectra of 7 were obtained at 0.19 or 0.24 mM when MeOH was used as solvent. b X-ray structures of chiral amine derivatives of 3. c 1H NMR analysis of the reaction between probe 3 and (S)-8 in the presence of Et3N (all 5.0 mM) in 0.8 mL of CDCl3. (1) Probe 3; (2) (S)-1-phenylethylamine; (3) reaction mixture of (S)-1-phenylethylamine, Et3N and probe 3 after 5 min; (4) reaction after 10 min; (5) after 15 min; and (6) isolated 3-nitro-4-((1-phenylethyl)amino)coumarin, 7, for comparison
The sensing with 3 exhibits the main elements of click chemistry19: it is fast, wide in scope, displays smooth substrate transformation with very high yield at room temperature, is compatible with a wide range of environmentally benign solvents such as methanol and acetonitrile, avoids formation of by-products, eliminates chromatographic or any type of work-up, is insensitive to air and moisture, and utilizes readily available starting materials, i.e., the coumarin probe 3. We anticipated that these preferable reaction characteristics in combination with the strong, red-shifted chiroptical readouts will generate unique sensing opportunities.
Comprehensive chirality and concentration sensing
A closer look at the sensing of 1-(2-naphthyl)ethylamine, 10, revealed that the irreversible substrate binding concurs with a drastic UV increase at 265 and 355 nm, while the absorption at 309 nm remains unchanged (Fig. 3). This unique feature allows ratiometric sensing of the amine concentration, for example by using the ratio of the relative absorption increase at 265 nm compared to the UV signal at 309 nm. We then conducted CD experiments and discovered that the induced CD maxima at 257 and 355 nm increase linearly with the substrate ee. The absolute configuration of 10 or another target compound can be assigned based on comparison of the sign of the induced CD signals with a reference and quantitative information about the substrate amount (concentration) and its enantiomeric composition is directly accessible from the UV changes and the CD amplitudes, respectively. This is particularly attractive because modern CD instruments generate UV and CD spectra simultaneously.
The robustness of the fast and quantitative Michael addition/elimination chemistry with a wide variety of chiral compounds in combination with the distinct chiroptical readouts of the coumarin sensor 3 suggested to us that sensing of complex mixtures, for example crude asymmetric reaction mixtures containing a variety of typically interfering compounds such as a chiral catalyst, additives, by-products and protic solvents, was within reach. We first decided to verify that the UV/CD responses of the coumarin probe allow reliable assignment of the absolute configuration together with accurate determination of the ee and concentration of micromolar samples of 10. Nine samples containing the amine in varying concentration and ee were prepared and subjected to our CCS assay (Table 1 and Supplementary Figures 70–74). In all cases, the absolute configuration of the major enantiomer was correctly identified by comparison of the CD signal with a reference and the concentrations and ee’s were determined with good accuracy. For example, the sensing analysis of the sample containing (S)-10 in 33.3% ee at 2.50 μM determined that the S-enantiomer was present in 36.0% ee at 2.70 μM (entry 5, Table 1). Generally, the click chemistry with coumarin 3 is complete within 1 h when the derivatization is conducted at 5 mM concentration which is convenient for adaption to small-scale asymmetric reaction development. The chiroptical analysis can then be performed without delay after dilution to micromolar concentrations. Simultaneous determination of the enantiomeric composition of two analytes present in the same sample is also possible. We applied the click sensor 3 to scalemic mixtures of 1-phenylethylamine, 8, and N-methyl-1-phenylethylamine, 17, following the general protocol described above. The chiroptical analysis of 10 samples containing these representative amines in drastically varying ee’s proved sufficiently accurate for HTS purposes with errors ranging from 0.6 to 7.6% ee (Supplementary Table 3 and Supplementary Figures 81–84).
Asymmetric reaction analysis
Finally, the chirality sensing method with chlorocoumarin 3 was applied to asymmetric reaction analysis (Table 2 and Supplementary Figures 75–80). We chose the iridium catalyzed hydrogenation of the N-methyl imine 48 to the secondary amine 17 for this purpose36,37,38,39,40. Several ligands 49–53 and catalyst loadings were varied to determine the value of chiroptical sensing and to compare it with traditional NMR/chiral high-performance liquid chromatography (HPLC) analysis. The inherent ruggedness of our click chemistry sensing approach together with the wide solvent compatibility allowed us to simply take 200 μL aliquots from the methanolic reaction mixtures for direct UV/CD analysis. Based on a conservative estimate, the analysis time per sample was 60 min and 6 mL of solvent waste for diluting the samples were generated. The vast majority of the analysis time is required for the reaction of the amine product with the probe. If necessary this can be accelerated at higher temperatures, however, one can easily conduct hundreds of these experiments in parallel using multi-well plate technology. In such a high-throughput screening (HTS) scenario, the analysis of hundreds of reaction mixtures would still take approximately 1 hour.
We developed a chiral HPLC method with Boc-protected 17 to verify the results from our sensing assay. The traditional NMR and chiral HPLC analysis of the enantioselective imine hydrogenation required more than 7 h and 540 mL of solvent waste were accumulated, which can be mostly attributed to the formation and purification of the Boc-protected derivative 54. Overall, the results obtained by both methods are in good agreement. For example, the reaction with 5 mol% of the Phox ligand derived Ir catalyst gave (S)-17 in 55.8% ee and quantitative yield according to NMR and HPLC analysis which compares well to the 59.8% ee and 96.0% yield determined by sensing (entry 1). The error margins of the chiroptical sensing are fairly small and acceptable, in particular if one would apply the sensing assay to HTS of hundreds of samples. The minimization of time and chemical waste compared to traditional methods underscores the efficiency, practicality, cost and environmental sustainability advantages of chiroptical sensing with the coumarin 3.
In summary, we have developed a rugged click chemistry probe that allows comprehensive chirality sensing of a large variety of aliphatic and aromatic primary and secondary amines, amino alcohols, alcohols, and amino acids without the common shortcomings encountered with assays based on dynamic covalent chemistry, metal coordination, and supramolecular assemblies. The fast and irreversible substrate binding with readily available 4-halocoumarin sensors has several attractive features, including wide application spectrum, distinct chiroptical signaling at high wavelengths, operational simplicity, elimination of by-product formation or complex dynamic equilibria, excellent solvent compatibility, and tolerance of air and water. The usefulness and practicality of this approach were demonstrated with the chiroptical sensing of the absolute configuration, concentration, and ee of several samples of 2-(2-naphthyl)ethylamine and by the direct analysis of crude reaction mixtures generated by iridium catalyzed asymmetric hydrogenation of a prochiral N-methyl imine to N-methyl 1-phenylethylamine, a challenging task for currently available sensing methods. Compared to existing methodology, the practicality, robustness and scope of comprehensive click chirality sensing with 4-halocoumarin conjugate acceptors are remarkable. This strategy is uniquely effective and addresses increasingly important time efficiency and sustainability aspects by enabling reaction scale miniaturization and adaption to HTE, i.e., multiwell plate CD/UV readers, which altogether streamline asymmetric reaction development and reduce labor, cost, energy consumption and waste production.
Methods
General CD sensing procedure
To test the general utility of probe 3 as chirality probe, CD spectra of the sensing experiments with chiral amines 8–19, amino alcohols 20–31, alcohols 32–33, amino acids 34–46 and were obtained. A solution of probe 3 (5.0 mM), a chiral amine or amino alcohol (5.0 mM) and Et3N (5.0 mM) in 2.0 mL of chloroform was stirred for 1 h and subjected to CD analysis after dilution to 0.24 mM unless otherwise noted. For the amino acid sensing, a solution of 3 (5.0 mM), amino acid (5.0 mM) and K2CO3 (10.0 mM) in 2.0 mL of acetonitrile–water (4:1) was stirred for 1 h and treated as described above. For alcohol sensing, a solution of 3 (10.0 mM), an alcohol (10.0 mM) and LiOt-Bu (20 mM) in 2.0 mL of tetrahydrofuran was stirred for 2 h prior to CD analysis. The CD spectra were collected with a standard sensitivity of 100 mdeg, a data pitch of 0.5 nm, a bandwidth of 1 nm, in a continuous scanning mode with a scanning speed of 500 nm/min and a response of 1 s, using a quartz cuvette (1 cm path length). The data were baseline corrected and smoothed using a binomial equation.
Comprehensive chirality sensing
Probe 3 (10.0 mM) and (S)-1-phenylethylamine, 10, in varying concentrations (0, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10.0 mM) were dissolved in the presence of Et3N (10.0 mM) in 2.0 mL of chloroform. To 10 μL of this solution, chloroform (2.0 mL) was added and the mixture was subjected to UV analysis. The UV absorbance at 355 and 265 nm increased as the concentration of 10 changed from 0 to 10 μM. Plotting and curve fitting of the UV absorbance change at 265 nm relative to 309 nm against the concentration (mM) of 10 showed a linear correlation (Fig. 3). An ee calibration curve was constructed using samples containing 1-(2-naphthyl)ethylamine, 10, with varying enantiomeric composition. Probe 3 (10.0 mM) and 10 (5.0 mM) with varying ee’s (+100, +80, +60, +40, +20, 0, −20, −40, −60, −80, and −100%) were dissolved in the presence of Et3N (10.0 mM) in 2.0 mL of chloroform. After 1 h, CD analysis was carried out by diluting 25 μL of the reaction mixture with chloroform (2.0 mL). The CD amplitudes at 355 and 257 nm were plotted against the sample enantiomeric excess showing a linear correrlation (Fig. 3). Nine scalemic samples of 10 at varying concentrations in chloroform were prepared and subjected to simultaneous analysis of the concentration, enantiomeric excess and absolute configuration using probe 3. First, a UV spectrum was obtained as described above and the concentration was calculated using regression analysis. Then, a CD spectrum was obtained as described above. The relevant intensities were used with linear regression equations to determine the enantiomeric excess. The absolute configuration was determined using the sign of the CD effects (Table 1).
Asymmetric reaction analysis
Bis(1,5-cyclooctadiene)diiridium(I) dichloride ([Ir(cod)Cl]2) (12.4 mg, 0.02 mmol) was added to the ligand, 49–53, (0.04 mmol) in dichloromethane and stirred for 30 min. N-Methyl-1-phenylethan-1-imine, 48, (100 mg, 0.75 mmol) and the preformed metal-ligand complex (0.04 mmol) were then combined in dichloromethane:methanol (8:1) (9 mL) and the mixture was stirred under 15 bar H2 pressure overnight. To 200 μL of the crude reaction mixture, 4-chloro-3-nitrocoumarin, 3, (10.0 mM), and Et3N (10.0 mM) were added in 2.0 mL of chloroform and stirred for 1 h. Then, 40 μL of this solution were diluted with chloroform (2.0 mL) and subjected to CD analysis to determine the absolute configuration based on the sign of the Cotton effect and the enantiomeric excess based on the CD amplitude. Another aliquot of 10 μL of the 200 μL solution was diluted with chloroform (2.0 mL) and subjected to UV analysis to determine the conversion of 48 to 17. The chiroptical analysis was performed in analogy to the CCS experiments (see SI for details). For NMR/HPLC analysis, a 200 μL portion of the crude reaction mixture was dissolved in CDCl3 and analyzed by 1H NMR spectroscopy do determine the conversion. Another 200 μL portion of the crude reaction mixture was filtered through a cotton plug and di-tert-butyl dicarbonate was added to the filtrate. Due to the presence of methanol in the reaction mixture, di-tert-butyl dicarbonate was used in excess (3 equivalents) and the reaction was allowed to run for 5 h. Then the reaction mixture was concentrated and purified via flash column chromatography on silica using 10–40% dichloromethane in hexanes to afford N-Boc-N-methyl-1-phenylethylamine. The enantiomeric excess of N-Boc-N-methyl-1-phenylethylamine was determined by chiral HPLC using S,S-Whelk-O 1 as chiral stationary phase unless otherwise noted. Mobile phase: hexanes IPA = 99:1, flow rate = 1.0 mL/min, UV = 214 nm, tR = 8.6 min (major) and tR = 9.6 min (minor).
Data availability
The data that support the findings of this study are available from the corresponding author upon request. The X-ray crystallographic coordinates for 4-chlorocoumarin, 1, 4-bromocoumarin, 2, 4-iodo-nitrocoumarin, 5, (S)-3-nitro-4-((1-phenylethyl)amino)coumarin, 7, and (R)-3-nitro-4-(N,α-dimethylbenzyl)amino)coumarin, 55, reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1853449, 1853447, 1853450, and 1853451. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
McNally, A., Prier, C. K. & MacMillan, D. W. C. Discovery of an α-amino C–H arylation reaction using the strategy of accelerated serendipity. Science 334, 1114–1117 (2011).
Santanilla, A. B. et al. Nanomolecular-scale high-throughput chemistry for the synthesis of complex molecules. Science 347, 49–53 (2015).
Collins, K. D., Gensch, T. & Glorius, F. Contemporary screening approaches to reaction discovery and development. Nat. Chem. 6, 859–871 (2014).
Leung, D., Kang, S. O. & Anslyn, E. V. Rapid determination of enantiomeric excess: a focus on optical approaches. Chem. Soc. Rev. 41, 448–479 (2012).
Wolf, C. & Bentley, K. W. Chirality sensing using stereodynamic probes with distinct electronic circular dichroism output. Chem. Soc. Rev. 42, 5408–5424 (2013).
Zardi, P., Wurst, K., Licini, G. & Zonta, C. Concentration-independent stereodynamic g-probe for chiroptical enantiomeric excess determination. J. Am. Chem. Soc. 139, 15616–15619 (2017).
Ni, C. et al. Dynamic covalent chemistry within biphenyl scaffolds: reversible covalent bonding, control of selectivity, and chirality sensing with one single system. Angew. Chem. Int. Ed. 57, 1300–1305 (2018).
Shabbir, S. H., Clinton, J. R. & Anslyn, E. V. A general protocol for creating high-throughput screening assays for reaction yield and enantiomeric excess applied to hydrobenzoin. Proc. Natl Acad. Sci. USA 106, 10487–10492 (2009).
Nieto, S., Dragna, J. M. & Anslyn, E. V. A facile circular dichroism protocol for rapid determination of enantiomeric excess and concentration of chiral primary amines. Chem. Eur. J. 16, 227–232 (2010).
Bentley, K. W., Proano, D. & Wolf, C. Chirality imprinting and direct asymmetric reaction screening using a stereodynamic Brønsted/Lewis acid receptor. Nat. Commun. 7, 12539 (2016).
Shcherbakova, E. G., Brega, V., Lynch, V. M., James, T. D. & Anzenbacher, P. High‐throughput assay for enantiomeric excess determination in 1,2‐ and 1,3‐diols and direct asymmetric reaction screening. Chem. Eur. J. 23, 10222–10229 (2017).
Bentley, K. W., Zhang, P. & Wolf, C. Miniature high-throughput chemosensing of yield, ee, and absolute configuration from crude reaction mixtures. Sci. Adv. 2, e1501162 (2016).
Biedermann, F. & Nau, W. M. Noncovalent chirality sensing ensembles for the detection and reaction monitoring of amino acids, peptides, proteins, and aromatic drugs. Angew. Chem. Int. Ed. 53, 5694–5699 (2014).
Feagin, T. A., Olsen, D. P., Headman, Z. C. & Heemstra, J. M. High-throughput enantiopurity analysis using enantiomeric DNA-based sensors. J. Am. Chem. Soc. 137, 4198–4206 (2015).
De los Santos, Z. A. & Wolf, C. Chiroptical asymmetric reaction screening via multicomponent self-assembly. J. Am. Chem. Soc. 138, 13517–13520 (2016).
Giuliano, M. W., Lin, C. Y., Romney, D. K., Miller, S. J. & Anslyn, E. V. A synergistic combinatorial and chiroptical study of peptide catalysts for asymmetric Baeyer–Villiger oxidation. Adv. Synth. Catal. 357, 2301–2309 (2015).
Joyce, L. A., Sherer, E. C. & Welch, C. J. Imine-based chiroptical sensing for analysis of chiral amines: from method design to synthetic application. Chem. Sci. 5, 2855–2861 (2014).
Jo, H. H., Gao, X., You, L., Anslyn, E. V. & Krische, M. J. Application of a high-throughput enantiomeric excess optical assay involving a dynamic covalent assembly: parallel asymmetric allylation and ee sensing of homoallylic alcohols. Chem. Sci. 6, 6747–6753 (2015).
Thanzeel, F. Y. & Wolf, C. Substrate-specific amino acid sensing using a molecular D/L-cysteine probe for comprehensive stereochemical analysis in aqueous solution. Angew. Chem. Int. Ed. 56, 7276–7281 (2017).
Kolb, H. C., Finn, M. G. & Sharpless, B. K. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Moses, J. E. & Moorhouse, A. D. The growing applications of click chemistry. Chem. Soc. Rev. 36, 1249–1262 (2007).
Baskin, J. M. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl Acad. Sci. USA 104, 16793 (2007). 16787.
Chang, P. V. et al. Copper-free click chemistry in living animals. Proc. Natl Acad. Sci. USA 107, 1821–1826 (2010).
Diehl, K. L. et al. Click and chemically triggered declick reactions through reversible amine and thiol coupling via a conjugate acceptor. Nat. Chem. 8, 968–973 (2016).
Li, S., Wu, P., Moses, J. E. & Sharpless, K. B. Multidimensional SuFEx click chemistry: sequential sulfur(VI) fluoride exchange connections of diverse modules launched from an SOF4 hub. Angew. Chem. Int. Ed. 56, 2903–2908 (2017).
Kukwikila, M., Gale, N., El-Sagheer, A. H., Brown, T. & Tavassoli, A. Assembly of a biocompatible triazole-linked gene by one-pot click-DNA ligation. Nat. Chem. 9, 1089–1098 (2017).
Gahtory, D., Sen, R., Kuzmyn, A. R., Escorihuela, J. & Zuilhof, H. Strain-promoted cycloaddition of cyclopropenes with o-quinones: a rapid click reaction. Angew. Chem. Int. Ed. 57, 10118–10122 (2018).
Ghosn, M. W. & Wolf, C. Chiral amplification with a stereodynamic triaryl probe: assignment of the absolute configuration and enantiomeric excess of amino alcohols. J. Am. Chem. Soc. 131, 16360–16361 (2009).
Iwaniuk, D. P. & Wolf, C. A stereodynamic probe providing a chiroptical response to substrate-controlled induction of an axially chiral arylacetylene framework. J. Am. Chem. Soc. 133, 2414–2417 (2011).
Dragna, J. M. et al. In situ assembly of octahedral Fe(II) complexes for the enantiomeric excess determination of chiral amines using circular dichroism spectroscopy. J. Am. Chem. Soc. 134, 4398–4407 (2012).
Bentley, K. W. & Wolf, C. Stereodynamic chemosensor with selective circular dichroism and fluorescence readout for in situ determination of absolute configuration, enantiomeric excess, and concentration of chiral compounds. J. Am. Chem. Soc. 135, 12200–12203 (2013).
Huang, Z., Yu, S., Wen, K., Yu, X. & Pu, L. Zn(II) promoted dramatic enhancement in the enantioselective fluorescent recognition of functional chiral amines by a chiral aldehyde. Chem. Sci. 5, 3457–3462 (2014).
Wen, K. et al. Rational design of a fluorescent sensor to simultaneously determine both the enantiomeric composition and the concentration of chiral functional amines. J. Am. Chem. Soc. 137, 4517–4524 (2015).
Pilicer, S. L., Bakhshi, P. R., Bentley, K. W. & Wolf, C. Biomimetic chirality sensing with pyridoxal-5’-phosphate. J. Am. Chem. Soc. 139, 1758–1761 (2017).
Park, E.-K., Park, B., Choi, J.-H., Choi, K. & Cho, M. Chirality transfer effects in proline-substituted coumarin compounds. J. Phys. Chem. 113, 11301–11305 (2009).
Verdaguer, X., Lange, U. E. W. & Buchwald, S. L. Amine additives greatly expand the scope of asymmetric hydrosilylation of imines. Angew. Chem. Int. Ed. 37, 1103–1107 (1998).
Klahn, M., Arndt, P., Spannenberg, A., Gansaeuer, A. & Rosenthal, U. Menthyl-substituted group 4 metallocene dihalides. Organometallics 27, 5846–5851 (2005).
Schnider, P. et al. Enantioselective hydrogenation of imines with chiral (phosphanodihydrooxazole)iridium catalysts. Chem. Eur. J. 3, 887–892 (1997).
Lindqvist, M. et al. Chiral molecular tweezers: synthesis and reactivity in asymmetric hydrogenation. J. Am. Chem. Soc. 137, 4038–4041 (2015).
Wakchaure, V. N., Kaib, P. S. J., Leutzsch, M. & List, B. Disulfonimide-catalyzed asymmetric reduction of N-alkyl imines. Angew. Chem. Int. Ed. 54, 11852–11856 (2015).
Acknowledgments
This work is dedicated to William H. Pirkle who was an extraordinary scholar, teacher, and mentor. We are grateful for financial support from the U.S. National Science Foundation (CHE-1464547 and CHE-1764135).
Author information
Authors and Affiliations
Contributions
F.Y.T., K.B. and C.W. designed and conceived the experiments. F.Y.T. and K.B performed the experiments and F.Y.T. analyzed the data with input from K.B. and C.W. All authors discussed the results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thanzeel, F.Y., Balaraman, K. & Wolf, C. Click chemistry enables quantitative chiroptical sensing of chiral compounds in protic media and complex mixtures. Nat Commun 9, 5323 (2018). https://doi.org/10.1038/s41467-018-07695-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-018-07695-9
- Springer Nature Limited
This article is cited by
-
Design of mechanical-robust phosphorescence materials through covalent click reaction
Nature Communications (2023)
-
Optical deciphering of multinary chiral compound mixtures through organic reaction based chemometric chirality sensing
Nature Communications (2021)