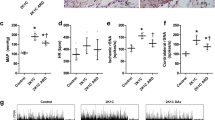
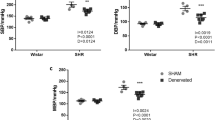
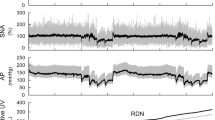
Abstract
The activation of sympathetic nervous system plays a critical role in the development of hypertension. The input from afferent renal nerves may affect central sympathetic outflow; however, its contribution to the development of hypertension remains unclear. We investigated the role of afferent renal nerves in acute and chronic blood pressure regulation using normotensive Wistar-Kyoto rats (WKY) and stroke-prone spontaneously hypertensive rats (SHRSP). Acute chemical stimulation of afferent renal nerves elicited larger increases in blood pressure and renal sympathetic nerve activity in young 9-week-old SHRSP compared to WKY. Selective afferent renal denervation (ARDN) and conventional total renal denervation (TRDN) ablating both afferent and efferent nerves in young SHRSP revealed that only TRDN, but not ARDN, chronically attenuated blood pressure elevation. ARDN did not affect plasma renin activity or plasma angiotensin II levels, whereas TRDN decreased both. Neither TRDN nor ARDN affected central sympathetic outflow and systemic sympathetic activity determined by neuronal activity in the parvocellular region of hypothalamic paraventricular nucleus and rostral ventrolateral medulla and by plasma and urinary norepinephrine levels, respectively. Renal injury was not apparent in young SHRSP compared with WKY, suggesting that renal afferent input might not be activated in young SHRSP. In conclusion, the chronic input from afferent renal nerves does not contribute to the development of hypertension in SHRSP despite the increased blood pressure response to the acute stimulation of afferent renal nerves. Efferent renal nerves may be involved in the development of hypertension via activation of the renin-angiotensin system in SHRSP.






Similar content being viewed by others
References
Hirooka Y. Sympathetic activation in hypertension: importance of the central nervous system. Am J Hypertens. 2020;33:914–26. https://doi.org/10.1093/ajh/hpaa074
Katsurada K, Ogoyama Y, Imai Y, Patel KP, Kario K. Renal denervation based on experimental rationale. Hypertens Res. 2021;44:1385–94. https://doi.org/10.1038/s41440-021-00746-7
Osborn JW, Foss JD. Renal nerves and long-term control of arterial pressure. Compr Physiol. 2017;7:263–320. https://doi.org/10.1002/cphy.c150047
Osborn JW, Tyshynsky R, Vulchanova L. Function of renal nerves in kidney physiology and pathophysiology. Annu Rev Physiol. 2021;83:429–50. https://doi.org/10.1146/annurev-physiol-031620-091656
Zheng H, Patel KP. Integration of renal sensory afferents at the level of the paraventricular nucleus dictating sympathetic outflow. Auton Neurosci. 2017;204:57–64. https://doi.org/10.1016/j.autneu.2016.08.008
Ye C, Qiu Y, Zhang F, Chen AD, Zhou H, Wang JJ, et al. Chemical stimulation of renal tissue induces sympathetic activation and a pressor response via the paraventricular nucleus in rats. Neurosci Bull. 2020;36:143–52. https://doi.org/10.1007/s12264-019-00417-1
Fujisawa Y, Nagai Y, Lei B, Nakano D, Fukui T, Hitomi H, et al. Roles of central renin-angiotensin system and afferent renal nerve in the control of systemic hemodynamics in rats. Hypertens Res. 2011;34:1228–32. https://doi.org/10.1038/hr.2011.115
Xu B, Zheng H, Liu X, Patel KP. Activation of afferent renal nerves modulates RVLM-projecting PVN neurons. Am J Physiol Heart Circ Physiol. 2015;308:H1103–111. https://doi.org/10.1152/ajpheart.00862.2014
Iyonaga T, Shinohara K, Mastuura T, Hirooka Y, Tsutsui H. Brain perivascular macrophages contribute to the development of hypertension in stroke-prone spontaneously hypertensive rats via sympathetic activation. Hypertens Res. 2020;43:99–110. https://doi.org/10.1038/s41440-019-0333-4
Shinohara K, Hirooka Y, Kishi T, Sunagawa K. Reduction of nitric oxide-mediated γ-amino butyric acid release in rostral ventrolateral medulla is involved in superoxide-induced sympathoexcitation of hypertensive rats. Circ J. 2012;76:2814–21. https://doi.org/10.1253/circj.CJ-12-0399
Nishihara M, Hirooka Y, Matsukawa R, Kishi T, Sunagawa K. Oxidative stress in the rostral ventrolateral medulla modulates excitatory and inhibitory inputs in spontaneously hypertensive rats. J Hypertens. 2012;30:97–106. https://doi.org/10.1097/HJH.0b013e32834e1df4
Mannoji H, Saku K, Nishikawa T, Tohyama T, Kamada K, Abe K, et al. Estimation of the baroreflex total loop gain by the power spectral analysis of continuous arterial pressure recordings. Am J Physiol Hear Circ Physiol. 2019;316:H828–H839. https://doi.org/10.1152/ajpheart.00681.2018
Zheng H, Katsurada K, Liu X, Knuepfer MM, Patel KP. Specific afferent renal denervation prevents reduction in neuronal nitric oxide synthase within the paraventricular nucleus in rats with chronic heart failure. Hypertension. 2018;72:667–75. https://doi.org/10.1161/HYPERTENSIONAHA.118.11071
Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, van Helden D, et al. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension. 2016;68:1415–23. https://doi.org/10.1161/HYPERTENSIONAHA.116.07850
Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Integr Comp Physiol. 2014;308:R112–R122. https://doi.org/10.1152/ajpregu.00427.2014
Ong J, Kinsman BJ, Sved AF, Rush BM, Tan RJ, Carattino MD, et al. Renal sensory nerves increase sympathetic nerve activity and blood pressure in 2-kidney 1-clip hypertensive mice. J Neurophysiol. 2019;122:358–67. https://doi.org/10.1152/jn.00173.2019
Nagaoka A, Kakihana M. Effects of renal sympathectomy on sodium and water excretion in stroke-prone spontaneously hypertensive rats. Jpn J Pharm. 1982;32:591–7. 10.37//0033-2909.I26.1.78
Nakagawa T, Hasegawa Y, Uekawa K, Ma M, Katayama T, Sueta D, et al. Renal denervation prevents stroke and brain injury via attenuation of oxidative stress in hypertensive rats. J Am Heart Assoc. 2013;2:e000375 https://doi.org/10.1161/JAHA.113.000375
Lappe RW, Webb RL, Brody MJ. Selective destruction of renal afferent versus efferent nerves in rats. Am J Physiol Regul Integr Comp Physiol. 1985;18:R634–637. https://doi.org/10.1152/ajpregu.1985.249.5.r634
Kurtz A. Control of renin synthesis and secretion. Am J Hypertens. 2012;25:839–47. https://doi.org/10.1038/ajh.2011.246
Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press. 2003;12:70–88. https://doi.org/10.1080/08037050310001057
Maeso R, Navarro-Cid J, Muñoz-García R, Rodrigo E, Ruilope LM, Lahera V, et al. Losartan reduces phenylephrine constrictor response in aortic rings from spontaneously hypertensive rats. Hypertension. 1996;28:967–72. https://doi.org/10.1161/01.HYP.28.6.967
Li JS, Touyz RM, Schiffrin EL. Effects of AT1 and AT2 angiotensin receptor antagonists in angiotensin II-infused rats. Hypertension. 1998;31:487–92. https://doi.org/10.1161/01.hyp.31.1.487
Tea BS, Der Sarkissian S, Touyz RM, Hamet P, DeBlois D. Proapoptotic and growth-inhibitory role of angiotensin II type 2 receptor in vascular smooth muscle cells of spontaneously hypertensive rats in vivo. Hypertension. 2000;35:1069–73. https://doi.org/10.1161/01.hyp.35.5.1069
Kobori H, Ichihara A, Suzuki H, Miyashita Y, Hayashi M, Saruta T. Thyroid hormone stimulates renin synthesis in rats without involving the sympathetic nervous system. Am J Physiol. 1997;272:E227–232. https://doi.org/10.1152/ajpendo.1997.272.2.E227
Qiu Y, Zheng F, Ye C, Chen AD, Wang JJ, Chen Q, et al. Angiotensin Type 1 receptors and superoxide anion production in hypothalamic paraventricular nucleus contribute to capsaicin-induced excitatory renal reflex and sympathetic activation. Neurosci Bull. 2020;36:463–74. https://doi.org/10.1007/s12264-019-00460-y
Chen AD, Zhang SJ, Yuan N, Xu Y, De W, Gao XY, et al. Angiotensin AT1 receptors in paraventricular nucleus contribute to sympathetic activation and enhanced cardiac sympathetic afferent reflex in renovascular hypertensive rats. Exp Physiol. 2011;96:94–103. https://doi.org/10.1113/expphysiol.2010.054353
Reja V, Goodchild AK, Phillips JK, Pilowsky PM. Upregulation of angiotensin AT1 receptor and intracellular kinase gene expression in hypertensive rats. Clin Exp Pharm Physiol. 2006;33:690–5. https://doi.org/10.1111/j.1440-1681.2006.04420.x
Milanez MIO, Veiga AC, Martins BS, Pontes RB, Bergamaschi CT, Campos RR, et al. Renal sensory activity regulates the γ-aminobutyric acidergic inputs to the paraventricular nucleus of the hypothalamus in goldblatt hypertension. Front Physiol. 2020;11:601237 https://doi.org/10.3389/fphys.2020.601237
Veiga AC, Milanez MIO, Ferreira GR, Lopes NR, Santos CP, de Angelis K, et al. Selective afferent renal denervation mitigates renal and splanchnic sympathetic nerve overactivity and renal function in chronic kidney disease-induced hypertension. J Hypertens. 2020;38:765–73. https://doi.org/10.1097/HJH.0000000000002304
Foss JD, Fiege J, Shimizu Y, Collister JP, Mayerhofer T, Wood L, et al. Role of afferent and efferent renal nerves in the development of AngII-salt hypertension in rats. Physiol Rep. 2018;6:e13602 https://doi.org/10.14814/phy2.13602
Foss JD, Fink GD, Osborn JW. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase Dahl S hypertension. Am J Physiol Integr Comp Physiol. 2015;310:R262–R267. https://doi.org/10.1152/ajpregu.00408.2015
Katsurada K, Shinohara K, Aoki J, Nanto S, Kario K. Renal denervation: basic and clinical evidence. Hypertens Res. 2022;45:198–209. https://doi.org/10.1038/s41440-021-00827-7
Xia M, Liu T, Chen D, Huang Y. Efficacy and safety of renal denervation for hypertension in patients with chronic kidney disease: a meta-analysis. Int J Hyperth. 2021;38:732–42. https://doi.org/10.1080/02656736.2021.1916100
Ahmad Y, Francis DP, Bhatt DL, Howard JP. Renal denervation for hypertension: a systematic review and meta-analysis of randomized, blinded, placebo-controlled trials. JACC Cardiovasc Inter. 2021;14:2614–24. https://doi.org/10.1016/j.jcin.2021.09.020
Ogoyama Y, Tada K, Abe M, Nanto S, Shibata H, Mukoyama M, et al. Effects of renal denervation on blood pressures in patients with hypertension: a systematic review and meta-analysis of randomized sham-controlled trials. Hypertens Res. 2022;45:210–20. https://doi.org/10.1038/s41440-021-00761-8
Kario K, Yokoi Y, Okamura K, Fujihara M, Ogoyama Y, Yamamoto E, et al. Catheter-based ultrasound renal denervation in patients with resistant hypertension: the randomized, controlled REQUIRE trial. Hypertens Res. 2022;45:221–31. https://doi.org/10.1038/s41440-021-00754-7
Mogi M, Maruhashi T, Higashi Y, Masuda T, Nagata D, Nagai M, et al. Update on hypertension research in 2021. Hypertens Res. 2022;45:1276–97. https://doi.org/10.1038/s41440-022-00967-4
Rey J, Townsend RR. Renal denervation: a review. Am J Kidney Dis. 2022;80:527–35. https://doi.org/10.1053/j.ajkd.2022.03.015
Banek CT, Gauthier MM, Baumann DC, van Helden D, Asirvatham-Jeyaraj N, Panoskaltsis-Mortari A, et al. Targeted afferent renal denervation reduces arterial pressure but not renal inflammation in established DOCA-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol. 2018;314:R883–R891. https://doi.org/10.1152/ajpregu.00416.2017
Kim S, Tokuyama M, Hosoi M, Yamamoto K. Adrenal and circulating renin-angiotensin system in stroke-prone hypertensive rats. Hypertension. 1992;20:280–91. https://doi.org/10.1161/01.HYP.20.3.280
Rudd MA, Grippo RS, Arendshorst WJ. Acute renal denervation produces a diuresis and natriuresis in young SHR but not WKY rats. Am J Physiol. 1986;251:F655–661. https://doi.org/10.1152/ajprenal.1986.251.4.F655
Machino T, Murakoshi N, Sato A, Xu D, Hoshi T, Kimura T, et al. Anti-hypertensive effect of radiofrequency renal denervation in spontaneously hypertensive rats. Life Sci. 2014;110:86–92. https://doi.org/10.1016/j.lfs.2014.06.015
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP21K08032 (KS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KS reports grants from Daiichi Sankyo, Nippon Boehringer Ingelheim, and Otsuka Medical Devices. HT reports grants and/or personal fees from Daiichi Sankyo, Novartis Pharma, Otsuka Pharmaceutical, Pfizer Japan, Mitsubishi Tanabe Pharma, Teijin Pharma, Nippon Boehringer Ingelheim, AstraZeneca, Ono Pharmaceutical, Kowa, IQVIA Service Japan, MEDINET, Medical Innovation Kyushu, Bayer Yakuhin, Johnson & Johnson, NEC, Nippon Rinsho and Japanese Heart Failure Society. Other authors report no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ikeda, S., Shinohara, K., Kashihara, S. et al. Contribution of afferent renal nerve signals to acute and chronic blood pressure regulation in stroke-prone spontaneously hypertensive rats. Hypertens Res 46, 268–279 (2023). https://doi.org/10.1038/s41440-022-01091-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-01091-z
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Renal sympathetic denervation improves pressure-natriuresis relationship in cardiorenal syndrome: insight from studies with Ren-2 transgenic hypertensive rats with volume overload induced using aorto-caval fistula
Hypertension Research (2024)
-
The effects of renal denervation on blood pressure, cardiac hypertrophy, and sympathetic activity during the established phase of hypertension in spontaneously hypertensive rats
Hypertension Research (2024)
-
Renal denervation in patients with chronic kidney disease: an approach using CO2 angiography
Hypertension Research (2024)
-
2023 update and perspectives
Hypertension Research (2024)
-
Renal denervation improves cardiac function independently of afterload and restores myocardial norepinephrine levels in a rodent heart failure model
Hypertension Research (2024)