Abstract
Two prospective multicenter studies demonstrated that a soluble fms-like tyrosine kinase 1 (sFlt-1)/placental growth factor (PlGF) ratio cutoff of ≤38 can rule out preeclampsia within 1 week with a negative predictive value (NPV) of 99.3% (PROGNOSIS) and 98.6% (PROGNOSIS Asia). We report a subanalysis of the Japanese cohort from the PROGNOSIS Asia study. Pregnant women with suspected preeclampsia between gestational weeks 18 + 0 days and 36 + 6 days were enrolled at eight Japanese sites. Primary objectives: Assess the performance of the Elecsys® sFlt-1/PlGF ratio cutoff ≤38 to rule out preeclampsia within 1 week and of the cutoff >38 to rule in preeclampsia within 4 weeks. Key secondary objectives: Prediction of maternal and fetal adverse outcomes (MAOs/FAOs) and their relationship with duration of pregnancy. Of 192 women enrolled, 180 (93.8%)/175 (91.1%) were evaluable for primary/combined endpoint analyses. Overall preeclampsia prevalence was 13.3%. A sFlt-1/PlGF ratio of ≤38 provided an NPV of 100% (95% confidence interval [CI], 97.5–100) for ruling out preeclampsia within 1 week, and a ratio of >38 provided a positive predictive value of 32.4% (95% CI, 18.0–49.8) for ruling in preeclampsia within 4 weeks. The area under the curve for the prediction of preeclampsia/maternal/fetal adverse outcomes within 1 week was 94.2% (95% CI, 89.3–97.8). After adjusting for gestational age and final preeclampsia status, Cox regression indicated a 2.8-fold greater risk of imminent delivery for women with a sFlt-1/PlGF ratio >38 versus ≤38. This subanalysis of Japanese women with suspicion of preeclampsia showed high predictive value for a Elecsys sFlt-1/PlGF ratio cutoff of 38 for short-term prediction of preeclampsia.
Similar content being viewed by others
Introduction
Preeclampsia is a heterogeneous, multiorgan disorder affecting 2–5% of pregnancies worldwide and at least 2.7% of singleton pregnancies in Japan [1,2,3,4,5,6]. The condition is associated with significant maternal and fetal mortality, with hypertensive disorders of pregnancy accounting for ~14% of maternal deaths globally [7].
Triage of women presenting with clinically suspected preeclampsia is challenging, and effective care requires identification and referral of women at high risk [8, 9]. The current “gold standard” for the diagnosis of preeclampsia is based on the presence of new-onset hypertension plus proteinuria and/or other maternal organ dysfunction [1, 10, 11]. However, the predictive value of blood pressure and other clinical characteristics for preeclampsia or adverse pregnancy outcomes is relatively low [12, 13]. Poor prediction of preeclampsia may lead to unnecessary hospitalization of women who will not develop the condition, while others who develop preeclampsia may be overlooked. Therefore, an improved method for the prediction of preeclampsia and associated adverse maternal/fetal outcomes is required.
Soluble fms-like tyrosine kinase 1 (sFlt-1), placental growth factor (PlGF), and vascular endothelial growth factor (VEGF) have been shown to play a pathogenic role in the development of preeclampsia [14]. sFlt-1, an antagonist of PlGF and VEGF, is upregulated in the preeclamptic placenta, leading to increased systemic levels of sFlt-1 and resultant decreases in the circulating levels of PlGF and VEGF [14]. The sFlt-1/PlGF ratio has been shown to be elevated in pregnant women 4–5 weeks prior to clinical onset of preeclampsia [15, 16] and is a reliable tool for discriminating between different types of pregnancy-related hypertensive disorders and for predicting imminent delivery [17, 18]. Two prospective multicenter studies have demonstrated that a sFlt-1/PlGF ratio cutoff of ≤38 can rule out preeclampsia within 1 week with a negative predictive value (NPV) of 99.3% (PROGNOSIS) and 98.6% (PROGNOSIS Asia) [19, 20]. Here, we report the findings from an exploratory subanalysis of the PROGNOSIS Asia study to examine the performance of the sFlt-1/PlGF ratio for the short-term prediction of preeclampsia in the Japanese cohort.
Methods
Study design
PROGNOSIS Asia was a prospective, blinded, multicenter, and observational study conducted across 25 sites in Asia between December 2014 and December 2016; the results of the primary analysis have been reported previously [20].
The Japanese cohort was enrolled at eight sites in Japan (June 2015–May 2016). Diagnostic criteria were based on the International Society for the Study of Hypertension in Pregnancy guidelines [21]. Key inclusion criteria were pregnant women ≥18 years of age at gestational week 18 + 0 days to 36 + 6 days presenting with suspected preeclampsia per protocol-defined criteria, previously published by Bian et al. [20]. Inclusion criteria were aligned with local practice guidelines, whereby the criterion relating to the lower limit of blood pressure was adapted to reflect Japanese practice and gestational week was adapted to 18 weeks (rather than 20 weeks of gestation for the other countries). Key exclusion criteria were manifest preeclampsia or confirmed diagnosis of hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome, multiple pregnancy, confirmed diagnosis of a fetal chromosomal abnormality, or having received treatment with an investigational medicine within 90 days.
The protocol was approved by local ethics committees and institutional review boards at each of the eight sites prior to study initiation. All participants provided written informed consent, and the study was conducted in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonization guidelines for Good Clinical Practice.
Study assessments
Assessments were made at visit 1 (baseline); visit 2 (7–14 days from baseline); visit 3 (24–32 days from baseline); at delivery; and at the postpartum visit. Unscheduled visits occurred in the event of pregnancy complications. Clinical data (medical history, clinical assessments) were collected at all study visits. Serum samples were collected at visits 1–3 according to a standardized operating procedure and stored frozen at −70 °C or −80 °C. Samples were analyzed at a College of American Pathologists-accredited central laboratory in Singapore (Covance Central Laboratory Service, Singapore), where maternal serum levels of sFlt-1 and PlGF were determined by fully automated Elecsys® sFlt-1 and PlGF immunoassays on a Cobas e 601 analyzer (Roche Diagnostics, Mannheim, Germany) [22]. The sFlt-1/PlGF ratio for each sample was calculated by the Roche biostatistics department (Penzberg, Germany), following transfer of all assay results at the end of the study.
Analysis objectives
The primary objectives were to validate the Elecsys sFlt-1/PlGF ratio cutoff of ≤38 to predict the absence of preeclampsia/eclampsia/HELLP syndrome within 1 week of the baseline visit and to validate the Elecsys sFlt-1/PlGF ratio cutoff of >38 to predict the occurrence of preeclampsia/eclampsia/HELLP syndrome within 4 weeks of the baseline visit. Key secondary objectives were to investigate the value of the sFlt-1/PlGF ratio for predicting maternal and fetal adverse outcomes (MAOs, FAOs) within 1 week or 4 weeks and to investigate the relationship between a sFlt-1/PlGF ratio of >38 and time to delivery and preterm delivery. The associations between the sFlt-1/PlGF ratio and the combined endpoint of preeclampsia and/or MAO and/or FAO were also examined. MAOs were defined as any preeclampsia-related adverse outcome other than preeclampsia/eclampsia/HELLP syndrome (e.g., maternal death, pulmonary edema, acute renal failure, cerebral hemorrhage); FAOs included perinatal/fetal death, delivery <34 weeks, fetal growth restriction, placental abruption, neonatal respiratory distress syndrome, necrotizing enterocolitis, and intraventricular hemorrhage.
Statistical analyses
Sample size was calculated for the entire study population of PROGNOSIS Asia [20], not for analyses in subset cohorts. The enrollment target for Japan was a minimum of 145 individuals.
Analyses were conducted using SAS version 9.4 (SAS, Cary, NC, USA) and R version 3.2.2 and version 3.4.0 (R Foundation, Vienna, Austria). Descriptive statistics were reported as medians and interquartile ranges for continuous data and as absolute and relative frequencies for count data. The predictive performance of the sFlt-1/PlGF ratio was determined for each objective by estimation of the NPV, positive predictive value (PPV), sensitivity, specificity, and area under the receiver-operator characteristic (ROC) curve, each with corresponding 95% confidence intervals (CI). A p value of < 0.05 defined a statistically significant difference. Cox regression was used to determine the impact of the sFlt-1/PlGF ratio on remaining pregnancy duration at the time of blood sampling, dichotomized by sFlt-1/PlGF ratios (≤38 vs. >38) and adjusted for gestational age and final preeclampsia status.
Results
Analysis population
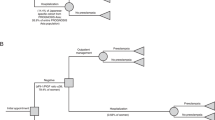
A total of 192 women were enrolled at eight sites in Japan; 180 (93.8%) were evaluable and included in the primary objective analyses (Fig. 1). The overall prevalence of preeclampsia was 13.3% (4.4% within 1 week; 8.9% within 4 weeks; Fig. 1), and the most common reasons for suspected preeclampsia were new onset of elevated blood pressure (48.3%), abnormal uterine perfusion (32.8%), and new onset of protein in the urine (31.1%; Table 1).
The characteristics of women who developed preeclampsia at any time and those who did not develop preeclampsia at any time are shown in Table 1. Median age and prepregnancy body mass index at baseline were comparable between women who did and did not develop preeclampsia. Median blood pressure was higher in women who developed preeclampsia than in those who did not develop preeclampsia (p < 0.001), whereas the median gestational age at delivery (p < 0.001) and median height and weight of the neonate (p = 0.019 and p = 0.002, respectively) were lower in women who developed preeclampsia than in those who did not develop preeclampsia.
Prediction of preeclampsia
Median sFlt-1/PlGF ratio values were higher in women who developed preeclampsia than in those who did not develop preeclampsia (Fig. 2A after 1 week (212.4 vs. 6.8; p < 0.001), 4 weeks (157.7 vs. 6.4; p < 0.001) and overall (88.8 vs. 6.4; p < 0.001).
Performance of the sFlt-1/PlGF ratio for predicting preeclampsia within 1 week and within 4 weeks. A Shows the distribution of sFlt-1/PlGF ratios at baseline and p values for participants who developed or did not develop preeclampsia within 1 week, within 4 weeks and overall.a B Shows the performance of the ratio for ruling out preeclampsia within 1 week (blue) and ruling in preeclampsia within 4 weeks (orange). aBoxes represent the median and interquartile range; the lower whisker represents the larger of the minimum ratios and the 25th quartile to 1.5× interquartile range, while the higher whisker represents the smaller of the maximum ratios and the 75th quartile to 1.5× interquartile range, in log-scale. AUC area under the curve, CI confidence interval, PE preeclampsia, PlGF placental growth factor, sFlt-1 soluble fms-like tyrosine kinase 1
The sFlt-1/PlGF ratio showed high sensitivity and specificity for ruling out preeclampsia within 1 week (ratio ≤38) and ruling in preeclampsia within 4 weeks (ratio >38) (Table 2). Based on a sFlt-1/PlGF ratio ≤38, the NPV for ruling out preeclampsia within 1 week was 100% (95% CI, 97.5–100.0), and the corresponding area under the ROC curve was 95.2% (95% CI, 91.4–98.2) (Fig. 2B). Based on a sFlt-1/PlGF ratio >38, the PPV for ruling in preeclampsia within 4 weeks was 32.4% (95% CI, 18.0–49.8), and the corresponding area under the ROC curve was 90.6% (95% CI, 84.5–95.5) (Fig. 2B).
Prediction of adverse outcomes
A total of 179 participants were eligible for analysis of MAOs. The predictive performance of the sFlt-1/PlGF ratio for MAOs could not be assessed, as only one participant experienced one or more MAOs (central serous chorioretinopathy, pulmonary edema, acute renal failure, and disseminated intravascular coagulation); the participant developed preeclampsia after 4 weeks and had a sFlt-1/PlGF ratio at baseline of 349.8.
A total of 176 participants were eligible for analysis of FAOs. One or more FAOs occurred in 6 individuals within 1 week and in 36 individuals within 4 weeks; at both timepoints, median sFlt-1/PlGF ratios were higher in participants with versus without an FAO (Table 3). Within participants with one or more FAOs, sFlt-1/PlGF ratios were higher in women ruled in for preeclampsia within 1 week and within 4 weeks than in women who were ruled out. The area under the ROC curve for any FAO within 4 weeks in all participants was 82.6% (95% CI, 75.0–89.5) (Table 3; Supplementary Fig. 1).
In total, 175 participants were eligible for analysis of the combined endpoint. In these individuals, a sFlt-1/PlGF ratio of 38 provided good separation of women with and without the combined endpoint of preeclampsia/eclampsia/HELLP syndrome and/or MAO and/or FAO within 1 or 4 weeks (both p < 0.001; Fig. 3A). The area under the ROC curve (95% CI) for predicting the combined endpoint within 1 week was 94.2% (89.3–97.8), and within 4 weeks, it was 85.9% (79.4–91.6) (Fig. 3B).
Performance of the sFlt-1/PlGF ratio for predicting preeclampsia/MAO/FAO within 1 week and within 4 weeks.a A shows the distribution of sFlt-1/PlGF ratios at baseline and p values for participants who developed or did not develop preeclampsia/MAO/FAO within 1 week and within 4 weeks.b B shows the performance of the ratio for predicting preeclampsia/MAO/FAO within 1 week (blue) and within 4 weeks (orange). a175 participants from Japan were eligible for this analysis. bBoxes represent the median and interquartile range; the lower whisker represents the larger of the minimum ratios and the 25th quartile to 1.5× interquartile range, while the higher whisker represents the smaller of the maximum ratios and the 75th quartile to 1.5× interquartile range, in log-scale. AUC area under the curve, CI confidence interval, FAO fetal adverse outcome, MAO maternal adverse outcome, PlGF placental growth factor, sFlt-1 soluble fms-like tyrosine kinase 1
Correlation between sFlt-1/PlGF and delivery
Based on data for 179 eligible participants, a sFlt-1/PlGF ratio >38 at baseline was associated with a shorter pregnancy duration on average, regardless of preeclampsia development (Supplementary Fig. 2). Cox regression analyses showed that the estimated likelihood of imminent delivery was 2.8-fold (95% CI, 1.8–4.2) higher in women with a sFlt-1/PlGF ratio >38 versus a ratio ≤38 after adjustment for gestational age and preeclampsia status. In women who developed preeclampsia, median gestational age did not reach that of full term (≥37 weeks), and among women who did not develop preeclampsia (n = 139), median sFlt-1/PlGF ratios were numerically higher in women with preterm deliveries (<37 weeks, either initiated by a physician [iatrogenic] or not [noniatrogenic]) than in women who delivered at term (≥37 weeks) (Supplementary Fig. 3).
Discussion
The present subanalysis validates the sFlt-1/PlGF ratio cutoff of 38 for short-term prediction of preeclampsia, maternal/fetal adverse outcomes and preterm delivery in Japanese women with clinically suspected preeclampsia. Importantly, a sFlt-1/PlGF ratio cutoff of ≤38 provided an NPV of 100% (95% CI, 97.5–100.0). This will enable clinicians in Japan to rule out preeclampsia within 1 week with a high degree of confidence, thus informing their decision making regarding hospitalization or outpatient monitoring. Additionally, a sFlt-1/PlGF ratio cutoff of >38 provided a PPV of 32.4% (95% CI, 18.0–49.8) to rule in preeclampsia within 4 weeks, which is higher than previously reported PPVs for predictors such as antepartum and intrapartum blood pressures combined (18–20%) and comparable to PPVs based on antepartum blood pressure only (22–36%) [12].
With the exception of slight differences in the inclusion criteria for the Japanese cohort (in line with local practice guidelines), the eligibility criteria in the Japanese and overall PROGNOSIS Asia study cohorts were well matched, and the same diagnostic criteria were applied to the entire population, permitting direct comparison [20]. In this regard, baseline characteristics in the Japanese cohort were generally similar to those for the overall population, including a similar prevalence of preeclampsia (13.3% versus 14.4%, respectively). Key measures of the predictive performance of the sFlt-1/PlGF ratio were also consistent in the Japanese and overall study cohorts, including the NPV for ruling out preeclampsia within 1 week (100% versus 98.6%, respectively), PPV for ruling in preeclampsia within 4 weeks (32.4% versus 30.3%), area under the ROC curve for any FAO within 4 weeks (82.6% versus 83.1%) and risk of imminent delivery in women with sFlt-1/PlGF ratio >38 versus ≤38 (2.8-fold higher versus 3.5-fold higher); for each of these measures, the 95% confidence intervals overlapped between cohorts [20].
Our results were also consistent with those of the PROGNOSIS study, which enrolled a predominantly Caucasian population and reported an incidence of preeclampsia and/or HELLP syndrome of 17.8% in the validation cohort [19]. The baseline characteristics of the Japanese cohort were similar to those of the PROGNOSIS validation cohort, although there were some differences. In the Japanese cohort, the median age (35 years versus 32 years) was higher than in the PROGNOSIS validation cohort of patients from non-Asian countries [19]. This is perhaps to be expected; advanced maternal age (AMA) (≥35 years at the birth of one’s first child) has become increasingly common in Japan, rising from a rate of 8.6% in 1990 to 25.9% in 2012 [23]. Among women of AMA, such as those within the Japanese cohort, there is some discourse surrounding levels of sFlt-1, which should be taken into consideration; it was recently reported that in AMA murine models resembling human AMA, serum sFlt-1 levels were significantly lower than in controls (p < 0.05), suggesting serum sFlt-1 levels are not necessarily reflective of preeclampsia pathogenesis in this setting [24]. This may have implications for the use of the sFlt-1/PlGF ratio in AMA women; however, further investigation in a human population is required. Other differences between the cohorts include the lower median BMI (22.2 kg/m2 versus 26.4 kg/m2) and lower rate of preeclampsia (13.3% versus 17.8%) noted in the Japanese cohort compared with the PROGNOSIS validation cohort [19]. However, the sFlt-1/PlGF ratio still demonstrated a comparable predictive performance between cohorts in terms of the NPV for ruling out preeclampsia within 1 week (Japanese cohort: 100% [95% CI, 97.5–100.0]; PROGNOSIS validation cohort: 99.3% [95% CI, 97.9–99.9]), and the PPV for ruling in preeclampsia within 4 weeks (32.4% versus 36.7%) and the area under the ROC curve values for the combined endpoint were similar for the PROGNOSIS Asia Japan cohort versus PROGNOSIS (1 week: 94.2% versus 88.3%; 4 weeks: 85.9% versus 86.4%), despite some differences between cohorts. For each of these measures, the 95% confidence intervals overlapped between cohorts.
Our findings are also consistent with the results of the randomized INSPIRE study, which recruited pregnant women (≥18 years; gestational weeks 24–37) with a clinical suspicion of preeclampsia at a single tertiary referral center in the United Kingdom [25]. The NPV for ruling out preeclampsia within 1 week was 100% in the present Japanese cohort versus 100% in INSPIRE (with standard clinical management plus sFlt-1/PlGF ratio; 99.2% for the ratio only) [25].
The findings of the present study are applicable to Japanese women presenting with clinically suspected preeclampsia, adding to growing evidence around the predictive value of the sFlt-1/PlGF ratio (cutoff 38) in Caucasian and Asian women [19, 20]. The varied baseline characteristics between the validation cohorts of PROGNOSIS Asia (including this subanalysis for Japan) and that of Zeisler et al. [19] provide support for universal use of the cutoff value of 38 for the Elecsys sFlt-1/PlGF immunoassay ratio, irrespective of individual characteristics. The PROGNOSIS Asia and PROGNOSIS studies both used the Elecsys sFlt-1 and PlGF immunoassays to determine the sFlt-1/PlGF ratio. Importantly, the optimal sFlt-1/PlGF ratio cutoff to predict preeclampsia may differ with immunoassays from other manufacturers. For example, it has been shown that the cutoffs used for the Elecsys sFlt-1/PlGF ratio are not transferrable to the Brahms Kryptor sFlt-1/PlGF immunoassay [26, 27].
The strengths of this work include analyses based on a well-defined sample cohort recruited from multiple sites across Japan and the use of fully automated immunoassays to derive the sFlt-1/PlGF ratio. Limitations include that the PROGNOSIS Asia study was powered for the primary analysis rather than for the present exploratory subanalysis and that only one MAO occurred, preventing separate evaluation of the predictive performance of the sFlt-1/PlGF ratio for MAOs. Additionally, PROGNOSIS and PROGNOSIS Asia were observational studies. Therefore, randomized trials, such as the INSPIRE study [25], are warranted to compare standard of care versus sFlt-1/PlGF-guided prediction for reducing hospitalizations and improving clinical outcomes in women presenting with clinical suspicion of preeclampsia.
Conclusions
This subanalysis of Japanese women with suspicion of preeclampsia enrolled in PROGNOSIS Asia showed the high predictive value of the Elecsys sFlt-1/PlGF ratio cutoff of 38 for short-term prediction of preeclampsia in this population, supporting its use alongside other diagnostic and clinical information. Adoption of the sFlt-1/PlGF ratio into clinical practice has the potential to improve both outcomes for pregnant women with suspected preeclampsia and fetal outcomes.
Data availability
The data that support the findings of this study are available from Roche Diagnostics Ltd but restrictions apply to the availability of these data, which were used under license for the current study, and therefore, are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Roche Diagnostics Ltd.
References
Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4:97–104.
Abalos E, Cuesta C, Carroli G, Qureshi Z, Widmer M, Vogel JP, et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121:14–24.
Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–7.
Kongwattanakul K, Saksiriwuttho P, Chaiyarach S, Thepsuthammarat K. Incidence, characteristics, maternal complications, and perinatal outcomes associated with preeclampsia with severe features and HELLP syndrome. Int J Womens Health. 2018;10:371–7.
Shiozaki A, Matsuda Y, Satoh S, Saito S. Comparison of risk factors for gestational hypertension and preeclampsia in Japanese singleton pregnancies. J Obstet Gynaecol Res. 2013;39:492–9.
Mayama M, Morikawa M, Umazume T, Nakagawa K, Hosokawa A, Yamaguchi M, et al. Increase in the number of patients diagnosed using the new classification of hypertensive disorders of pregnancy in Japan. J Obstet Gynaecol Res. 2019;45:1118–26.
Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–33.
Mikami Y, Takai Y, Era S, Ono Y, Saitoh M, Baba K, et al. Provisional criteria for the diagnosis of hypertension in pregnancy using home blood pressure measurements. Hypertens Res. 2017;40:679–84.
Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124:1094–112.
Watanabe K, Matsubara K, Nakamoto O, Ushijima J, Ohkuchi A, Koide K, et al. Outline of the new definition and classification of “Hypertensive Disorders of Pregnancy (HDP)”; a revised JSSHP statement of 2005. Hypertens Res Pregnancy. 2018;6:33–7.
Takagi K, Yamasaki M, Nakamoto O, Saito S, Suzuki H, Seki H, et al. A review of best practice guide 2015 for care and treatment of hypertension in pregnancy. Hypertens Res Pregnancy. 2015;3:65–103.
Zhang J, Klebanoff MA, Roberts JM. Prediction of adverse outcomes by common definitions of hypertension in pregnancy. Obstet Gynecol. 2001;97:261–7.
North RA, McCowan LM, Dekker GA, Poston L, Chan EH, Stewart AW, et al. Clinical risk prediction for pre-eclampsia in nulliparous women: development of model in international prospective cohort. BMJ. 2011;342:d1875.
Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Investig. 2003;111:649–58.
Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83.
Ohkuchi A, Hirashima C, Takahashi K, Suzuki H, Matsubara S, Suzuki M. Onset threshold of the plasma levels of soluble fms-like tyrosine kinase 1/placental growth factor ratio for predicting the imminent onset of preeclampsia within 4 weeks after blood sampling at 19-31 weeks of gestation. Hypertens Res. 2013;36:1073–80.
Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e51–8.
Stepan H, Hund M, Andraczek T. Combining biomarkers to predict pregnancy complications and redefine preeclampsia: the angiogenic-placental syndrome. Hypertension. 2020;75:918–26.
Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22.
Bian X, Biswas A, Huang X, Lee KJ, Li TK, Masuyama H, et al. Short-term prediction of adverse outcomes using the sFlt-1 (soluble fms-like tyrosine kinase 1)/PlGF (placental growth factor) ratio in Asian women with suspected preeclampsia. Hypertension. 2019;74:164–72.
Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. 2001;20:IX–XIV.
Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202:161.e1–11.
Murakami K, Turale S, Skirton H, Doris F, Tsujino K, Ito M, et al. Experiences regarding maternal age-specific risks and prenatal testing of women of advanced maternal age in Japan. Nurs Health Sci. 2016;18:8–14.
Furuya K, Kumasawa K, Nakamura H, Nishimori K, Kimura T. Novel biomarker profiles in experimental aged maternal mice with hypertensive disorders of pregnancy. Hypertens Res. 2019;42:29–39.
Cerdeira AS, O’Sullivan J, Ohuma EO, Harrington D, Szafranski P, Black R, et al. Randomized interventional study on prediction of preeclampsia/eclampsia in women with suspected preeclampsia: INSPIRE. Hypertension. 2019;74:983–90.
Stepan H, Hund M, Dilba P, Sillman J, Schlembach D. Elecsys® and Kryptor immunoassays for the measurement of sFlt-1 and PlGF to aid preeclampsia diagnosis: are they comparable? Clin Chem Lab Med. 2019;57:1339–48.
Lefevre G, Hertig A, Guibourdenche J, Levy P, Bailleul S, Drouin D, et al. Decision-making based on sFlt-1/PlGF ratios: are immunoassay results interchangeable for diagnosis or prognosis of preeclampsia?. Clin Chem Lab Med. 2020. https://doi.org/10.1515/cclm-2020-0084.
Acknowledgements
The authors wish to acknowledge Toshio Nakayama (Department of Obstetrics and Gynecology, The University of Tokyo Hospital) for recruitment of study participants at The University of Tokyo Hospital. This study was supported in the initial phase by Deirdre Allegranza from Roche Diagnostics International Ltd, Rotkreuz, Switzerland. W.D.J. Verhagen-Kamerbeek of Roche Diagnostics provided clinical science support throughout the study; support for study conduct, monitoring, and measurement of sFlt-1 and PlGF was provided by the Contract Research Organization Covance, Inc. Third-party medical writing assistance, under the direction of the authors, was provided by David Evans, Ph.D., and Chloe Fletcher, MSc (Ashfield MedComms [Macclesfield, UK], an Ashfield Health company) and was funded by Roche Diagnostics International Ltd (Rotkreuz, Switzerland). COBAS, COBAS E, and ELECSYS are trademarks of Roche. The trade name of ELECSYS in Japan is ECLUSYS.
Funding
This study was funded by Roche Diagnostics International Ltd (Rotkreuz, Switzerland).
Author information
Authors and Affiliations
Contributions
AD, SG, MH: study design and concept development, analysis and interpretation of data. AO, SS, TY, HM, HM, KK, JY, TN: collection and interpretation of data. All authors drafted the paper and approved the final version for submission.
Corresponding author
Ethics declarations
Conflict of interest
KK, HM, HM, TN, and JY report no competing interests. AO, TY, and SS received fees from Roche Diagnostics Ltd during the conduct of the study. AD and SG are employees of Roche Diagnostics Ltd. MH is an employee of Roche Diagnostics Ltd and holds stock in F. Hoffmann-La Roche. MH also reports being an inventor of patents related to the sFlt-1/PlGF or endoglin/PlGF ratio to rule out the onset of preeclampsia in pregnant women within a certain time period (PCT/EP2013/063115) and the dynamics of the sFlt-1 or endoglin/PlGF ratio as an indicator for imminent preeclampsia and HELLP syndrome (PCT/EP2012/072157).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ohkuchi, A., Saito, S., Yamamoto, T. et al. Short-term prediction of preeclampsia using the sFlt-1/PlGF ratio: a subanalysis of pregnant Japanese women from the PROGNOSIS Asia study. Hypertens Res 44, 813–821 (2021). https://doi.org/10.1038/s41440-021-00629-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-021-00629-x
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Maternal angiogenic factor disruptions prior to clinical diagnosis of preeclampsia: insights from the REVAMP study
Hypertension Research (2024)
-
Prediction of preterm preeclampsia risk in Asians using a simple two-item assessment in early pregnancy
Hypertension Research (2024)
-
Update on Hypertension Research in 2021
Hypertension Research (2022)
-
Economic evaluation of the sFlt-1/PlGF ratio for the short-term prediction of preeclampsia in a Japanese cohort of the PROGNOSIS Asia study
Hypertension Research (2021)