Abstract
Pigmentation is an excellent trait to examine patterns of evolutionary change because it is often under natural selection. Benthic and limnetic threespine stickleback (Gasterosteus aculeatus) exhibit distinct pigmentation phenotypes, likely an adaptation to occupation of divergent niches. The genetic architecture of pigmentation in vertebrates appears to be complex. Prior QTL mapping of threespine stickleback pigmentation phenotypes has identified several candidate loci. However—relative to other morphological phenotypes (e.g., spines or lateral plates)—the genetic architecture of threespine stickleback pigmentation remains understudied. Here, we performed QTL mapping for two melanic pigmentation traits (melanophore density and lateral barring) using benthic-limnetic F2 crosses. The two traits mapped to different chromosomes, suggesting a distinct genetic basis. The resulting QTLs were additive, but explained a relatively small fraction of the total variance (~6%). QTLs maps differed by F1 family, suggesting variation in genetic architecture or ability to detect loci of small effect. Functional analysis identified enriched pathways for candidate loci. Several of the resulting candidate loci for pigmentation, including three loci in enriched pathways (bco1, sulf1, and tyms) have been previously indicated to affect pigmentation in other vertebrates. These findings add to a growing body of evidence suggesting pigmentation is often polygenic.
Similar content being viewed by others
Introduction
Across the animal kingdom, pigmentation serves as an important communication signal (or disruptor) intra- and interspecifically and thus is often under natural and sexual selection (Cuthill et al. 2017; Hubbard et al. 2010; Orteu and Jiggins 2020; Protas and Patel 2008). Pigmentation and patterning are critical for many key biological functions or interactions–including mate choice, thermoregulation, microbial resistance, crypsis, toxicity warning, and mimicry (Cuthill et al. 2017; Protas and Patel 2008). These signals are often generated through a combination of pigment cells and reflective structures, and both pigmentation and patterning are under strong genetic control (Jablonski and Chaplin 2017; Lopes et al. 2016; Luo et al. 2021; Nachman et al. 2003). As such, animal pigmentation genetics has emerged as a robust model system to study the phenotype-genotype relationship. The interest in this trait has led to the identification of hundreds of loci and pathways involved in vertebrate pigmentation (Elkin et al. 2022; Hidalgo et al. 2022; Kelsh 2004; Lynn Lamoreux et al. 2010). However, studies on the genetic basis of pigmentation are often biased toward certain taxonomic groups (e.g., mice, humans), populations, and/or candidate loci (e.g., mc1r, agouti) (Elkin et al. 2022; Tapanes et al. 2022)). For example, ~70% of known pigmentation candidate genes emerged from mammalian studies, but only 5% are associated with fish (Elkin et al. 2022). Additional taxa and loci must be studied and identified to gain a more comprehensive and unbiased understanding of the genetic architecture of pigmentation.
Through characterization of the genetic architecture of pigmentation, we gain insight into evolutionary phenomena, including the predictability of phenotypic and genotypic evolution as well as the origins of adaptive genetic variation (Cuthill et al. 2017; Elkin et al. 2022; Martin and Orgogozo 2013). So far, work suggests that often the same loci are co-opted across vast evolutionary scales to produce convergent pigmentation phenotypes (Crawford et al. 2017; Lamason et al. 2005; Miller et al. 2007; Saenko et al. 2015)). For example, oca2 underlies melanin deficiency in humans and corn snakes. Frequent identification of major effect genes (e.g. mc1r) in studies of specific populations (i.e., mice, European people) led to the assumption that pigmentation has a simple genetic architecture, with mutations of large effect generating key phenotypes (Hubbard et al. 2010; Protas and Patel 2008; Quillen et al. 2019). However, recent evidence suggests the underlying genetic architecture of this trait may frequently be complex (highly polygenic) (Anderson et al. 2009; Jones et al. 2018). Further, pigmentation can exhibit rapid phenotypic and genetic change, quickly evolving within a handful of generations, and adapting to new environments (Barrett et al. 2019; Jones et al. 2018). To fully understand pigmentation in light of evolutionary change, we must be able to robustly characterize the genetic architecture in more than a handful of organisms.
The threespine stickleback (Gasterosteus aculeautus; hereafter referred to as ‘stickleback’) offers an opportunity to study key evolutionary processes and patterns, such as—evolutionary predictability and adaptation, and with ample genetic resources it is possible to characterize genetic architecture of adaptive traits. Marine stickleback repeatedly and rapidly colonized newly formed freshwater habitats at the end of the Pleistocene (≈12,000 years ago) (Bell and Foster, 1994). Within lakes and streams, stickleback independently adapted to the local ecological conditions–often diverging along a benthic-limnetic axis. Within a handful of lakes there has been the evolution of sympatric benthic and limnetic ecotypes which utilize the littoral and pelagic regions of the lakes, respectively (Schluter and McPhail 1992). These sympatric ecotypes have diverged both genetically and phenotypically in response to their divergent niches (Jones et al. 2012; Peichel et al. 2001; Schluter and McPhail 1992). Notably, phenotypic divergence involves suites of trophic (Schluter and McPhail 1992) and defensive traits (Vamosi and Schluter 2004). However, the ecotypes have also diverged in several pigmentation traits (Clarke and Schluter 2011; Greenwood et al. 2011; Gygax et al. 2018; Miller et al. 2007).
Limnetic fish exhibit greater ventral pigmentation (Miller et al. 2007) and more lateral barring than benthic fish (Greenwood et al. 2011). Increased brightness has been associated with increased use of limnetic resources (French et al. 2018; Bolnick and Ballare 2020; Lavin and McPhail, 1986). Additionally, green pigmentation in the dorsal region is more prevalent in benthic fish (Clarke and Schluter 2011; Gygax et al. 2018). Males of each ecotype also differ in their nuptial coloration—limnetic males exhibit redder throat patches relative to benthic stickleback, and often have an intensely blue iris (Boughman 2001). Differences in pigmentation are predicted to be adaptive as there is covariance with the spectral qualities of each ecotype’s primary habitat (littoral vs. pelagic) (Clarke and Schluter 2011; Rennison et al. 2016); and the preferred nest sites of the two ecotypes also differ in spectral quality (Boughman 2001). The visual sensitivities of the two ecotypes also exhibit divergence (Boughman 2001; Rennison et al. 2016), suggesting differential perception of intra- and inter-specific pigment signals could contribute to pigmentation divergence (Boughman 2001). Further, there are distinct predation regimes between the habitats (Vamosi and Schluter 2004) and differential exposure to a vertebrate predator has been found to be associated to divergence of pigmentation (Gygax et al. 2018), suggesting selection due to crypsis may also drive the evolution of pigment differences.
Quantitative trait mapping (QTL) studies for some stickleback pigmentation traits have successfully identified candidate genes or genomic regions. So far, work using marine-freshwater pairs has characterized candidate regions for two pigmentation traits: lateral barring and ventral melanism (Greenwood et al. 2011; Greenwood et al. 2012). Candidate regions have also been found for both male and female nuptial coloration, specifically male red throat chroma was mapped in a benthic-limnetic pair (Malek et al. 2012) and red throat and pelvic spine pigmentation in females from allopatric stickleback populations (Yong et al. 2015). Yet, in general, we know little about the genetic architecture of pigmentation traits of stickleback or how the genetic architecture varies across populations. More than 1000 QTL have been identified for various stickleback phenotypes (behavioral, morphological, or life history), but only 20 (1.7%) are associated with pigmentation traits. Furthermore, of the 27 threespine stickleback QTL studies included in a 2017 meta-analysis of stickleback QTL, only four studies mapped pigment traits (Peichel and Marques 2017). However, gene expression studies have also been useful in the identification of pigment-associated genes (McKinnon et al. 2022).
Here, we conducted a QTL mapping study of two melanin-based pigmentation phenotypes—melanophore density and lateral barring using threespine stickleback benthic-limnetic F2 crosses. We focused on these traits as there is experimental evidence that melanism and lateral barring are adaptive phenotypes, diverging in response to differential predation pressures (Gygax et al. 2018), which aids in vertebrate predator avoidance. Once candidate regions were identified, functional enrichment analyses were used to further characterize the resulting loci.
Materials and Methods
Four F1 crosses were made in the Spring of 2011 using four benthic females and four limnetic males collected from Paxton Lake on Texada Island, British Columbia, Canada. These F1 families were reared in 100 L tanks under standard laboratory conditions for nine months. Once the fish reached reproductive maturity in the Spring of 2012, each F1 family was split between a pair of semi-natural ponds (n = 8 ponds), which were located on the University of British Columbia campus in Vancouver, Canada. The pond-rearing facility and cross-design have been previously described (Arnegard et al. 2014; Rennison et al. 2019). Each pond was 25 m × 15 m, and included a vegetated littoral zone as well as a deep-water habitat of 6 m (S1). The ponds contained typical natural food resources and invertebrate predators. Once in the ponds, the F1 fish reproduced naturally from May – July 2012. In the fall of 2012, a sample of the resulting juvenile F2 fish was taken from each pond (S2). Immediately following collection by minnow trap or net, fish were euthanized using MS-222 and preserved in 95% ethanol. The fish were fin-clipped for DNA extraction and subsequent genotyping.
Pigmentation variation
We phenotyped two pigmentation traits in our F2 sample (N = 400)—melanophore density and lateral barring (Fig. 1). Using Adobe Photoshop, we estimated melanocyte density by manually counting the number of visible melanocytes on the fin junction using the ‘count tool’ and dividing the resulting count value by the total area (in mm) (similar to (Miller et al. 2007)) (S3). We estimated lateral barring by converting each photo to black and white and estimating the absolute difference between a light and dark patch on the ventral flank (S4). This was done by selecting two squares 11 × 11 pixels in size, and placing the first point on the dark bar superior to the start of the anal fin. If the fish exhibited a clear barring pattern, the second square was placed on the brighter region between the two dark bars. If the fish lacked a clear barring pattern, we selected two squares at an average distance typical of two dark bars (≈1.12 mm) and took the absolute difference between both points. To account for potential differences in lighting conditions across each photo, we divided the absolute difference by the average grey value of the photo, and then multiplied that number by 100 (similar to (Greenwood et al. 2011)).
Since some previous pigmentation work has relied on scoring phenotypes on living fish (Greenwood et al. 2011; Gygax et al. 2018; Clarke and Schluter 2011), we first sought to test the validity of scoring melanic pigmentation on stained and preserved fish. Our verification sample included live wild marine (Sooke, Courtenay, Sayward estuaries) and freshwater (Mohun, Comox, and Muchalat lakes) populations (N = 170) that were collected in British Columbia in Spring 2021. We photographed fish prior to euthanasia against an X-rite color checker passport using a Nikon D500 mounted with the AF-S DX NIKKOR 16–80 mm f/2.8–4E ED VR lens (S5). We set a manual white balance by calibrating the camera with a grey standard in the field. All fish were photographed against a blue background following established protocols (Gygax et al. 2018; Stevens et al. 2007). Instead of melanophore density, we scored brightness as a proxy for overall pigmentation as brightness is a good measure of the relative prevalence of black pigmentation. This was necessary because the melanophore density was difficult to score on many of the marine individuals due to the presence of other colors (e.g., silver iridescence) and bony elements. We estimated an individual’s brightness using the Red-Green-Blue (RGB) color model sampled from the skin at the fin junction. We scored lateral barring following the same methodology used on preserved specimens (described above). Following tissue fixation in formalin and alizarin red staining following the protocol of Peichel et al. 2001, the fish were re-photographed and scored for melanophore density and lateral barring. A Pearson correlation test was used for both traits in order to assess if there was a meaningful relationship between pigmentation estimates obtained from stained versus living fish. A negative (melanophore density vs brightness) or positive correlation (lateral barring) between pre and post-staining measurements (at p < 0.05) indicated our stained pigment measurements indeed captured a meaningful aspect of biological reality.
QTL mapping
The underlying genotype data was generated for and used in a previously described experiment (Rennison et al. 2019). DNA was extracted from each F2 individual’s fin clip (N = 400, 50 from each pond) using a standard phenol-chloroform extraction protocol. DNA was also extracted from the F1 parents and F0 grandparents. Libraries were prepared using the PstI enzyme following a genotyping by sequencing protocol (as in (Elshire et al. 2011)) and sequenced on an Illumina Hi-Seq 2000 platform. A standard reference-based bioinformatics pipeline was used to identify sequence variants (single nucleotide polymorphisms, SNPs) (see archived code from Rennison et al. 2019 for full details). Briefly, after demultiplexing, Trimmomatic (Bolder et al. 2014) was used to filter out low-quality sequences and adapter contamination. Reads were aligned to the stickleback reference genome (Jones et al. 2012) using BWA v0.7.9a (Li and Durbin 2009) with subsequent realignment using STAMPY v1.0.23 (Lunter and Goddson, 2011). For genotyping the GATK v3.3.0 (McKenna et al. 2010) best practices workflow (DePristo et al. 2011) was followed except that the MarkDuplicates step was omitted. RealignTargetCreator and IndelRealigner were used to realign reads around indels and HaplotypeCaller identified single nucleotide polymorphisms (SNPs) in individuals. Joint genotyping was done across all individuals using GenotypeGVCFs. The results were written to a single VCF file containing all variable sites. This file was filtered for a minimum quality score (of 20) and depth of coverage (minimum of 8 reads and maximum of 100,000) before use in downstream analyses.
A pedigree was built using the MasterBayes R package and a set of 1799 SNPs, which had minimal (<10%) missing data across all individuals. In order to have fully informative markers, only SNPs that were homozygous for alternative alleles in the benthic and limnetic grandparents of each F2 cross were used. These SNPs were then used to calculate pairwise recombination frequencies and create a genetic map using JoinMap version 3.0 (Ooijen and Voorrips, 2001). In total, 398 F2 progeny from the four F1 crosses were used for mapping, with many F2 families. F2 genotypes were coded according to the population code for outbred crosses, allowing segregation of up to four alleles per locus (cross-pollinator). The JMGRP module of JoinMap was used with a LOD score threshold of 4.0 to assign 2243 loci to 33 linkage groups. For each linkage group, a map was created with the JMMAP module. Mapping was done using the Kosambi function with a LOD threshold of 1.0, recombination threshold of 0.499, jump threshold of 5.0, and no fixed order. Two rounds of mapping were performed, with a ripple performed after each marker was added to the map.
QTL mapping was performed using Haley-Knott regressions across the F1 families (all-family QTL) and individual sex and family were set as covariates in the R/qtl package (Broman and Sen 2009) with 2037 SNP markers spread across the 21 chromosomes. The marker density was on average 4.2 SNP markers per kilobase (kb) (mean range across chromosomes 2.3 – 5.9 SNPs/kb). To test if there was any variation among the four F1 families in the genetic basis of divergent pigmentation, we also performed QTL mapping independently for each family (within-family QTLs) with sex as a covariate, and sample sizes of 93–99 individuals per F1 family. We calculated the percentage variance explained (PVE) for each candidate locus using the equation: PVE = 1 – (10^(−2*(LOD/n))), where LOD is the estimated LOD score and n is the sample size. The significance threshold for each phenotype was estimated using permutation testing with 10,000 iterations. Overall phenotypic heritability for the two pigmentation traits was calculated by estimating a kinship matrix using the kinship2 R package and the est_herit function in the qtl2 package.
Functional enrichment testing
We identified candidate genes from within the 1.5 LOD interval region surrounding the QTL peaks; the resulting candidates were then explored through comparisons to existing candidates from other taxa and using functional enrichment testing. First, we determined whether any of the candidate loci found within our QTL peaks had been previously associated with pigmentation in other vertebrates. To this end, we determined what genes fell within the chromosomal regions associated with the significant peaks in the all-family QTL. We searched for matches between our candidate gene list and pigmentation loci using previously curated and published pigmentation lists (Baxter et al. 2019; McKinnon et al. 2022). Second, we performed functional enrichment of Gene ontology terms associated with the candidate gene list to assess if any functional pathways were overly represented. We used the biomaRt package to extract gene position, gene IDs, HGNC symbols, and associated gene ontology (GO) terms from the stickleback genome (Durinck et al. 2009). The GO pathways associated with each gene ID of interest in our study were extracted and topGO was used to test for functional enrichment across our set of candidates (Alexa and Rahnenführer 2023). To test for significant enrichment, a Fisher’s exact test based on annotated gene counts was run that took into consideration GO hierarchy, and the algorithm was set to weight01. Significant GO terms were visualized using ‘showSigOfNodes’ and we identified which and how many genes in our candidate gene list were associated with these pathways.
Results
Pigmentation variation
The pigmentation phenotypes characterized in our F2 mapping population of stained fish appeared to capture a biologically relevant pigment trait present in live wild-caught fish (Fig. 2). There was evidence of a significant negative correlation between melanophore density of stained fish and brightness at the fin junction measured from living fish (r = −0.41, p < 0.0001). Thus, the “brighter” the operculum (i.e., the less dark), the lower the melanophore density. There was also a significantly positive correlation between lateral barring in living and stained fish (r = 0.71, p < 0.0001). The strength of the relationship between these variables varied amongst the populations for both lateral barring (0.63 to 0.36) and melanophore density (−0.26 to −0.54).
Within the benthic-limnetic F2 hybrid individuals used for QTL mapping, we did not find any relationship between the two surveyed pigmentation traits (melanophore density and lateral barring) (r = 0.01; p > 0.05). This suggests that these phenotypes are largely independent of each other. Across the F1 mapping families there were statistically significant differences in melanophore density (F = 6.79; p < 0.0001) and lateral barring (F = 9.45; p < 0.0001). In general, families two and three exhibited greater melanophore density at the fin junction, while families two and four displayed more lateral barring (Fig. 3).
QTL mapping
Melanophore density
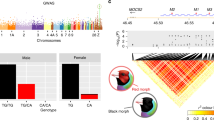
When mapping melanophore density across all four F1 families, there was a single significant QTL peak, which was located on chromosome 8 (Figs. 4A, S6). Examination of the 1.5-LOD support interval placed the peak in a region encompassing ≈8 Mb, between markers ChVIII:7120492 and ChVIII:14925951. The alleles at this QTL had predominantly additive effects (Fig. 4B), although when family four was analyzed independently it exhibited a non-additive effect (Fig. 4C). Across families, this QTL explained ~5% of the total phenotypic variance. Within each family the variance explained ranged from 7.4 – 10.2% (Family 1 = 7.42%; Family 2 = 10.21%; Family 3 = 7.85%; Family 4 = 9.03%). QTL mapping conducted within each F1 family identified an additional peak in family 1 for melanophore density on chromosome 18 between markers ChXVIII:2652629 and ChXVIII:12506627 (Table 1) that explained 9% of the phenotypic variance. The total heritability of this trait was 0.28 when estimated across all families and markers.
Lateral Barring
In the all F1 family QTL analysis for lateral barring, a significant candidate peak was identified on chromosome 21. Examination of the 1.5-LOD support interval places the peak in a region encompassing ≈7 Mb, between markers chrXXI:3061663 and chrXXI:10192631 (Figs. 5A, S7). Similar to mapping for melanophore density, the effects were additive in the all-family analysis (Fig. 5C). The overall percent variance explained was 4.26%, and phenotypic variance explained again differed among families ranging from 4.5 – 11% (Family 1 = 4.62%; Family 2 = 11.03%; Family 3 = 11.24%; Family 4 = 5.22%). One additional candidate on chromosome 16 was identified in the F1 family 2 analysis (see Table 2). The total heritability of this trait was 0.35 across all families and markers.
Functional enrichment
Across both traits, we identified 1,099 loci associated within significant QTL intervals in both the all-family and within-family QTLs. Across the all-family crosses, there were 638 genes within the 1.5 LOD interval across both outlier chromosomal regions, and approximately 5% were previously identified pigmentation genes. Among the candidates resulting from our all-F1 family map of melanophore density, we identified 350 candidate genes, and 11 of these genes (~4%) were previously shown to be associated with pigmentation in other vertebrates. For lateral barring, we identified 288 candidates in the all-family cross, and of these, 8 genes (~3%) were previously known vertebrate pigmentation loci.
To further investigate our set of candidates, we performed functional enrichment testing separately on candidate regions on chromosomes eight and twenty-one, for melanophore density and lateral barring, respectively. Using the lists of 140 and 119 annotated candidate genes we identified 22 significantly enriched pathways (Table 3). From the annotated chromosome 8 candidates, there were 8 enriched pathways: five involved in catalytic activity, one in transporter activity, one in binding, and the last in molecular function regulation (S8). Among the chromosome 21 candidates, there were 14 pathways significantly enriched: three involved in binding, and the rest involved with catalytic activity (S9). Within these enriched pathways, three of the genes have been previously associated with pigmentation pathways (Table 3).
Discussion and Conclusion
We investigated the genetic basis of two melanin pigmentation phenotypes, lateral barring and melanophore density, using a large sample of benthic-limnetic F2 hybrid stickleback. QTL mapping was conducted across and within families; the resulting candidates were compared to known pigmentation genes and their functions were explored using GO term analysis. Using this approach, we found that the two pigmentation traits were uncorrelated and mapped to distinct genetic regions (Figs. 4A and 5A), suggesting they are independent traits. Melanocyte density mapped to chromosome 8 (Fig. 4A), and degree of lateral barring mapped to chromosome 21 (Fig. 5A). A previous QTL study of marine-freshwater stickleback crosses found candidates for melanization of the gills and ventral flank map to the kitlg locus on chromosome 19 (Miller et al. 2007). Prior work examining the degree of lateral barring in marine-freshwater crosses identified candidate regions on chromosomes 1, 6, and 11 (Greenwood et al. 2011). This suggests that distinct genes may underly different components of melanism across the body and/or that the genetic architecture of lateral barring may differ between marine-freshwater populations relative to benthic-limnetic populations. Alternatively, the distinct loci underlying lateral barring in marine-freshwater pairs could be due to a failure to detect small effects in the benthic-limnetic crosses. Our results indicate that the effects on the QTL were additive (Figs. 4B and 5B), which is in line with previous findings (Miller et al. 2014). The identified loci are of relatively small effect, suggesting that these traits are likely polygenic.
Large effect loci have been identified for several key ecological traits in stickleback including lateral plate count (>76% variance explained), neuromast pattern (>39% variance explained) and pelvic spine length ( > 65% variance explained) (Colosimo et al. 2005; Erickson et al. 2016; Wark et al. 2012). Yet, small effect loci also contribute to these traits, and variance in other important stickleback traits, including other defense traits (e.g. dorsal spine length), trophic traits (e.g. gill raker number and length, tooth number), and body shape have been shown to have a highly polygenic architecture with many loci of relatively small effect contributing to phenotypic variation (Erickson et al. 2016; Miller et al. 2014; Peichel and Marques 2017). Prior work on melanization and degree of barring found a combination of relatively small effect loci (6.6–11.7% variance explained) and moderate effect loci (~20% variance explained) (Greenwood et al. 2011; Greenwood et al. 2012). In contrast, a large effect locus, kitlg, explains >56% of the variance in gill melanic pigments in marine-freshwater (Miller et al. 2007). Variability in the complexity of the architecture of pigmentation has also been found in other taxa. For example, in Peromyscus mice and several lizard species, differences in pigmentary loss or gain has been attributed to a single mutation (Hoekstra et al. 2006; Nachman et al. 2003; Rosenblum et al. 2010), while in Drosophila, the degree of melanization is often associated with a suite of small effect genes (Dembeck et al. 2015).
Within a single species, differences in genetic background can impact the phenotype through epistatic interactions. For example, in beach mice, lighter coloration associated with one gene (mc1r) is not apparent unless another gene (asip) also increases its expression (Steiner et al. 2008). The phenotypic and genetic effects of pigmentation loci will thus vary among and between populations and species (Hubbard et al. 2010; Manceau et al. 2010). Our results indicate the variation between families in their expressed phenotypes, effect sizes, and dominance effects (Figs. 3, 5C), which could be due to epistasis. A similar pattern of family-level variation was detected previously when mapping skeletal traits in benthic-limnetic F2 crosses (Rennison et al. 2019). The observed variation in mapped QTLs among F1 families could result from the presence of different segregating variances present in the pure benthic and limnetic parents used for each F1 cross. Alternatively, the variation in F1 families may be a result of stochastic differences in the power of detection of these relatively small effect loci. With only 100 individuals per family, loci near the significance cut-off could fall just above the threshold in one family and below in another. Differences in the fraction of missing data across individuals for each family could also contribute to the pattern of variable detection. Unfortunately, due to the absence of inter F1 family crosses and the relatively small sample sizes of the individual families we did not have the power or experimental framework to investigate these potential epistatic effects in this experiment.
From the candidate QTL regions for these two pigmentation traits, several new candidate pigmentation genes were identified for benthic and limnetic stickleback (S6). Three of the candidate loci (sulf1, bco1, tyms) were associated with functionally enriched pathways (Table 3). Of these, bco1 has been previously associated with fish carotenoid pigmentation, including in threespine stickleback (Huang et al. 2021; McKinnon et al. 2022). Another candidate, tyms, is known to lead to abnormal pigmentary patterns in zebrafish (Amsterdam et al. 2004; Phenotype Annotation (1994–2006), 2006). While all three of these genes are associated with pigmentation phenotypes in other vertebrates, only one is associated with other stickleback pigmentation phenotypes. Of the genes found proximate to our QTL peaks, >500 had no prior known role in pigmentation and only 30 (~5.5%) were functionally enriched. Thus, our survey expands this candidate list of potential loci underlying pigmentation evolution.
This study demonstrates that quantification/characterization of melanic pigmentation in stained stickleback provides a likely functionally relevant estimation of melanic traits in living fish. Previous pigmentation work on stickleback has been largely limited to phenotyping conducted using photos from living fish, specimens phenotyped immediately following euthanasia, or surveys of internal structures (Greenwood et al. 2011; Malek et al. 2012; McKinnon et al. 2022; Yong et al. 2015). Our finding that pigmentation phenotypes collected from preserved and stained specimens produce biologically meaningful data opens up additional opportunities to study pigmentation using museum collections or in instances where live photographs would be difficult to collect. Stickleback pigmentation genetics remains understudied relative to other traits (Reid et al. 2021), and fish pigmentation genetics is generally understudied relative to other vertebrates (Elkin et al. 2022). This is likely due in part to pigmentation being more challenging to quantify in fish than in other vertebrates. For example, while mammals have only one type of chromatophore, fish have six different kinds (black melanophores, yellow-orange xanthophores, red erythrophores, light-reflecting iridophores, white leucophores, blue cyanophores) (Cal et al. 2017; Kelsh 2004). Many chromatophores exhibit plasticity to environmental conditions (e.g., red erythrophores influenced by carotenoid availability in the diet (Pike et al. 2011). However, the extent to which pigment phenotypes can change depends on the concentrations of melanosomes (Logan et al. 2006). As such, melanic traits may be more static and thus more easily phenotyped. As the list of pigmentation QTLs grows for threespine stickleback—a model in evolutionary biology—it can aid us in understanding the predictability of phenotypic and genotypic evolution and the origins of adaptive genetic variation.
Data archiving
Underlying data and code are archived on GitHub: https://github.com/djrennison/Heredity_pigment.
References
Alexa A, Rahnenführer J (2023) Gene set enrichment analysis with topGO. Biocond Improv 27:1–26
Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N (2004) Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci USA 101(35):12792–12797
Anderson TM, vonHoldt BM, Candille SI, Musiani M, Greco C, Stahler DR et al. (2009) Molecular and evolutionary history of melanism in North American gray wolves. Science 323(5919):1339–1343
Arnegard ME, McGee MD, Matthews B, Marchinko KB, Conte GL, Kabir S et al. (2014) Genetics of ecological divergence during speciation. Nature 511(7509):307–311
Barrett RDH, Laurent S, Mallarino R, Pfeifer SP, Xu CCY, Foll M et al. (2019) Linking a mutation to survival in wild mice. Science 363(6426):499–504
Baxter LL, Watkins-Chow DE, Pavan WJ, Loftus SK (2019) A curated gene list for expanding the horizons of pigmentation biology. Pigment Cell Melanoma Res 32(3):348–358
Bell, MA, and Foster, SA (Eds.). (1994). The Evolutionary Biology of the Threespine Stickleback. Oxford University Press
Bolder AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Bolnick DI, Ballare KM (2020) Resource diversity promotes among-individual diet variation, but not genomic diversity, in lake stickleback. Ecol Lett 23(3):495–505
Boughman JW (2001) Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411(6840):944–948
Broman KW, Sen S (2009) A guide to QTL mapping with R/qtl. Springer Science and Business Media
Cal L, Suarez-Bregua P, Cerdá-Reverter JM, Braasch I, Rotllant J (2017) Fish pigmentation and the melanocortin system. Comp Biochem Physiol 211:26–33
Clarke JM, Schluter D (2011) Colour plasticity and background matching in a threespine stickleback species pair. Biol J Linn Soc 102(4):902–914
Colosimo PF, Hosemann KE, Balabhadra S, Villarreal Jr G, Dickson M, Grimwood J et al. (2005) Widespread parallel evolution in sticklebacks by repeated fixation of Ectodysplasin alleles. Science 307(5717):1928–1933
Crawford NG, Kelly DE, Hansen MEB, Beltrame MH, Fan S, Bowman SL et al. (2017) Loci associated with skin pigmentation identified in African populations. Science 358(6365):eaan8433
Cuthill, IC, Allen, WL, Arbuckle, K, Caspers, B, Chaplin, G, Hauber, ME, et al. (2017). The biology of color. Science, 357(6350)
Dembeck LM, Huang W, Magwire MM, Lawrence F, Lyman RF, Mackay TFC (2015) Genetic Architecture of Abdominal Pigmentation in Drosophila melanogaster. PLoS Genet 11(5):e1005163
DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C et al. (2011) A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43:491–498
Durinck S, Spellman PT, Birney E, Huber W (2009) Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc 4(8):1184–1191
Elkin, J, Martin, A, Courtier-Orgogozo, V, and Emília Santos, M (2022). Meta-analysis of the genetic loci of pigment pattern evolution in vertebrates. In bioRxiv (p. 2022.01.01.474697)
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PloS One 6(5):e19379
Erickson PA, Glazer AM, Killingbeck EE, Agoglia RM, Baek J, Carsanaro SM et al. (2016) Partially repeatable genetic basis of benthic adaptation in threespine sticklebacks. Evolution 70(4):887–902
French CM, Ingram T, Bolnick DI (2018) Geographical variation in colour of female threespine stickleback (Gasterosteus aculeatus). PeerJ 6:e4807
Greenwood AK, Jones FC, Chan YF, Brady SD, Absher DM, Grimwood J et al. (2011) The genetic basis of divergent pigment patterns in juvenile threespine sticklebacks. Heredity 107(2):155–166
Greenwood AnnaK, Cech JN, Peichel CL (2012) Molecular and developmental contributions to divergent pigment patterns in marine and freshwater sticklebacks. Evol Dev 14(4):351–362
Gygax M, Rentsch AK, Rudman SM, Rennison DJ (2018) Differential predation alters pigmentation in threespine stickleback (Gasterosteus aculeatus). J Evolut Biol 31(10):1589–1598
Hidalgo M, Curantz C, Quenech’Du N, Neguer J, Beck S, Mohammad A, Manceau M (2022) A conserved molecular template underlies color pattern diversity in estrildid finches. Sci Adv 8(35):eabm5800
Hoekstra HE, Hirschmann RJ, Bundey RA, Insel PA, Crossland JP (2006) A single amino acid mutation contributes to adaptive beach mouse color pattern. Science 313(5783):101–104
Huang D, Lewis VM, Foster TN, Toomey MB, Corbo JC, Parichy DM (2021) Development and genetics of red coloration in the zebrafish relative Danio albolineatus. ELife 10:e70253
Hubbard JK, Uy JAC, Hauber ME, Hoekstra HE, Safran RJ (2010) Vertebrate pigmentation: from underlying genes to adaptive function. Trends Genet: TIG 26(5):231–239
Jablonski NG, Chaplin G (2017). The colours of humanity: the evolution of pigmentation in the human lineage. Philos Trans Royal Soc Lond. Series B, 372(1724).
Jones FC, Grabherr MG, Chan YF, Russell P, Mauceli E, Johnson J et al. (2012) The genomic basis of adaptive evolution in threespine sticklebacks. Nature 484(7392):55–61
Jones MR, Mills LS, Alves PC, Callahan CM, Alves JM, Lafferty DJR et al. (2018) Adaptive introgression underlies polymorphic seasonal camouflage in snowshoe hares. Science 360(6395):1355–1358
Kelsh RN (2004) Genetics and evolution of pigment patterns in fish. Pigment Cell Res 17(4):326–336
Lamason RL, Mohideen M-APK, Mest JR, Wong AC, Norton HL, Aros MC et al. (2005) SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 310(5755)):1782–1786
Lavin PA, McPhail JD (1986) Adaptive divergence of trophic phenotype among freshwater populations of the threespine stickleback (Gasterosteus aculeatus). Can J Fish Aquat Sci J Can Des Sci Halieut et Aquat 43(12):2455–2463
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760
Logan DW, Burn SF, Jackson IJ (2006) Regulation of pigmentation in zebrafish melanophores. Pigment Cell Res/Spons Eur Soc Pigment Cell Res Int Pigment Cell Soc 19(3):206–213
Lopes RJ, Johnson JD, Toomey MB, Ferreira MS, Araujo PM, Melo-Ferreira J et al. (2016) Genetic basis for red coloration in birds. Curr Biol 26(11):1427–1434
Lunter G, Goddson M (2011) STAMPY: a statistical algorithm for sensitive and fast mapping of Illumina sequence reads. Genome Res 21:936–939
Luo M, Lu G, Yin H, Wang L, Atuganile M, Dong Z (2021) Fish pigmentation and coloration: Molecular mechanisms and aquaculture perspectives. Rev Aquac 13(4):2395–2412
Lynn Lamoreux, M, Delmas, V, Larue, L, and Bennett, D (2010). The colors of mice: A model genetic network. John Wiley and Sons
Malek TB, Boughman JW, Dworkin I, Peichel CL (2012) Admixture mapping of male nuptial colour and body shape in a recently formed hybrid population of threespine stickleback. Mol Ecol 21(21):5265–5279
Manceau M, Domingues VS, Linnen CR, Rosenblum EB, Hoekstra HE (2010) Convergence in pigmentation at multiple levels: mutations, genes and function. Philos Trans R Soc Lond Ser B 365(1552):2439–2450
Martin A, Orgogozo V (2013) The loci of repeated evolution: A catalog of genetic hotspots of phenotypic variation. Evolution 67(5):1235–1250
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernystsky A et al. (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303
McKinnon JS, Newsome WB, Balakrishnan CN (2022) Gene expression in male and female stickleback from populations with convergent and divergent throat coloration. Ecol Evol 12(5):e8860
Miller CT, Beleza S, Pollen AA, Schluter D, Kittles RA, Shriver MD, Kingsley DM (2007) Cis-regulatory changes in Kit ligand expression and parallel evolution of pigmentation in sticklebacks and humans. Cell 131(6):1179–1189
Miller CT, Glazer AM, Summers BR, Blackman BK, Norman AR, Shapiro MD et al. (2014) Modular skeletal evolution in sticklebacks is controlled by additive and clustered quantitative trait loci. Genetics 197(1):405–420
Nachman MW, Hoekstra HE, D’Agostino SL (2003) The genetic basis of adaptive melanism in pocket mice. Proc Natl Acad Sci USA 100(9):5268–5273
Orteu A, Jiggins CD (2020) The genomics of coloration provides insights into adaptive evolution. Nat Rev Genet 21(8):461–475
Ooijen JWV, Voorrips RE (2001) JoinMap 3.0, Software for the calculation of genetic linkage maps. Plant Research International, Wagemingen
Peichel, Nereng CL, Ohgi KS, Cole KA, Colosimo BL, Buerkle PF et al. (2001) The genetic architecture of divergence between threespine stickleback species. Nature 414(6866):901–905
Peichel CL, Marques DA (2017) The genetic and molecular architecture of phenotypic diversity in sticklebacks. Philos Trans R Soc Lond Ser B Biol Sci 372(1713):20150486
Pike TW, Bjerkeng B, Blount JD, Lindström J, Metcalfe NB (2011) How integument colour reflects its carotenoid content: A stickleback’s perspective. Funct Ecol 25(1):297–304
Protas ME, Patel NH (2008) Evolution of coloration patterns. Annu Rev Cell Dev Biol 24:425–446
Quillen EE, Norton HL, Parra EJ, Lona-Durazo F, Ang KC, Illiescu FM et al. (2019) Shades of complexity: New perspectives on the evolution and genetic architecture of human skin. Am J Phys Anthropol 168(Suppl 67):4–26
Reid K, Bell MA, Veeramah KR (2021) Threespine stickleback: A model system for evolutionary genomics. Annu Rev Genom Hum Genet 22:357–383
Rennison DJ, Owens GL, Heckman N, Schluter D, Veen T (2016) Rapid adaptive evolution of colour vision in the threespine stickleback radiation. Proc R Soc B 283(1830):20160242
Rennison DJ, Rudman SM, Schluter D (2019) Genetics of adaptation: Experimental test of a biotic mechanism driving divergence in traits and genes. Evol Lett 3(5):513–520
Rosenblum EB, Römpler H, Schöneberg T, Hoekstra HE (2010) Molecular and functional basis of phenotypic convergence in white lizards at White Sands. Proc Natl Acad Sci USA 107(5):2113–2117
Saenko SV, Lamichhaney S, Barrio AM, Rafati N, Andersson L, Milinkovitch MC (2015) Amelanism in the corn snake is associated with the insertion of an LTR-retrotransposon in the OCA2 gene. Sci Rep. 5(1):1–9
Schluter D, McPhail JD (1992) Ecological character displacement and speciation in sticklebacks. Am Nat 140(1):85–108
Steiner CC, Römpler H, Boettger LM, Schöneberg T, Hoekstra HE (2008) The genetic basis of phenotypic convergence in beach mice: Similar pigment patterns but different genes. Mol Biol Evol 26(1):35–45
Stevens M, Párraga A, Cuthill IC, Partridge JC, Troscianko TS (2007) Using digital photography to study animal coloration. Biol J Linn Soc 90(2):211–237
Tapanes, E, Lasisi, T, Kamilar, JM, Bradley, BJ (2022). Genomics and cellular biology of the primate pigmentation: lessons from other taxa. Program of the 91st Annual Meeting of the American Association of Biological Anthropologists, 1–209.
Vamosi SM, Schluter D (2004) Character shifts in the defensive armor of sympatric sticklebacks. Evolution 58(2):376–385
Wark AR, Mills MG, Dang L-H, Chan YF, Jones FC, Brady SD et al. (2012) Genetic architecture of variation in the lateral line sensory system of threespine sticklebacks. G3 2(9):1047–1056
Yong L, Peichel CL, McKinnon JS (2015) Genetic architecture of conspicuous red ornaments in female threespine stickleback. G3 6(3):579–588
Acknowledgements
The authors wish to thank Vishwa Pandya and John Villalpando for their assistance with phenotypic data collection.
Author information
Authors and Affiliations
Contributions
DJR conceived the study, and ET developed the phenotyping protocols to carry out the work. DJR performed the genotyping and ET performed the QTL mapping. DJR and ET interpreted the results, and contributed to the writing, and editing of the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
All experiments were performed in accordance with the relevant guidelines and regulations. Animal rearing and experimentation protocol were was conducted under animal care permit #A11-0402, which was approved by the University of British Columbia following the guidelines of the Canadian Council on Animal Care.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Associate editor: Lounès Chikhi.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tapanes, E., Rennison, D.J. The genetic basis of divergent melanic pigmentation in benthic and limnetic threespine stickleback. Heredity (2024). https://doi.org/10.1038/s41437-024-00706-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41437-024-00706-0
- Springer Nature Switzerland AG