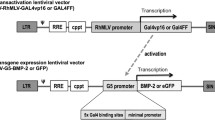
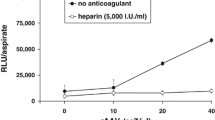
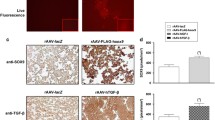
Abstract
Adeno-associated viral vectors (AAV) are unique in their ability to transduce a variety of both dividing and nondividing cells, with significantly lower risk of random genomic integration and with no known pathogenicity in humans, but their role in ex vivo regional gene therapy for bone repair has not been definitively established. The goal of this study was to test the ability of AAV vectors carrying the cDNA for BMP-2 to transduce human mesenchymal stem cells (MSCs), produce BMP-2, and induce osteogenesis in vitro as compared with lentiviral gene therapy with a two-step transcriptional amplification system lentiviral vector (LV-TSTA). To this end, we created two AAV vectors (serotypes 2 and 6) expressing the target transgene; eGFP or BMP-2. Transduction of human MSCs isolated from bone marrow (BMSCs) or adipose tissue (ASCs) with AAV2-eGFP and AAV6-eGFP led to low transduction efficiency (BMSCs: 3.57% and 8.82%, respectively, ASCs: 6.17 and 20.2%, respectively) and mean fluorescence intensity as seen with FACS analysis 7 days following transduction, even at MOIs as high as 106. In contrast, strong eGFP expression was detectable in all of the cell types post transduction with LV-TSTA-eGFP. Transduction with BMP-2 producing vectors led to minimal BMP-2 production in AAV-transduced cells 2 and 7 days following transduction. In addition, transduction of ASCs and BMSCs with AAV2-BMP-2 and AAV6-BMP-2 did not enhance their osteogenic potential as seen with an alizarin red assay. In contrast, the LV-TSTA-BMP-2-transduced cells were characterized by an abundant BMP-2 production and induction of the osteogenic phenotype in vitro (p < 0.001 vs. AAV2 and 6). Our results demonstrate that the AAV2 and AAV6 vectors cannot induce a significant transgene expression in human BMSCs and ASCs, even at MOIs as high as 106. The LV-TSTA vector is significantly superior in transducing human MSCs; thus this vector would be preferable when developing an ex vivo regional gene therapy strategy for clinical use in orthopedic surgery applications.





Similar content being viewed by others
References
Brinker MR. Non-unions: evaluation and treatment. In: Browner BD, Levine AM, Jupiter JB, Trafton PG, editors, Skeletal Trauma: basic science, management, and reconstruction, 4th Ed. Philadelphia: W.B. Saunders; 2009. p. 615–707, Chapter 22.
Schlundt C, Bucher CH, Tsitsilonis S, Schell H, Duda GN, Schmidt-Bleek K. Clinical and research approaches to treat non-union fracture. Curr Osteoporos Rep. 2018;16:155–68.
Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury. 2007;38:S75–80.
Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technol Assess. 2007;11:1–150.
Bougioukli S, Evans CH, Alluri RK, Ghivizzani SC, Lieberman JR. Gene therapy to enhance bone and cartilage repair in orthopaedic surgery. Curr Gene Ther. 2018;18:154–70.
Singh N, Frey NV, Grupp SA, Maude SL. CAR T cell therapy in acute lymphoblastic leukemia and potential for chronic lymphocytic leukemia. Curr Treat Options Oncol. 2016;17:28.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Voelker R. Gene therapy for vision loss. JAMA. 2018;319:434.
Hoy SM. Onasemnogene abeparvovec: first global approval. Drugs. 2019;79:1255–62.
Higano CS, Armstrong AJ, Sartor AO, Vogelzang NJ, Kantoff PW, McLeod DG, et al. Real-world outcomes of sipuleucel-T treatment in PROCEED, a prospective registry of men with metastatic castration-resistant prostate cancer. Cancer. 2019;125:4172–80.
Approved Cellular and Gene Therapy Products https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products. Accessed 7 Dec 2019.
Kofron MD, Laurencin CT. Bone tissue engineering by gene delivery. Adv Drug Deliv Rev. 2006;58:555–76.
Phillips JE, Gersbach CA, García AJ. Virus-based gene therapy strategies for bone regeneration. Biomaterials. 2007;28:211–29.
Evans CH. Gene delivery to bone. Adv Drug Deliv Rev. 2012;64:1331–40.
Sugiyama O, An DS, Kung SP, Feeley BT, Gamradt S, Liu NQ, et al. Lentivirus-mediated gene transfer induces long-term transgene expression of BMP-2 in vitro and new bone formation in vivo. Mol Ther. 2005;11:390–8.
Feeley BT, Conduah AH, Sugiyama O, Krenek L, Chen IS, Lieberman JR. In vivo molecular imaging of adenoviral versus lentiviral gene therapy in two bone formation models. J Orthop Res. 2006;24:1709–21.
Bougioukli S, Sugiyama O, Alluri RK, Yoho R, Oakes DA, Lieberman JR. In vitro evaluation of a lentiviral two-step transcriptional amplification system using GAL4FF transactivator for gene therapy applications in bone repair. Gene Ther. 2018;25:260–8.
Bougioukli S, Sugiyama O, Pannell W, Ortega B, Tan MH, Tang AH, et al. Gene therapy for bone repair using human cells: superior osteogenic potential of bone morphogenetic protein 2-transduced mesenchymal stem cells derived from adipose tissue compared to bone marrow. Hum Gene Ther. 2018;29:507–19.
Hsu WK, Sugiyama O, Park SH, Conduah A, Feeley BT, Liu NQ, et al. Lentiviral mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone. 2007;40:931–8.
Virk MS, Sugiyama O, Park SH, Gambhir SS, Adams DJ, Drissi H, et al. “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Mol Ther. 2011;19:960–8.
Pensak M, Hong S, Dukas A, Tinsley B, Drissi H, Tang A, et al. The role of transduced bone marrow cells overexpressing BMP-2 in healing critical-sized defects in a mouse femur. Gene Ther. 2015;22:467–75.
Bougioukli S, Jain A, Sugiyama O, Tinsley BA, Tang AH, Tan MH, et al. Combination therapy with BMP-2 and a systemic RANKL inhibitor enhances bone healing in a mouse critical-sized femoral defect. Bone. 2016;84:93–103.
Bougioukli S, Alluri R, Pannell W, Sugiyama O, Vega A, Tang A, et al. Ex vivo gene therapy using human bone marrow cells overexpressing BMP-2: “Next-day” gene therapy versus standard “two-step” approach. Bone. 2019;128:115032.
Bokhoven MC. Measurement of insertional mutagenesis by retroviral and lentiviral vectors. Doctoral thesis. University of London; 2008. https://discovery.ucl.ac.uk/id/eprint/1444113/1/U591415.pdf.
Chen Y, Luk KD, Cheung KM, Xu R, Lin MC, Lu WW, et al. Gene therapy for new bone formation using adeno-associated viral bone morphogenetic protein-2 vectors. Gene Ther. 2003;10:1345–53.
FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. https://www.fda.gov/news-events/press-announcements/fda-approves-novel-gene-therapy-treat-patients-rare-form-inherited-vision-loss. Accessed 31 Dec 2019.
FDA Approves Zolgensma, Landmark AAV-Delivered Gene Therapy to Treat Spinal Muscular Atrophy. 2019 Annual ASGCT meeting. https://www.asgct.org/research/news/may-2019/fda-approves-zolgensma-gene-therapy. Accessed 31 Dec 2019.
The US National Institute of Health. Adeno-associated virus. https://clinicaltrials.gov/ct2/results?term=adeno+associated. Accessed 22 Dec 2019.
Ferreira V, Petry H, Salmon F. Immune responses to AAV-vectors, the glybera example from bench to bedside. Front Immunol. 2014;5:82.
Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371:1994–2004.
Weleber RG, Pennesi ME, Wilson DJ, Kaushal S, Erker LR, Jensen L, et al. Results at 2 years after gene therapy for RPE65-deficient leber congenital amaurosis and severe early-childhood-onset retinal dystrophy. Ophthalmology. 2016;123:1606–20.
Ke J, Zheng LW, Cheung LK. Orthopaedic gene therapy using recombinant adeno-associated virus vectors. Arch Oral Biol. 2011;56:619–28.
Tian K, Qi M, Wang L, Li Z, Xu J, Li Y, et al. Two-stage therapeutic utility of ectopically formed bone tissue in skeletal muscle induced by adeno-associated virus containing bone morphogenetic protein-4 gene. J Orthop Surg Res. 2015;10:86.
Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291–7.
Zhu M, Heydarkhan-Hagvall S, Hedrick M, Benhaim P, Zuk P. Manual isolation of adipose-derived stem cells from human lipoaspirates. J Vis Exp. 2013;79:e50585.
Rogers GL, Chen H, Morales H, Cannon PM. Homologous recombination-based genome editing by clade F AAVs is inefficient in the absence of a targeted DNA break. Mol. Ther. 2019;27:1–11.
Iyer M, Wu L, Carey M, Wang Y, Smallwood A, Gambhir SS. Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proc Natl Acad Sci USA. 2001;98:14595–600.
Kang Y, Liao WM, Yuan ZH, Sheng PY, Zhang LJ, Yuan XW, et al. In vitro and in vivo induction of bone formation based on adeno-associated virus-mediated BMP-7 gene therapy using human adipose-derived mesenchymal stem cells. Acta Pharmacol Sin. 2007;28:839–49.
Vakhshori V, Bougioukli S, Sugiyama O, Tang A, Yoho R, Lieberman JR. Cryopreservation of human adipose-derived stem cells for use in ex vivo regional gene therapy for bone repair. Hum Gene Ther Methods. 2018;29:269–77.
Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84.
Pensak MJ, Lieberman JR. Gene therapy for bone regeneration. Curr Pharm Des. 2013;19:3466–73.
Zhang X, Godbey WT. Viral vectors for gene delivery in tissue engineering. Adv Drug Deliv Rev. 2006;58:515–34.
Ellis BL, Hirsch ML, Barker JC, Connelly JP, Steininger RJ 3rd, Porteus MH. A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with Nine natural adeno-associated virus (AAV1-9) and one engineered adeno-associated virus serotype. Virol J. 2013;10:74.
Kumar SR, Markusic DM, Biswas M, High KA, Herzog RW. Clinical development of gene therapy: results and lessons from recent successes. Mol Ther Methods Clin Dev. 2016;3:16034.
http://www.genetherapynet.com/glybera.html. Accessed 10 Nov 2019.
Luk KD, Chen Y, Cheung KM, Kung HF, Lu WW, Leong JC. Adeno-associated virus-mediated bone morphogenetic protein-4 gene therapy for in vivo bone formation. Biochem Biophys Res Commun. 2003;308:636–45.
Chen Y, Luk KD, Cheung KM, Lu WW, An XM, Ng SS, et al. Combination of adeno-associated virus and adenovirus vectors expressing bone morphogenetic protein-2 produces enhanced osteogenic activity in immunocompetent rats. Biochem Biophys Res Commun. 2004;317:675–81.
Li JZ, Li H, Hankins GR, Lieu AS, Noh E, Jacobson L, et al. Different osteogenic potentials of recombinant human BMP-6 adeno-associated virus and adenovirus in two rat strains. Tissue Eng. 2006;12:209–19.
Ben Arav A, Pelled G, Zilberman Y, Kimelman-Bleich N, Gazit Z, Schwarz EM, et al. Adeno-associated virus-coated allografts: a novel approach for cranioplasty. J Tissue Eng Regen Med. 2012;6:43–50.
Koefoed M, Ito H, Gromov K, Reynolds DG, Awad HA, Rubery PT, et al. Biological effects of rAAV-caAlk2 coating on structural allograft healing. Mol Ther. 2005;12:212–8.
Brown N, Song L, Kollu NR, Hirsch ML. Adeno-associated virus vectors and stem cells: friends or foes? Hum Gene Ther. 2017;28:450–63.
Ito H, Goater JJ, Tiyapatanaputi P, Rubery PT, O’Keefe RJ, Schwarz EM. Light-activated gene transduction of recombinant adeno-associated virus in human mesenchymal stem cells. Gene Ther. 2004;11:34–41.
Ju XD, Lou SQ, Wang WG, Peng JQ, Tian H. Effect of hydroxyurea and etoposide on transduction of human bone marrow mesenchymal stem and progenitor cell by adeno-associated virus vectors. Acta Pharmacol Sin. 2004;25:196–202.
Stender S, Murphy M, O’Brien T, Stengaard C, Ulrich-Vinther M, Søballe K, et al. Adeno-associated viral vector transduction of human mesenchymal stem cells. Eur Cell Mater. 2007;13:93–9.
McMahon JM, Conroy S, Lyons M, Greiser U, O’shea C, Strappe P, et al. Gene transfer into rat mesenchymal stem cells: a comparative study of viral and nonviral vectors. Stem Cells Dev. 2006;15:87–96.
Alaee F, Sugiyama O, Virk MS, Tang Y, Wang B, Lieberman JR. In vitro evaluation of a double-stranded self-complementary adeno-associated virus type2 vector in bone marrow stromal cells for bone healing. Genet Vaccines Ther. 2011;9:4.
Hansen J, Qing K, Kwon HJ, Mah C, Srivastava A. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J. Virol. 2000;74:992–6.
Li M, Jayandharan GR, Li B, Ling C, Ma W, Srivastava A, et al. High-efficiency transduction of fibroblasts and mesenchymal stem cells by tyrosine-mutant AAV2 vectors for their potential use in cellular therapy. Hum Gene Ther. 2010;21:1527–43.
Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–45.
Hauck B, Zhao W, High K, Xiao W. Intracellular viral processing, not single-stranded DNA accumulation, is crucial for recombinant adeno-associated virus transduction. J Virol. 2004;78:13678–86.
Sharma P, Wimalawansa SM, Gould GC, Johnson RM, Excoffon KJ. Adeno-associated virus 5 transduces adipose-derived stem cells with greater efficacy than other adeno-associated viral serotypes. Hum Gene Ther Methods. 2016;27:219–27.
Virk MS, Conduah A, Park SH, Liu N, Sugiyama O, Cuomo A, et al. Influence of short-term adenoviral vector and prolonged lentiviral vector mediated bone morphogenetic protein-2 expression on the quality of bone repair in a rat femoral defect model. Bone. 2008;42:921–31.
Acknowledgements
This project was supported by an NIH RO1 research grant to JRL [R01AR057076].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SB, MC, VV, HM, OS, DAO, DL, and PC have no conflicts to report. JRL has received royalties from Depuy Inc, and is a shareholder in Hip Innovation Technologies, Inc.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bougioukli, S., Chateau, M., Morales, H. et al. Limited potential of AAV-mediated gene therapy in transducing human mesenchymal stem cells for bone repair applications. Gene Ther 28, 729–739 (2021). https://doi.org/10.1038/s41434-020-0182-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-020-0182-4
- Springer Nature Limited