Abstract
Background
Understanding and mitigating the societal economic impact of vision impairment (VI) is important for achieving the Sustainable Development Goals.
Aim
To estimate the prevalent societal economic impact of presenting VI in Trinidad and Tobago using bottom-up cost and utilisation data from the 2014 National Eye Survey of Trinidad and Tobago.
Methods
We took a societal perspective to combine comprehensive, individual-level cost and utilisation data, with population-based prevalence estimates for VI, and additional data from a contemporaneous national eyecare system survey. We included direct (medical and non-medical) and indirect (productivity loss) costs, and intangible losses in total cost estimates, presented in 2014 Trinidad & Tobago (TT) dollars and UK sterling equivalent. We considered but excluded transfer payments and dead weight losses. Sensitivity analyses explored impact on total cost of parameter uncertainty and assumptions.
Results
Individual utilisation and cost data were available for 65.5% (n = 2792/4263) and 59.0% (n = 2516/4263) eligible participants aged ≥40 years, respectively. Participant mean age was 58.4(SD 11.8, range 40–103) years, 56.3% were female. We estimated total societal cost of VI in 2014 at UK£365,650,241 (TT$3,842,324,655), equivalent to £675 per capita (population ≥40 years). Loss of wellbeing accounted for 73.3%. Excluding this, the economic cost was UK£97,547,222 (TT$1,025,045,399), of which indirect costs accounted for 70.5%, followed by direct medical costs (17.9%), and direct non-medical costs (11.6%).
Conclusion
This study provides a comprehensive estimate of the economic impact of vision loss in a Caribbean country, and highlights the extent to which affected individuals and their families bear the societal economic cost of vision impairment.
Similar content being viewed by others
Understanding and mitigating the societal economic impacts of vision impairment (VI) will be critically important for achieving the Sustainable Development Goals (SDGs) [1,2,3]. The global cost of VI was estimated at US$3 trillion in 2010, and projected to rise 20% by 2020 [4]. More recently, annual, global productivity losses from VI (<6/18) were estimated in the 15–64 year old population in 2018 at $410.7 billion purchasing power parity, equivalent to 0.3% of global Gross Domestic Product [5, 6]. Despite the importance of this problem, there has been little population-based observational research to support detailed estimation of the economic impact of VI in different countries [6]. Considered from a societal perspective, economic cost encompasses not only the direct costs resulting from eye care services, treatments and non-medical costs, and indirect costs resulting from lost income (productivity losses), but also wellbeing impacts on affected individuals and their carers. The Disability Adjusted Life Year (DALY) was developed to capture such intangible effects of disease [7]. Globally, there were an estimated 22.6million DALYs associated with vision impairment in 2019, accounting for 0.88% of DALYs from all causes [8].
Cost-of-illness studies estimate the economic burden associated with a disease or health state, through describing, itemising, valuing and summing the associated costs [9]. Their usefulness to policy makers has been debated since their inception over 50 years ago, with arguments advanced by proponents [10, 11], and critics [12]. Arguably, cost-of-illness studies provide the most valuable insight when designed as descriptive, bottom-up studies that capture, “the true cost to society”, “envisage the different subjects who bear the costs”, and explain sources of cost variability, to help direct further investment or research effectively [13]. Through highlighting the impact of disease on both the population and the economy, they can also provide a basis for prioritising research funding across different disease areas [14]. Every country has a unique population and political, social welfare, employment and healthcare systems, which influence the economic impact of VI. Previous systematic reviews of cost of vision loss studies reveal very few based on primary observational research, with no prior data from national eye surveys, and frequent omission of the productivity loss associated with informal care [5, 6, 15]. Study heterogeneity has precluded meta-analyses [6, 15]. Additionally, there are no data on the economic impact of vision loss in the Caribbean [6, 15].
To address this, we included an economic questionnaire in the National Eye Survey of Trinidad and Tobago (NESTT, 2014), the most comprehensive population-based eye survey undertaken in the Caribbean region for over two decades [16, 17], and in a contemporaneous national eyecare health system survey [18]. Herein we estimate the total societal economic cost in 2014 resulting from presenting VI involving the better-seeing eye, adhering to cost categories outlined by Cost of Vision Loss Consensus Guidelines (2010) [19]. Additional descriptive data are provided to give more nuanced insight into the economic and wellbeing impacts of VI in Trinidad and Tobago in 2014, and access to low vision support.
Methods
Full details on the methodology and main results from the NESTT (2014) are provided elsewhere [17, 18, 20]. In brief, we sampled 9913 eligible people aged 5 years and above, residing in 3556 households (95.9% coverage) within 120 clusters, using multi-stage, random cluster sampling with probability-proportional-to-size methods, including 4263 people aged 40 years and above. In 3589 (84.2%) responders, we measured uniocular presenting distance, and binocular presenting near visual acuity outside the household, according to a standardised measurement protocol [17]. The demographics of NESTT participants aged 40 years and above were similar to the 2011 Census data for the same age group [21], with mean age of 57.1 (sd 11.8) years, 54.3% female (versus 50.6% in Census), most of South Asian (43.7% versus 35.4% in Census) or African (40.0% versus 34.2% in Census) ancestry with the remainder of predominantly mixed race (15.2% versus 22.8% in Census), and 4.4% (versus 4.6% in Census) resided in Tobago [16]. Adults (≥40 years) were invited for comprehensive clinic-based assessment, including administration of multiple questionnaires, administered using the Epi Info software package (version 3.5.4, Centers for Disease Control and Prevention, Atlanta, GA, USA).
Socioeconomic variables
We administered a socioeconomic questionnaire (Supplementary Table 1), adapted for inclusion in this study [17, 21, 22]. In the clinical assessment, we recorded spectacle and contact lens ownership, surgical and laser history. We recorded prescription topical ophthalmic medication use over the past 3 months. We asked participants with best-corrected visual acuity worse than 6/18 about access to low vision services and use of low vision aids.
Statistical methods
We performed statistical analyses using standard statistical software (StataCorp.2013.Stata Statistical Software:Release 13.1.College Station,TX:StataCorp LP). We report crude estimates for means or proportions in participants. We report ‘adjusted’ estimates for means or proportions in the population-representative sample. We adjusted crude estimates using STATA’s ‘svy’ command suite, to account for multi-level survey design (by island and cluster), with weighting for selection probability, and variable response rate by cluster (weight = 1/response rate). Post-stratification adjustment used the national Census (2011) for the non-institutional population (stratified by 15 municipalities, gender and 5-year age categories). We applied finite population corrections to the first and second sampling stages, including the total number of enumeration districts, and households, by island.
We used multi-level mixed effects logistic regression models (STATA ‘melogit’) to account for multi-level survey design by island and cluster, adjusted for age and sex, and study design, to explore odds of: presenting VI (<6/12) by employment group or educational attainment or literacy, utilising any eye care services, having health insurance, and of using the public sector exclusively for eye care, by vision category.
Cases of vision impairment
We estimated total cases in each vision category [23], by applying adjusted prevalence estimates to the 2014 mid-year population aged 40 years and above (541,894 people) [24].
Classification of economic impact
We estimated direct and indirect costs in each category (See Table 1) [19]. We took a societal perspective to estimate all prevalent costs associated with VI in adults (≥40 years) in Trinidad and Tobago in 2014, regardless of who incurred them. We included costs to individuals, family and friends providing informal care, the Government, health care system, and employers (in the form of productivity losses) [25].
Definition and estimation of intangible effects
To estimate DALYs experienced by the population aged 40 years and above in 2014, we adopted the approach taken by the World Health Organization and Institute of Health Metrics for the Global Burden of Disease Study to estimate loss of wellbeing. In this, prevalent years lived with disability (YLD) are estimated, and years of life lost (YLL) assigned a value of zero to reflect an assumption that VI is not directly associated with premature mortality [26]. To calculate prevalent YLD, we multiplied prevalent cases by the disability weight, with no discounting for time or unequal age weights. After consideration of all available weights [27], we chose the latest WHO disability weights, of 0.047 for near VI (with normal distance vision), 0.005 for mild distance VI, 0.089 for moderate distance VI, 0.314 for severe distance VI, and 0.338 for blindness [26], but explored alternatives at the high [7] and low [28] extremes in sensitivity analysis. We reported impact of VI on quality-adjusted life years previously [29].
Trinidad and Tobago is a high-income non-OECD (Organisation for Economic Co-operation and Development) country, with no published in-country estimation of the value of a statistical life (VSL). Taking the life expectancy at birth to be 70.52 [30], and using a VSL derived for Trinidad and Tobago in 2015, of US$3,035,000, we estimated the value of one lost year of wellbeing in 2015 to be US$43,037, without discounting [31]. We applied the World Bank GDP deflator to yield a VSL in 2014 of $42,483 [32], equivalent to TT$272,316.
Sensitivity analysis
In sensitivity analysis we varied multiple parameters within their 95% confidence interval to explore impact on total cost of various assumptions. Where primary data were not available, or available for only a subgroup of participants, we varied the parameter by +/−50%.
Allocation of costs
Some costs are easier to attribute than others. For direct costs, we applied public or private sector unit costs depending on the participant’s stated preferred provider; if participants reported using both sectors for eye care, we applied an average of the public and private sector unit costs. We assumed that the Government, via the regional health authorities, bore the cost of public sector eye care and treatments made available on the Chronic Disease Assistance Program (CDAP). We assumed that individuals bore the cost of transportation, all refractive correction, and private eye care services and treatments. We assumed insured individuals had a 30% co-payment, based on survey responses.
Conversion of unit costs
We inflated unit costs in this analysis, where necessary, to 2014 values, using the World Bank Gross Domestic Product deflator [32], which takes into account fluctuating exchange rates and different purchasing power of different currencies and inflation rates. We then converted into TT$ using The World Bank Official Exchange Rate (LCU per US$ period average) in 2014, which was TT$1 = UK£0.0952 [33].
Ethics approval
We obtained Ethics Committee approval from the Ministry of Health of the Government of the Republic of Trinidad and Tobago, the University of the West Indies (Trinidad), and Anglia Ruskin University (UK). The study adhered to the tenets of the Declaration of Helsinki. All participants provided written informed consent.
Results
We estimated eyecare service utilisation (Supplementary Table 2) and indirect costs from the NESTT medical and ophthalmic questionnaires (2792 (65.5%) completed), and socioeconomic questionnaire (n = 2,516 (59.0%) completed). Participants included 72.5% (161/222) of all participants with mild VI, 73.5% (158/215) with moderate or severe VI and 41.9% (13/31) of those who were blind in the better-seeing eye. We obtained direct costs in the contemporaneous eyecare system survey (Supplementary Table 3) [18].
Total economic cost of vision impairment and eye care
The total societal cost of VI amongst adults 40 years and above in Trinidad and Tobago in 2014 was TT$3,842,324,655 (UK£365,650,241), with loss of wellbeing accounting for 73.3% of total cost (Table 2). Excluding this, the economic cost was TT$1,025,045,399, of which indirect costs accounted for 70.5%, followed by direct medical costs (17.9%) and direct non-medical costs (11.6%). These equated to a one-year per capita cost to every member of the adult population (≥40 years) of £674.76 including lost wellbeing, and £180.01 excluding lost wellbeing, of which £32.19 were direct medical costs, £20.96 were direct non-medical costs, and £126.86 were indirect costs. Supplementary Tables 2–15 present detailed estimates underlying these summary costs, and Table 16 details excluded transfer payments.
Estimation of cases with vision impairment in 2014
We estimated 64,431 (95% CI 54,623–74,077) cases of distance VI amongst adults 40 years and above in 2014 [16]. Of these, 86.1% (95% CI 82.9 to 88.8), equivalent to 55,481 (95% CI 53,401–57,221) cases, were potentially avoidable. In addition, there were an estimated 120,842 (95% CI 112,715–128,971) cases of avoidable near VI resulting from uncorrected presbyopia.
Sensitivity analyses
A Tornado chart (Fig. 1) illustrates the outcome of a 1-way deterministic sensitivity analysis exploring impact of parameter uncertainty on the base cost estimate, excluding loss of wellbeing. Applying alternative disability weights from the Global Burden of Disease (GBD) Study (2013) resulted in substantial reduction in cost associated with lost wellbeing, to just 3485 DALYs, equivalent to TT$941,661,752 [26]. In contrast, applying the original GBD Study disability weights increased the estimate to 12,259 DALYs, equivalent to TT$3,338,445,299 [7].
Differential economic impacts by vision level
Whilst we could not ascertain individual participant income to permit direct monetary estimation of productivity loss, data from 2663 responders revealed an association between greater levels of presenting VI and lower household income (Supplementary Table 17). The employment rate varied by category of presenting vision, from 73.2% (95% CI 70.9–75.4) in those with both normal distance and near vision (n = 1110/1561), to 41.4% (95% CI 30.6–52.2) in those with moderate or severe vision impairment [MSVI] (n = 28/69), with no employment amongst blind individuals (n = 0/5) (Supplementary Table 11). This yielded individual productivity losses ranging from TT$2,367 in those with near VI only, to TT$36,869 in those who were blind, whilst those with normal distance and near vision had a productivity gain of TT$2639 compared to the population median.
The three most frequent occupational categories in Trinidad and Tobago in 2014 using the Information Commissioner’s Office classification were professionals (37.6%), elementary occupations (20.6%), and service workers or market sales people (18.2%), whether VI or normally sighted (Supplementary Table 18). In a multivariable model, the odds of having presenting VI (<6/12) were significantly higher amongst people employed in the service industry (OR 2.7, 95% CI 1.3–5.4) and people employed in elementary jobs (OR 2.9, 95% CI 1.5–5.8), as compared to professionals (p = 0.011). Considering other variables potentially associated with occupational opportunity, an active email address was reported by 31.4% (56) with normal vision, 9.5% [17] with mild VI, 4.4% [8] with MSVI, and no one with blindness. Access to a family car was reported by 75.5% (1712) with normal vision, 64.3% (128) with mild VI, 61.9% (122) with MSVI, and 50.0% [14] who were blind.
Individuals with vision impairment had significantly lower educational attainment than those with normal vision (See Supplementary Table 19). In a multivariable model, the odds of presenting with vision <6/12 in the better seeing eye were significantly lower amongst those who had completed secondary school (OR 0.43, 95% CI 0.31–0.59), post-secondary (OR 0.44, 95% CI 0.25–0.77) or university (OR 0.30, 95% CI 0.15–0.62), as compared to those who had completed only primary school (p < 0.0001). Illiteracy was reported by 0.74%(11/1483) of people with normal distance vision, compared to over 2.5% of those with mild VI (5/199), MSVI (5/196) and blindness (1/30). In a multivariable model the odds of presenting with vision <6/12 were significantly reduced in those who were literate (OR 0.26, 95% CI 0.12–0.58), compared to those who were illiterate (p < 0.001).
Eye care access by vision level
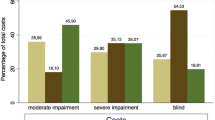
Eye care service utilisation in the past 12 months varied from around 30% in those with normal vision, mild VI and MSVI, to 18% in those with near VI and 7% in those who were blind (Supplementary Table 20). A multivariable model revealed that use of eye services was significantly more likely in women (OR 1.36, 95% CI 1.13–1.64), and amongst people older than 60 years, whereas those with near VI (OR 0.49, 95% CI 0.39–0.61), or blindness (OR 0.11, 95% CI 0.01–0.85), were significantly less likely to report utilising any eye care.
Health insurance coverage also varied significantly by vision level, ranging from 24.6% (95% CI 21.8–27.6) in those with normal distance and near vision, to 0% in blind participants (p < 0.001) (Supplementary Table 20). Median (IQR) out-of-pocket-expenditure (OOPE) on eye care ranged from TT$100 (100–150) for optometry sight tests, reported by 16.0% (402) in the past 12 months, to TT$10,000 (1600–13000) for day case surgery, reported by 0.7% [18] (Supplementary Table 21). Any OOPE on eye care services in the past 12 months was reported by 25.3% (95% CI 22.9–27.8) of those with normal vision, and by no one who was blind (Supplementary Table 21). OOPE on topical ophthalmic medications were incurred by 19.3% (95% CI 9.9–34.1) of blind people, compared to only 4.7% (95% CI 3.7–6.0) amongst those with normal vision.
Discussion
This study provides the first estimate of the societal cost of presenting VI in Trinidad and Tobago. We estimate a prevalent cost of TT$3.8 billion (UK£365.7 million) to the general adult population 40 years and above in the year 2014, with loss of wellbeing accounting for 73.3%, followed by indirect costs, then direct medical and non-medical costs. Affected individuals and their families bore the major share of total cost (97.6%). The cost of VI per capita was £675(including wellbeing loss) or £180 (excluding wellbeing loss). These were equivalent to approximately 6.0% and 1.6% of the Gross National Income per capita (GNIpc = £11,212, $US18,380, GNI at Atlas Method), respectively. Monthly household income was reported as less than TT$9999 (£952, US$1,562) by 80% (n = 1985). This could indicate a bias toward lower self-reported income in our study, as average monthly household income of TT$9202 was reported in the 2008/2009 Household Budget Survey [34], or that the GNI represents skewed income distribution and substantial inequality; Trinidad and Tobago has reserves of oil and natural gas, and the last GINI coefficient estimate for Trinidad and Tobago was 40.2 in 1992 [35]. It is likely the economic burden of vision impairment fell disproportionately on lower income households, with 91% MSVI and 96% blind participants residing in households with monthly income <TT$9999, compared to 75% with normal vision. Visually impaired participants were significantly more likely to have lower educational attainment, and were less likely to have access to the internet or a family car.
The findings of this predominantly bottom-up, observational cost of vision loss study broadly agree with other studies in high-income countries. These similarly report that intangible effects, including estimates relating to loss of wellbeing, make the greatest contribution to the overall economic impact of VI and blindness [15]. After these, the next highest costs typically result from productivity losses, followed by caregiving, recurrent hospitalisations and use of medical and supportive devices, with drug costs not adding significantly in studies published before 2012 [15]. Direct comparisons of absolute costs are challenging because consensus on what to include in cost of vision loss studies was unavailable prior to 2010 [19], and there is still no standardisation of cost tools, with investigators frequently devising their own [15, 36]. In Trinidad and Tobago in 2014, direct expenditure on topical ophthalmic medications contributed significantly to total direct costs, and glaucoma was the leading cause of vision loss [16]. Given the large volumes of topical ophthalmic medications involved, there may be considerable benefit to the Ministry of Health intervening to reduce acquisition cost.
Costs in other domains were lower than expected for a high-income country, indicating a possible gap in eyecare service provision relative to the needs of the population. For example, no participant in 2014 had received laser refractive surgery, or anti-VEGF therapy (only available in the private sector) [18]. The latter was emerging as standard-of-care for numerous eye diseases internationally at that time. However, NESTT was not powered to reliably determine the prevalence of uncommon events. The relatively low unit cost of ophthalmology outpatient services may reflect historic underinvestment in public sector ophthalmic equipment. In 2014, only three of five public hospital eye departments in Trinidad and Tobago had visual field analysers, none had OCT imaging devices, and whilst two had FFA devices, they did not have sufficient staff for routine use [18]. We also observed few state-funded prevention programmes, with no national screening approach for congenital cataract or retinopathy of prematurity, responsible for some cases of adult VI. A pilot screening programme for diabetic retinopathy (DR) was completed in 2013, and a school vision screening programme was in the process of being implemented [18]. Low vision services were relatively underdeveloped for a high-income country, with only one low vision participant reporting previous assessment, and less than 10% reporting access to low vision aids. No blind people were employed, indicating potential opportunity to strengthen workplace-based enablement policies.
Societal direct costs are anticipated to have risen significantly in the decade following the 2014 national survey, through a combination of increased demand for eyecare services from an aging population, catch-up in ophthalmic management practices relative to other high-income countries driving a need for investment in equipment, and demand for higher-cost medicines and laser and surgical treatments.
Which disability weights are most applicable to VI remains controversial [27]. Our sensitivity analysis highlighted that disability weights from difference sources yield estimates of the cost associated with lost wellbeing which vary 4-fold, between 3485 DALYs (equivalent to TT$941,661,752) [26] and 12,259 DALYs (equivalent to TT$3,338,445,299) [7].
The study had a number of limitations. In common with previous studies, this study was limited to costs incurred by those aged 40 years and above [37,38,39]. Secondly, the survey did not include the institutionalised population, or consider costs associated with long-term care placement resulting from VI. Thirdly, the cost and utilisation estimates are subject to non-response bias (response rates 59–66%). Blind people may have been less likely to participate than normally sighted people, on account of transport difficulty, lack of someone to accompany them, frailty, or lack of perceived value to participating in the eye survey, and those blind people who attended clinic may have differed significantly from those who were housebound. This may account for our conservative estimate of informal care needs (23% blind people required a mean 8.4 h per day). A systematic review reported average informal care hours ranging from 5.8 h per week for persons with vision >20/32 to 94.1 h per week for persons with vision <20/250 [15]. Fourthly, the data were at risk of recall bias, with participants asked to recall episodes and costs occurring over the past 12 months. Some may have forgotten an entire episode of eyecare, or incorrectly recalled whether it occurred within or prior to the 12-month period in question [25, 40]. In common with many other cost studies, this analysis does not include transfer payments or estimation of dead-weight losses. This study was also unable to consider the opportunity cost of being a carer. Furthermore, direct health costs arising from falls, fractures, motor vehicle accidents, exacerbation of diabetic complications due to difficulty self-managing, and depression relating to VI were not explicitly measured in this survey. These have contributed substantially to direct costs in some studies [15]. Finally, it is important to highlight that whilst we estimated an assumed value of purchasing a DALY, and explored this in sensitivity analysis, as recommended by Frick et al. [19] cost-of-illness studies do not typically attach monetary values to the DALY or QALY impact of disease. We acknowledge that attempting to value statistical life – the amount that groups of individuals are willing to pay for a marginal change in their likelihood of death – is fraught with conceptual and ethical limitations.
Our study was subject to a number of assumptions. The market price for labour was assumed to be a reasonable approximation of the opportunity cost of the employment gap resulting from VI [25]. Furthermore, we assumed that market prices including an element of profit, such as private sector service and drug costs, were based on a fair rate of return on investment, such that they reflected societal opportunity costs [41].
These cost estimates provide an important benchmark and baseline data for cost-effectiveness analyses in Trinidad and Tobago. For example, to explore the potential value of widening access to low vision aids, workforce enablement programs, widening access to DR screening [42], widening access to affordable spectacles for effective refractive error coverage [43], or investment to improve effective cataract surgical coverage [44]. More generally, there is pressing need for international consensus on cost of VI study design and measurement tools, to facilitate comparison between countries [6, 15, 19, 45].
Conclusion
This cost of vision loss study finds that VI in Trinidad and Tobago in 2014 had a significant societal economic impact, with affected individuals and their families bearing the majority of associated costs, including lost wellbeing, informal care costs, productivity losses and other opportunity costs. This highlights that policy decisions based on direct costs to the health sector alone would fail to apportion appropriate societal and research resources to addressing VI – at least 86% of which is potentially avoidable through interventions to prevent and treat eye disease [16]. We identified potential areas for investment, and elements that may drive rising future eyecare costs, including the aging demographic, emerging high-cost medicines and imaging equipment. Given the high proportion of indirect costs and intangible effects associated with VI, and Trinidad and Tobago’s pluralistic eye care system, policy makers may need to consider intervening proactively to correct market failures that limit timely access to sight-saving interventions in the public sector.
Summary
What is known about this topic?
-
Understanding and mitigating the societal economic impacts of vision impairment (VI) will be critical to achieving the Sustainable Development Goals.
-
Previous systematic reviews reveal considerable heterogeneity in the methodology and findings of cost-of-VI studies, with few based on individual-level cost and utilisation data from primary observational studies, including population-based eye surveys.
-
Consensus Guidelines were proposed by Frick et al in 2010 to support greater standardisation, but leave some issues unaddressed.
-
There were no data on the economic impact of vision impairment in the Caribbean.
What this study adds
-
We estimated the total societal cost of VI in 2014 at UK£365,650,241 (TT$3,842,324,655), equivalent to £675 per capita (in the population >40 years).
-
Loss of wellbeing accounted for 73.3% of total cost, followed by indirect costs, direct medical costs and direct non-medical costs, highlighting the extent to which individuals and their families bear the societal economic cost of VI.
-
This study provides detailed methodological outline showing how economic variables can be readily included in survey questionnaires, potentially enhancing the impact of population-based eye survey research.
Data availability
Further analyses on the unpublished data set are in progress with manuscripts in preparation. Data access is governed by the NESTT Steering Committee, chaired by a representative of the Faculty of Medical Science at the University of the West Indies (St Augustine, Trinidad) and academic partners in the Vision and Eye Research Unit at Anglia Ruskin University (Cambridge, UK), and The School of Life Course and Population Sciences, King’s College London.
References
Zhang JH, Ramke J, Jan C, Bascaran C, Mwangi N, Furtado JM, et al. Advancing the Sustainable Development Goals through improving eye health: a scoping review. Lancet Planet Health. 2022;6:e270–80.
Burton MJ, Ramke J, Marques AP, Bourne RRA, Congdon N, Jones I, et al. The Lancet Global Health Commission on Global Eye Health: vision beyond 2020. Lancet Glob Health. 2021;9:e489–551.
Congdon N, Reddy PA, Mackenzie G, Golgate P, Wen Q, Clarke M. Presbyopia and the sustainable development goals. Lancet Glob Health. 2018;6:e1067.
Gordois A, Cutler H, Pezzullo L, Gordon K, Cruess A, Winyard S, et al. An estimation of the worldwide economic and health burden of visual impairment. Glob Public Health. 2012;7:465–81.
Marques AP, Ramke J, Cairns J, Butt T, Zhang JH, Faal HB, et al. Estimating the global cost of vision impairment and its major causes: protocol for a systematic review. BMJ Open. 2020;10:e036689.
Marques AP, Ramke J, Cairns J, Butt T, Zhang JH, Muirhead D, et al. Global economic productivity losses from vision impairment and blindness. EClinicalMedicine. 2021;35:100852.
Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72:429–45.
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22.
Rice DP. Estimating the cost of illness. Am J Public Health Nations Health. 1967;57:424–40.
Behrens C, Henke K. Cost of illness studies: no aid to decision making: Reply to Shiell et al. (Health Policy, 8 (1987) 317–323. Health Policy. 1988;10:137–41.
Rice DP. Cost-of-illness studies: fact or fiction? Lancet. 1994;344:1519–20.
Koopmanschap MA. Cost-of-illness studies Useful health policy?. PharmacoEconomics. 1998;14:143–8.
Tarricone R. Cost-of-illness analysis: what room in health economics?. Health Policy. 2006;77:51–63.
Luengo-Fernandez R, Leal J, Gray A. UK research spend in 2008 and 2012: comparing stroke, cancer, coronary heart disease and dementia. BMJ Open. 2015;5:e006648.
Koberlein J, Beifus K, Schaffert C, Finger RP. The economic burden of visual impairment and blindness: a systematic review. BMJ Open. 2013;3:e003471.
Braithwaite T, Verlander NQ, Peto T, Bartholomew D, Deomansingh F, Bridgemohan P, et al. National Eye Survey of Trinidad and Tobago (NESTT): prevalence, causes and risk factors for presenting vision impairment in adults over 40 years. Br J Ophthalmol. 2020;104:74–80.
Braithwaite T, Verlander NQ, Bartholomew D, Bridgemohan P, McNally K, Roach A, et al. The National Eye Survey of Trinidad and Tobago (NESTT): rationale, objectives and methodology. Ophthalmic Epidemiol. 2017;24:116–129.
Braithwaite T, Winford B, Bailey H, Bridgemohan P, Bartholomew D, Singh D, et al. Health system dynamics analysis of eyecare services in Trinidad and Tobago and progress towards Vision 2020 Goals. Health Policy Plan. 2018;33:70–84.
Frick KD, Kymes SM, Lee PP, Matchar DB, Pezzullo ML, Rein DB, et al. The cost of visual impairment: purposes perspectives, and guidances. Invest Ophthalmol Vis Sci. 2010;51:1801–5.
Roach A, Braithwaite T, Carrington C, Marcellin E, Sharma S, Hingorani A, et al. Addressing ethical challenges in the Genetics Substudy of the National Eye Survey of Trinidad and Tobago (GSNESTT). App Transl Genom. 2016;9:6–14.
Ministry of Planning and Sustainable Development Government of the Republic of Trinidad and Tobago. Trinidad and Tobago 2011 Population and Housing Census Demographic Report. The Central Statistics Office; 2012.
Clarke P, Gray A, Legood R, Briggs A, Holman R. The impact of diabetes-related complications on healthcare costs: results from the United Kingdom Prospective Diabetes Study (UKPDS Study No. 65). Diabet Med J Br Diabet Assoc. 2003;20:442–50.
Bourne R, Price H, Taylor H, Leasher J, Keeffe J, Glanville J, et al. New systematic review methodology for visual impairment and blindness for the 2010 global burden of disease study. Ophthalmic Epidemiol. 2013;20:33–9.
Central Statistical Office. Population Mid Year Estimates. 2014. http://cso.gov.tt/data/?productID=31-Population-Mid-Year-Estimates2014. Accessed 14 December 2016.
Gray A, Clarke PM, Wolstenholme JL, Wordsworth S. Chapter 6: Defining, measuring and valuing costs. In: Gray AM, Briggs A, editor. Applied Methods of Cost-effectiveness Analysis in Health Care. Oxford: Oxford University Press; 2011.
Department of Health Statistics and Information Systems World Health Organisation. WHO methods and data sources for global burden of disease estimates 2000-2011. Geneva: World Health Organization; 2013. http://www.who.int/healthinfo/statistics/GlobalDALYmethods_2000_2011.pdf?ua=1.
Braithwaite T, Taylor H, Bourne R, Keeffe J, Pesudovs K. Does blindness count? Disability weights for vision loss. Clin Exp Ophthalmol. 2017;45:217–20.
Salomon JA, Haagsma JA, Davis A, de Noordhout CM, Polinder S, Havelaar AH, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob health. 2015;3:e712–23.
Braithwaite T, Bailey H, Bartholomew D, Saei A, Pesudovs K, Ramsewak SS, et al. Impact of vision loss on health-related quality of life in Trinidad and Tobago. Ophthalmology. 2019;126:1055–8.
PanAmerican Health Organisation. Trinidad and Tobago Indicator Profile, Group of Resources, Services and Coverage Indicators. Pan American Health Organization; 2014. http://ais.paho.org/phip/viz/indicatorprofilebydomain.asp.
Viscusi WK, Masterman CJ. Income elasticities and global values of a statistical life. J Benefit Cost Anal. 2017;8:226–50.
The World Bank. Gross Domestic Product deflator (base year varies by country): World Bank national accounts data, and OECD National Accounts data files: World Bank Group. 2018. Available from: https://data.worldbank.org/indicator/NY.GDP.DEFL.ZS?end=2015&start=2014.
The World Bank. Official exchange rate (LCU per US$, period average). 2018. Available from: https://data.worldbank.org/indicator/PA.NUS.FCRF?end=2014&start=2004.
Central Statistical Office of the Ministry of Planning and Development. Household Budget Survey. Available from: https://cso.gov.tt/household-budgetary-survey-hbs/2008-2009.
World Bank Poverty and Inequality Platform. Gini Index - Trinidad and Tobago. 1992. Available from: https://data.worldbank.org/indicator/SI.POV.GINI?locations=TT2023. Accessed 29 November 2023.
Lofland JH, Pizzi L, Frick KD. A review of health-related workplace productivity loss instruments. PharmacoEconomics. 2004;22:165–84.
Frick KD, Gower EW, Kempen JH, Wolff JL. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol. 2007;125:544–50.
Rein DB, Zhang P, Wirth KE, Lee PP, Hoerger TJ, McCall N, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124:1754–60.
Javitt JC, Zhou Z, Willke RJ. Association between vision loss and higher medical care costs in Medicare beneficiaries costs are greater for those with progressive vision loss. Ophthalmology. 2007;114:238–45.
Clarke PM, Fiebig DG, Gerdtham UG. Optimal recall length in survey design. J Health Econ. 2008;27:1275–84.
Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998;36:778–92.
Southwest Regional Health Authority. 10,000 Diabetic Retinopathy Screenings San Fernando, Trinidad & Tobago. 2018. Available from: https://www.swrha.co.tt/content/10-000-diabetic-retinopathy-screenings.
Bourne RRA, Cicinelli MV, Sedighi T, Tapply IH, McCormick I, Jonas JB, et al. Effective refractive error coverage in adults aged 50 years and older: estimates from population-based surveys in 61 countries. Lancet Glob health. 2022;10:e1754–63.
McCormick I, Butcher R, Evans JR, Mactaggart IZ, Limburg H, Jolley E, et al. Effective cataract surgical coverage in adults aged 50 years and older: estimates from population-based surveys in 55 countries. Lancet Glob health. 2022;10:e1744–53.
Bloom BS, Bruno DJ, Maman DY, Jayadevappa R. Usefulness of US cost-of-illness studies in healthcare decision making. PharmacoEconomics. 2001;19:207–13.
Lafuma A, Brezin A, Lopatriello S, Hieke K, Hutchinson J, Mimaud V, et al. Evaluation of non-medical costs associated with visual impairment in four European countries: France, Italy, Germany and the UK. PharmacoEconomics. 2006;24:193–205.
Wittenborn JS, Zhang X, Feagan CW, Crouse WL, Shrestha S, Kemper AR, et al. The economic burden of vision loss and eye disorders among the United States population younger than 40 years. Ophthalmology. 2013;120:1728–35.
Pezzullo L, Streatfeild J, Simkiss P, Shickle D. The economic impact of sight loss and blindness in the UK adult population. BMC Health Serv Res. 2018;18:63.
Royal National Institute of Blind People Shop. 2018. Available from: http://shop.rnib.org.uk/.
Larg A, Moss JR. Cost-of-illness studies: a guide to critical evaluation. PharmacoEconomics. 2011;29:653–71.
Central Statistical Office of the Ministry of Planning and Development. Table 70B: Persons with Job by Occupational Group, Sex and Monthly Income Group. Port of Spain, Trinidad and Tobago: Central Statistics Office; 2014.
Taylor HR, Pezzullo ML, Keeffe JE. The economic impact and cost of visual impairment in Australia. Br J Ophthalmol. 2006;90:272–5.
Koopmanschap MA, van Ineveld BM. Towards a new approach for estimating indirect costs of disease. Soc Sci Med. 1992;34:1005–10.
van den Berg B, Brouwer WB, Koopmanschap MA. Economic valuation of informal care. An overview of methods and applications. Eur J Health Econ. 2004;5:36–45.
Acknowledgements
The authors wish to thank the Ministry of Health of the Government of the Republic of Trinidad and Tobago, the Minister of Health, and the Regional Health Authorities, for their support. We also thank the administrative staff at the University of the West Indies and Anglia Ruskin University. We are most grateful to Diane Williams and the team at Medilex Ltd (USA) for their technical support and equipment sponsorship, and for additional sponsorship by Precision Vision Ltd (USA) and Core Distribution Ltd (Trinidad). Finally, our sincere thanks to everyone in the National Eye Survey of Trinidad and Tobago Study Group: Allingham, R., Applewaite, C., Badal, K., Bailey, H., Ballah, R., Bartholomew, D., Bhagan, A., Bourne, R., Braithwaite, T., Bridgemohan, C., Bridgemohan, P., Bruce, M., Carrington, C., Carter, K., Cesair, A., Crowley, T., Daulat-Araujo, J., De Freitas, A., Deomansingh, F., Dineen, B., Dowlath, K., Farrier, J., Fraser, A., Gray, A., Hauser, M., Hingorani, A., Hingorani, M., Jonas, J., Lynch, N., Maharaj, V., Marcellin, E., McNally, K., Misir, A., Mohan, J., Narine, M., Newsom, W., Noel, N., Pablo-Casas, J., Pardhan, S., Parker, M., Pascall, A., Persad, M., Pesudovs, K., Peto, T., Pulchan, B., Ramlal, B., Ramsewak, S.S., Ramsewak, S.S., Ravello, R., Roach, A., Robinson, L., Sharma, A., Sharma, R., Sharma, S., Singh, D., Teelucksingh, S., Thomas, A., Tripathi, V., Verlander, N., Winford, B.
Funding
Financial Support for the study was received from Ministry of Health of the Government of Trinidad and Tobago, and Fight for Sight UK (Grant ref 1339/40). The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
TB, AG, RB, Samuel SR, SS, DS were responsible for study conception. RB, Samuel SR, SS, DS, TB and Shivaa SR were responsible for securing ethical approvals and/or study funding. TB, AG, HB, RB, VT, and AG were responsible for study design. TB, DB, VM, AF, FD, Shivaa SR were responsible for recruiting and assessing study participants and data entry. TB, VT, HB, RB, AG were responsible for data analysis and interpreting results. TB drafted the manuscript. All authors provided critical revision of the manuscript and approval of the final submission.
Corresponding author
Ethics declarations
Competing interests
TB holds an unpaid role on the Association for Research in Vision and Ophthalmology Annual Program Management Committee. HB received grants from Roche Central America (Grant ref 000046018), EuroQol Research Foundation (Grant ref 341-RA, 357-RA), support for travel to meetings from the EuroQol Research Foundation, and consulting fees from the Centre for Health Economics, University of the West Indies. HB holds unpaid roles on the Caribbean Health Economics Executive committee, and the advisory board for Pathcheck – Institute for Technology and Global Health. The remaining authors report no disclosures or conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Braithwaite, T., Bailey, H., Bartholomew, D. et al. The societal economic impact of vision impairment in adults 40 years and above: findings from the National Eye Survey of Trinidad and Tobago. Eye (2023). https://doi.org/10.1038/s41433-023-02860-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41433-023-02860-x
- Springer Nature Limited