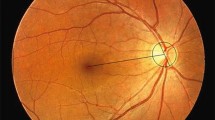
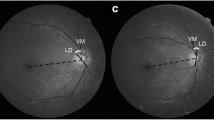
Abstract
Background
Which phenotypes are we able to recognize in the optic nerve of patients with primary open angle glaucoma?
Methods
Retrospective interventional case series. 885 eyes from 885 patients at an outpatient tertiary care centre who met specified criteria for POAG were included. Disc photographs were classified by three glaucoma specialists into the following phenotypes according to their predominant characteristics: (1) concentric rim thinning, (2) focal rim thinning, (3) acquired pit of the optic nerve (APON), (4) tilted, (5) extensive peripapillary atrophy (PPA), and (6) broad rim thinning. Demographic, medical, and ocular data were collected. Kruskal–Wallis was used as a non-parametric test and pairwise comparison was performed by using Wilcoxon rank sum test corrected.
Results
Phenotypic distribution was as follows: 398(45%) focal thinning, 153(18%) concentric thinning, 153(17%) broad thinning, 109(12%) tilted, 47(5%) extensive PPA and 25(3%) APON. Phenotypic traits of interest included a higher proportion of female patients with the focal thinning phenotype (p = 0.015); myopia (p = 0.000), Asian race (OR: 8.8, p = 0.000), and younger age (p = 0.000) were associated with the tilted phenotype; the concentric thinning patients had thicker RNFL (p = 0.000), higher MD (p = 0.008) and lower PSD (p = 0.043) than broad thinning, despite no difference in disc sizes (p = 0.849). The focal thinning group had a localized VF pattern with high PSD compared to concentric thinning (p = 0.005).
Conclusion
We report six phenotypic classifications of POAG patients with demographic and ocular differences between phenotypes. Future refinement of phenotypes should allow enhanced identification of genetic associations and improved individualization of patient care.



Similar content being viewed by others
Data availability
All data are available from the corresponding author upon a reasonable request.
References
Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Investig Ophthalmol Vis Sci. 1997;38:83–91.
Weinreb RN, Tee Khaw P. Primary open-angle glaucoma. Lancet (Lond, Engl). 2004;363:1711–20. https://doi.org/10.1016/S0140-6736(04)16257-0
Caprioli, J.: Clinical evaluation of the optic nerve in glaucoma. Tr. Am Ophth. Soc. 1994;92:589–641.
Nicolela MT, Drance SM. Various glaucomatous optic nerve appearances: Clinical correlations. Ophthalmology. 1996;103:640–9. https://doi.org/10.1016/S0161-6420(96)30640-4
Radius RL, Maumenee AE, Green WR. Pit-like changes of the optic nerve head in open-angle glaucoma. Br J Ophthalmol. 1978;62:389–93. https://doi.org/10.1136/BJO.62.6.389
Javitt JC, Spaeth GL, Katz LJ, Poryzees E, Addiego R. Acquired Pits of the Optic Nerve: Increased Prevalence in Patients with Low-tension Glaucoma. Ophthalmology. 1990;97:1038–44. https://doi.org/10.1016/S0161-6420(90)32466-1
Ugurlu S, Weitzman M, Nduaguba C, Caprioli J. Acquired pit of the optic nerve: a risk factor for progression of glaucoma. Am J Ophthalmol. 1998;125:457–64. https://doi.org/10.1016/S0002-9394(99)80185-8
Nduaguba C, Ugurlu S, Caprioli J. Acquired pits of the optic nerve in glaucoma: Prevalence and associated visual field loss. Acta Ophthalmol Scand. 1998;76:273–7. https://doi.org/10.1034/J.1600-0420.1998.760304.X
Jonas JB, Nguyen XN, Gusek GC, Naumann GO. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric data. Invest Ophthalmol Vis Sci. 1989;30:908–18.
Dandona L, Dandona R. What is the global burden of visual impairment? BMC Med. 2006;4. https://doi.org/10.1186/1741-7015-4-6
R: The R Project for Statistical Computing. Accessed June 1, 2022. https://www.r-project.org/
Abdillah A, Sutisna A, Tarjiah I, Fitria D, Widiyarto T. Application of Multinomial Logistic Regression to analyze learning difficulties in statistics courses. In: Journal of Physics: Conference Series. 1490. Institute of Physics Publishing; 2020. https://doi.org/10.1088/1742-6596/1490/1/012012
Lex A, Gehlenborg N, Strobelt H, Vuillemot R, Pfister H. UpSet: Visualization of intersecting sets. IEEE Trans Vis Comput Graph. 2014;20:1983–92. https://doi.org/10.1109/TVCG.2014.2346248
Broadway DC, Nicolela MT, Drance SM. Optic disk appearances in primary open-angle glaucoma. Survey Ophthalmol. 1999;43. https://doi.org/10.1016/S0039-6257(99)00007-7
Tan NYQ, Sng CCA, Jonas JB, Wong TY, Jansonius NM, Ang M. Glaucoma in myopia: diagnostic dilemmas. Br J Ophthalmol. 2019;103:1347–55. https://doi.org/10.1136/BJOPHTHALMOL-2018-313530
Spaeth GL. A new classification of glaucoma including focal glaucoma. Survey Ophthalmol. 1994;38. https://doi.org/10.1016/0039-6257(94)90042-6
Geijssen HC, Greve EL. The spectrum of primary open angle glaucoma I: Senile sclerotic glaucoma versus high tension glaucoma. Ophthalmic Surg. 1987;18:207–13. https://doi.org/10.3928/1542-8877-19870301-11
Downs JC, Girkin CA. Lamina Cribrosa in Glaucoma. https://doi.org/10.1097/ICU.0000000000000354
Albon J, Purslow PP, Karwatowski WSS, Easty DL. Age related compliance of the lamina cribrosa in human eyes. Br J Ophthalmol. 2000;84:318–23. https://doi.org/10.1136/BJO.84.3.318
Zukerman R, Harris A, Vercellin AV, Siesky B, Pasquale LR, Ciulla TA. Molecular Genetics of Glaucoma: Subtype and Ethnicity Considerations. Genes. 2020;12:1–36. https://doi.org/10.3390/GENES12010055
Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106:2010–5. https://doi.org/10.1016/S0161-6420(99)90416-5
Jonas JB, Wang YX, Dong L, Panda-Jonas S. High Myopia and Glaucoma-Like Optic Neuropathy. Asia-Pac J Ophthalmol (Phila, Pa). 2020;9:234–8. https://doi.org/10.1097/APO.0000000000000288
Yo C. Asian Americans: myopia and refractive surgery. Int Ophthalmol Clin. 2003;43:173–87. https://doi.org/10.1097/00004397-200343040-00015
Geijssen HC, Greve EL. Focal ischaemic normal pressure glaucoma versus high pressure glaucoma. Doc Ophthalmol Adv Ophthalmol. 1990;75:291–302. https://doi.org/10.1007/BF00164843
Nakazawa T, Fuse N, Omodaka K, Aizawa N, Kuwahara S, Nishida K. Different types of optic disc shape in patients with advanced open-angle glaucoma. Jpn J Ophthalmol. 2010;54:291–5. https://doi.org/10.1007/S10384-010-0816-Y
Park IK, Kim KW, Moon NJ, Shin JH, Chun YS. Comparison of superior and inferior visual field asymmetry between normal-tension and high-tension glaucoma. J Glaucoma. 2021;30:648–55. https://doi.org/10.1097/IJG.0000000000001872
Park JH, Yoo C, Park J, Kim YY. Visual Field Defects in Young Patients With Open-angle Glaucoma: Comparison Between High-tension and Normal-tension Glaucoma. J Glaucoma. 2017;26:541–7. https://doi.org/10.1097/IJG.0000000000000667
Ganeshrao SB, Senthil S, Choudhari N, Durga SS, Garudadri CS. Comparison of visual field progression rates among the high tension glaucoma, primary angle closure glaucoma, and normal tension glaucoma. Investig Ophthalmol Vis Sci. 2019;60:889–900. https://doi.org/10.1167/iovs.18-25421
Häntzschel J, Terai N, Sorgenfrei F, Haustein M, Pillunat K, Pillunat LE. Morphological and functional differences between normal-tension and high-tension glaucoma. Acta Ophthalmol. 2013;91. https://doi.org/10.1111/aos.12061
Caprioli J, Spaeth GL. Comparison of visual field defects in the low-tension glaucomas with those in the high-tension glaucomas. Am J Ophthalmol. 1984;97:730–7. https://doi.org/10.1016/0002-9394(84)90505-1
Ekici E, Moghimi S, Hou H, Proudfoot J, Zangwill LM, Do JL, et al. Central visual field defects in patients with distinct glaucomatous optic disc phenotypes. Am J Ophthalmol. 2021;223:229–40. https://doi.org/10.1016/j.ajo.2020.10.015
Mahmoudinezhad G, Lin M, Rabiolo A, Morales E, Hirunpatravong P, Sharifipour F, et al. Rate of visual field decay in glaucomatous eyes with acquired pits of the optic nerve. Br J Ophthalmol. 2021;105:381–6. https://doi.org/10.1136/bjophthalmol-2020-315980
Moghimi S, Hosseini H, Riddle J, et al. Measurement of optic disc size and rim area with spectral-domain OCT and scanning laser ophthalmoscopy. Investig Ophthalmol Vis Sci. 2012;53:4519–30. https://doi.org/10.1167/iovs.11-8362
Caprioli J, Miller JM. Optic disc rim area is related to disc size in normal subjects. Arch Ophthalmol. 1987;105:1683–5. https://doi.org/10.1001/archopht.1987.01060120081030
Schuman JS, Hee MR, Puliafito CA, Wong C, Pedut-Kloizman T, Lin CP, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113:586–96. https://doi.org/10.1001/archopht.1995.01100050054031
Swaminathan SS, Jammal AA, Berchuck SI, Medeiros FA. Rapid initial OCT RNFL thinning is predictive of faster visual field loss during extended follow-up in glaucoma. Am J Ophthalmol. 2021;229:100. https://doi.org/10.1016/J.AJO.2021.03.019
Miki A, Medeiros FA, Weinreb RN, Jain S, He F, Sharpsten L, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology. 2014;121:1350–8. https://doi.org/10.1016/j.ophtha.2014.01.017
Sung KR, Kim S, Lee Y, Yun SC, Na JH. Retinal nerve fiber layer normative classification by optical coherence tomography for prediction of future visual field loss. Investig Ophthalmol Vis Sci. 2011;52:2634–9. https://doi.org/10.1167/IOVS.10-6246
Sehi M, Zhang X, Greenfield DS, Chung Y, Wollstein G, Francis BA, et al; Advanced Imaging for Glaucoma Study Group. Retinal nerve fiber layer atrophy is associated with visual field loss over time in glaucoma suspect and glaucomatous eyes. Am J Ophthalmol. 2013;155:73–82.e1. https://doi.org/10.1016/j.ajo.2012.07.005
David RCC, Moghimi S, Ekici E, Do JL, Hou H, Proudfoot JA, et al. Rates of retinal nerve fiber layer thinning in distinct glaucomatous optic disc phenotypes in early glaucoma. Am J Ophthalmol. 2021;229:8–17. https://doi.org/10.1016/j.ajo.2021.04.010
Lalezary M, Medeiros FA, Weinreb RN, Bowd C, Sample PA, Tavares IM, et al. Baseline optical coherence tomography predicts the development of glaucomatous change in glaucoma suspects. Am J Ophthalmol. 2006;142:576–82. https://doi.org/10.1016/j.ajo.2006.05.004
Enders P, Adler W, Schaub F, Hermann MM, Dietlein T, Cursiefen C, et al. Novel Bruch’s membrane opening minimum rim area equalizes disc size dependency and offers high diagnostic power for glaucoma. Invest Ophthalmol Vis Sci. 2016;57:6596–603. https://doi.org/10.1167/iovs.16-20561
Park DY, Lee EJ, Han JC, Kee C. Applicability of ISNT Rule Using BMO-MRW to Differentiate Between Healthy and Glaucomatous Eyes. J Glaucoma. 2018;27:610–6. https://doi.org/10.1097/IJG.0000000000000970
Kabbara SW, Zangwill LM, Mundae R, Hammel N, Bowd C, Medeiros FA, et al. Comparing optical coherence tomography radial and cube scan patterns for measuring Bruch’s membrane opening minimum rim width (BMO-MRW) in glaucoma and healthy eyes: cross-sectional and longitudinal analysis. Br J Ophthalmol. 2018;102:344–51. https://doi.org/10.1136/bjophthalmol-2016-310111
Gmeiner JMD, Schrems WA, Mardin CY, Laemmer R, Kruse FE, Schrems-Hoesl LM. Comparison of Bruch’s Membrane Opening Minimum Rim Width and Peripapillary Retinal Nerve Fiber Layer Thickness in Early Glaucoma Assessment. Investig Ophthalmol Vis Sci. 2016;57:OCT575–OCT584. https://doi.org/10.1167/IOVS.15-18906
Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J. 2013;4:14. https://doi.org/10.1186/1878-5085-4-14
Leske MC. Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. https://doi.org/10.1097/ICU.0b013e32831eef82
Graf-Grauwiller T, Stiimpfig D, Flammer J, Words K. Original Paper • Travail original Originalarbeit Do Beta-Blockers Cause Vasospasm? Beta-blockers Timolol Betaxolol Laser-Doppler velocimetry. Ophthalmologica 1993;206:45–50.
Anderson DR, Drance SM, Schulzer M; Collaborative Normal-Tension Glaucoma Study Group. Natural history of normal-tension glaucoma. Ophthalmology. 2001;108:247–53. https://doi.org/10.1016/s0161-6420(00)00518-2
Jonas JB, Papastathopoulos KI. Pressure-dependent changes of the optic disk in primary open-angle glaucoma. Am J Ophthalmol. 1995;119:313–7. https://doi.org/10.1016/S0002-9394(14)71173-0
Anderson DR, Drance SM, Schulzer M. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–97. https://doi.org/10.1016/S0002-9394(98)00223-2
Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S. A randomized trial of brimonidine versus timolol in preserving visual function: Results from the low-pressure glaucoma treatment study. Am J Ophthalmol. 2011;151:671–81. https://doi.org/10.1016/J.AJO.2010.09.026
Hayreh SS, Podhajsky P, Zimmerman MB. Beta-blocker eyedrops and nocturnal arterial hypotension. Am J Ophthalmol. 1999;128:301–9. https://doi.org/10.1016/S0002-9394(99)00160-9
De Moraes CG, Liebmann JM, Greenfield DS, Gardiner SK, Ritch R, Krupin T. Risk factors for visual field progression in the low-pressure glaucoma treatment study. Am J Ophthalmol. 2012;154:702–11. https://doi.org/10.1016/j.ajo.2012.04.015
Burgoyne CF, Crawford Downs J, Bellezza AJ, Francis Suh JK, Hart RT. The optic nerve head as a biomechanical structure: A new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retinal Eye Res. 2005;24:39–73. https://doi.org/10.1016/J.PRETEYERES.2004.06.001
Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic Nerve Damage in Human Glaucoma: II. The Site of Injury and Susceptibility to Damage. Arch Ophthalmol. 1981;99:635–49. https://doi.org/10.1001/ARCHOPHT.1981.03930010635009
Gharahkhani P, Jorgenson E, Hysi P, Khawaja AP, Pendergrass S, Han X, O, et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat Commun. 2021;12:1258. https://doi.org/10.1038/s41467-020-20851-4
Genetics of Glaucoma in People of African Descent (GGLAD) Consortium; Hauser MA, Allingham RR, Aung T, Van Der Heide CJ, Taylor KD, Rotter JI, et al. Association of genetic variants with primary open-angle glaucoma among individuals with african ancestry. JAMA. 2019 Nov 5;322(17):1682–91. https://doi.org/10.1001/jama.2019.16161.
Guo X, Rotter JI. Genome-Wide Association Studies. J Am Med Assoc. 2019;322:1705–6. https://doi.org/10.1001/jama.2019.16479
Osman W, Low SK, Takahashi A, Kubo M, Nakamura Y. A genome-wide association study in the Japanese population confirms 9p21 and 14q23 as susceptibility loci for primary open angle glaucoma. Hum Mol Genet. 2012;21:2836–42. https://doi.org/10.1093/HMG/DDS103
Taylor KD, Guo X, Zangwill LM, Liebmann JM, Girkin CA, Feldman RM, et al. Genetic architecture of primary open-angle glaucoma in individuals of African descent: the African descent and glaucoma evaluation study III. Ophthalmology. 2019;126:38–48. https://doi.org/10.1016/j.ophtha.2018.10.031
Acknowledgements
The authors thank Fei Yu Ph.D., for his expertize and assistance with statistical analyses.
Funding
Research to Prevent Blindness, New York, NY; The Payden Glaucoma Research Fund; The Simms/Mann Family Foundation. The funding organizations had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
All authors contributed to this study. Conception and design: JC, LG, ADG, DSV; Data collection: LG, EB; Formal analysis and interpretation of data: JC, LG, EM; Methodology: JC, LG, ADG, DSV; Project administration: JC, LG, EB; Supervision: JC, EB; Validation: JC; Writing paper: JC, LG, ADG, EB; Writing—review & editing: JC, LG, ADG, EB, SD, EM.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grassi, L., Salazar Vega, D., De Gainza, A. et al. Phenotypic expressions of the optic disc in primary open-angle glaucoma. Eye 37, 3839–3846 (2023). https://doi.org/10.1038/s41433-023-02627-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02627-4
- Springer Nature Limited