Abstract
Objectives
To inspect the inter-reader agreement of different diagnostic modalities in identifying choroidal neovascularization (CNV) activity secondary to angioid streaks (AS) and to analyze the prevalence of subretinal hyper-reflective material (SHRM) in active CNV.
Methods
Retrospective study of patients with AS with active CNV; optical coherence tomography (OCT), OCT angiography (OCTA), fundus fluorescein angiography (FFA), and indocyanine green angiography (ICGA) from each patient were collected. Agreement between two readers using different diagnostic modalities is presented as free-marginal kappa (k) and 95% confidence interval (CI).
Results
This study included 19 eyes of 12 patients with active CNV (5 naive and 14 previously treated). Agreement among readers on CNV activity was excellent for OCT (k =0.88; 95% CI 0.71–1.00), good for FFA (k = 0.70; 95% CI 0.46–0.94) and ICGA (k = 0.58; 95% CI 0.31–0.84), and poor using OCTA (k = 0.39; 95% CI 0.11–0.68). SHRM was the most common OCT finding associated with active CNV (100%); fuzzy borders were present in 53% of SHRM cases at baseline.
Conclusions
Identification of CNV activity in AS is challenging; OCT was the best modality to inspect active CNV. The identification of SHRM contributed to recognizing active CNV. Further studies are needed to assess the role of SHRM in anticipating prognosis and guiding treatment of CNV secondary to AS.
Similar content being viewed by others
Introduction
Angioid streaks (AS) are sharp interruptions of the Bruch’s membrane (BM) radiating from the optic disc [1, 2]. AS are frequently complicated by choroidal neovascularization (CNV) [3, 4].
Signs of neovascular activity include retinal haemorrhage, retinal leakage on fundus fluorescein angiography (FFA), hyperfluorescent vascular network on indocyanine green angiography (ICGA), increased retinal thickness and intraretinal or subretinal fluid on optical coherence tomography (OCT) [5, 6]. Subretinal hyper-reflective material (SHRM) on OCT, presenting as hyper-reflective material between the external retina and the retinal pigment epithelium (RPE), has been recently described as a sign of activity in CNV secondary to age-related degeneration (AMD) [7,8,9]. In particular, SHRM with fuzzy borders well correlates with leakage on FFA [10].
OCT angiography (OCTA) has also been included in the multimodal assessment of CNV in AS. The aspect of the CNV network on OCTA might provide clues for guiding CNV treatment, as the presence of branching vessels, peripheral arcades, loops, and peripheral halo have been associated with neovascular activity [11, 12].
The presence of these features on multimodal imaging and their variation with time serves as a clinical criterion for initiating and monitoring the response to anti-vascular endothelial growth factor (VEGF) agents; however, a comprehensive analysis of their role in identifying CNV activity in AS is lacking. Moreover, the detection of the aforementioned signs may be challenging in eyes with AS due to extensive degenerative and atrophic changes at the posterior pole. Pattern dystrophy-like deposits [13], chronic subretinal fluid [14], BM undulations [15], tubulations, RPE alterations [16] and inflammatory changes might confound the clinical picture [17, 18].
The primary aim of this study was to assess the concordance of readers in identifying AS-related CNV activity using different diagnostic modalities, with particular attention to the presence of SHRM on OCT and the aspect of the neovascular network on OCTA.
Methods
This was a retrospective analysis including patients with AS presenting at the Ophthalmology Department of the San Raffaele Hospital, Vita-Salute University (Milan, Italy) from January 2016 to January 2017. Patients signed a written consent approved by the local ethical committee and adhering to the tenets of the Declaration of Helsinki.
Patients with active CNV related to AS, either naive or treated, subfoveal or extrafoveal and at least 12 months of follow-up were recruited. Only subjects with multimodal imaging at baseline, including OCT, OCTA, FFA, and ICGA, were included. Exclusion criteria were insufficient media clarity to obtain high-quality imaging; CNV secondary to any retinal disorder other than AS; previous intraocular surgery, with except of uneventful cataract extraction at least 6 months before baseline visit; age <18 years.
All patients underwent monthly ophthalmologic examination in the first year after baseline visit. Each visit included measurement of best-corrected visual acuity (BCVA) using ETDRS charts, slit-lamp biomicroscopy, and OCT (Heidelberg Spectralis Heidelberg, Germany), acquired with a follow-up mode. Central macular thickness (CMT) was automatically calculated by the Spectralis device at each acquisition. Fundus photography, OCTA (swept-source DRI OCT Triton, Topcon Corporation, Japan; or AngioPlex® CIRRUS model 5000, Carl Zeiss Meditec, Inc., Dublin, USA), FFA and ICGA (Heidelberg Spectralis Heidelberg, Germany) were obtained at the baseline visit and then repeated at physician discretion. A single intravitreal injection of aflibercept was performed at baseline and patients were retreated with a pro-re-nata regimen, based on visual acuity and/or multimodal imaging findings.
The definition of CNV activity was carried out in the presence of new haemorrhage on biomicroscopic examination, increased CMT, new or worsened intraretinal/subretinal fluid on OCT, leakage on FFA, and late-phase plaque on ICGA. The presence of SHRM at baseline was annotated for each eye and its borders were classified as follows: “sharp” if more than 75% of the borders were sharply traceable, “mixed” if 25% to 75% of the borders were traceable, and “fuzzy” if less than 25% of borders were identified [9]. SHRM was defined as “layered” if internal layers of different reflectivity were discernible, and “non-layered” otherwise. Hyper-reflective spots within the SHRM, defined as at least three hyper-reflective dots of at least 20 microns in diameter and reflectivity comparable to the retinal nerve fibre layer with moderate backscattering, were manually counted [19, 20]. The maximum height of SHRM was calculated with the in-built OCT calliper.
OCTA slabs were selected based on the best visualization of the entire CNV network, after removal of the superficial vessels’ projections. OCTA slabs were analyzed according to the presence of the following features: branching peripheral vessels (defined as thin tangled capillaries), peripheral arcades (peripheral connections between tiny branching vessels), loops (defined as inner anastomoses between tiny vessels), perilesional halo (defined as a hypo-reflective region on the choriocapillaris slab surrounding the CNV network) [11].
The primary outcome was to analyze the prevalence of signs of CNV activity on OCT, OCTA, FFA, and ICGA and the agreement among these modalities. Qualitative and quantitative features on each modality were judged by 2 retina specialists (AM and CG). Uncertainty regarding judgement was clarified by a senior judge (MBP).
Statistical analyses were performed with SPSS 18 (IBM, Armonk, NY). Descriptive statistic was presented as mean ± standard deviation. The BCVA was converted into a LogMAR scale for statistical calculations. The inter-rater agreement between the readers for each imaging modality was calculated using Randolph’s free-marginal multi-rater kappa; overall agreement (OA) and 95% confidence interval (CI) are provided. Kappa values <0.40 were defined as “poor”, between 0.40 and 0.75 as “good”, and >0.75 as “excellent” [21].
Results
Baseline characteristics
This study included 19 eyes of 12 patients, mean age 53 ± 13 years, with 4 females (33.3%). The CNV characteristics are listed in Table 1.
Baseline BCVA was 0.50 ± 0.3 LogMAR, with mean CMT of 243 ± 113 µm. All the patients presented at least 1 sign of CNV activity on multimodal imaging; the prevalence of each feature is depicted in Table 2.
On OCT, the most frequent sign was the presence of SHRM (Fig. 1). SHRM featured fuzzy borders in 53% of cases, mixed borders in 31%, and sharp borders in 16%. Other OCT findings included intraretinal (58%) and subretinal fluid (58%). Dye-leakage on FFA was present in 79% of eyes. On OCTA, a vascular branching plaque and perilesional dark halo were observed in 63% and 58% of cases, respectively.
A Cross-sectional optical coherence tomography (OCT) of an eye with angioid streaks and abnormal SHRM with sharp borders between the retinal pigment epithelium (RPE) and the Bruch’s membrane; hypo-reflective subretinal space is present where vitelliform material reabsorbed [13]. B The development of SHRM with fuzzy borders. C The disappearance of SHRM with fuzzy borders after treatment with one intravitreal injection of anti-VEGF.9.
Anti-VEGF treatment outcomes
The mean number of intravitreal anti-VEGF injections administered over 12 months was 4 ± 2; at last follow-up, BCVA was 0.42 ± 0.4 LogMAR (p = 0.49), with a mean CMT of 217 ± 110 µm (p = 0.477).
At the final assessment, 8 eyes had still at least one sign of active CNV; subretinal fluid persisted in 5 eyes of 3 patients, while intraretinal fluid persisted in 8 eyes of 7 patients. SHRM disappeared or changed its characteristics after anti-VEGF treatment; the majority of eyes presented SHRM with sharp borders (58%) at the exit visit, 37% had SHRM with mixed borders, and only 5% had a fuzzy contour. Mean SHRM height reduced from 219 ± 123 µm at baseline to 197 ± 139 µm (p = 0.59) at the end of the 12-month follow-up. On OCTA, vascular branching and perilesional halo reduced to 47% and 26%, respectively.
Agreement analysis
Agreement among readers on CNV activity varied according to the imaging modality chosen (Figs. 2 and 3). Using OCT, the agreement was excellent (OA = 93.94%; free-marginal kappa = 0.88; 95% CI 0.71–1.00). On FFA and ICGA, the agreement was good (OA = 84.85%; free-marginal kappa = 0.70; 95% CI 0.46–0.94 and OA = 78.79%; free-marginal kappa = 0.58; 95% CI 0.31–0.84, respectively). On OCTA, the agreement was poor (OA = 69.70%; free-marginal kappa = 0.39; 95% CI 0.11–0.68).
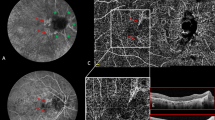
Top panels show on the left; the cross-sectional optical coherence tomography (OCT) of an eye with angioid streaks and abnormal SHRM with sharp borders between the retinal pigment epithelium (RPE) and the Bruch’s membrane; top panels on the right show OCT angiography (OCTA) revealing the presence of CNV with branching vessels, peripheral arcades, loops, and peripheral halo. Middle panels show on the left the development of SHRM with fuzzy borders (arrow) on OCT; OCTA does not reveal significant changes compared to the image above in the area of the new SHRM (arrows). Bottom panels illustrate early and late fluorescein angiography, on the left, and indocyanine green angiography, on the right, with minimal leakage and staining of the SHRM with fuzzy borders (arrows).
Top panels show on the left the cross-sectional optical coherence tomography (OCT) of an eye with angioid streaks and abnormal SHRM with sharp borders between the retinal pigment epithelium (RPE) and the Bruch’s membrane; top panels on the right show OCT angiography (OCTA) revealing the presence of CNV with peripheral arcades, and peripheral halo. Middle panels show on the left the development of SHRM with fuzzy borders (arrow) on OCT; OCTA reveals the development of a new neovascular network with branching vessels, loops and peripheral halo in the area of the new SHRM (arrows). Bottom panels illustrate early and late fluorescein angiography, on the left, and indocyanine green angiography, on the right, with significant leakage and staining of the SHRM with fuzzy borders (arrows).
The combined agreement on CNV activity when considering FFA and OCT only was good (OA = 81.8%; Free-marginal kappa = 0.64; 95% CI 0.31–0.97). When the four different imaging modalities were considered together, the agreement among the two readers was poor (OA = 69.7%; Free-marginal kappa = 0.39 with 95% CI 0.15, 0.64).
Discussion
In this study, we observed relevant discrepancies between different imaging modalities in detecting CNV activity in AS. The OCT was the most powerful modality, having the highest rate of agreement between the two readers; the presence of SHRM with fuzzy and mixed borders was the most frequent sign associated with CNV activity on OCT. These results may have important implications on the management of CNV in AS and in refining the treatment protocols.
The detection of CNV activity in AS is not always straightforward using fundus examination or multimodal imaging, due to the atrophic and fibrotic changes occurring over time. This might contribute to explain why both clinical trials and real-life studies have failed in showing clinically significant increase in VA using anti-VEGF in this condition [13, 22,23,24,25].
The majority of the previous investigations considered retinal haemorrhages, leakage on FFA, and intraretinal or subretinal fluid on OCT as parameters of activity. In this analysis, we included additional parameters on multimodal imaging to evaluate the neovascular activity, as the presence of SHRM and the features of CNV on OCTA. SHRM turned out to be the most frequent tomographic sign of CNV activity. The analysis of borders of the SHRM added some clues on CNV activity; fuzzy or mixed borders were more frequent at baseline (53% and 31%, respectively). After treatment, more than half of included eyes featured SHRM with sharp borders, while only 5% had a fuzzy silhouette. Previous studies on AMD eyes have demonstrated SHRM to be associated with higher risk of subretinal fibrosis and worse VA after anti-VEGF course [26]. AS-related SHRM might be less likely to disappear after anti-VEGF therapy because of an increased fibrotic component at baseline or over the treatment course. Noteworthily, in eyes in which SHRM did not completely disappear, a new area of SHRM with fuzzy borders was often visible on the top of old sharply bordered SHRM in the setting of neovascular activity relapse (Fig. 2).
Intraretinal and subretinal fluid were recognized in 58% and 58% of patients at baseline, respectively. Subretinal fluid persisted in a few eyes despite treatment (Fig. 1); chronic subretinal fluid, resistant to anti-VEGF agents, has been previously described in eyes with reabsorbed vitelliform material in the setting of AS-related pattern dystrophy [13]. The presence of hyperfluorescent lesions other than active CNV, including degenerative/atrophic changes of the RPE or the BM might partially explain the only fair agreement found using FFA and ICGA. Furthermore, in the context of a long-standing CNV, staining of fibrotic areas might also be misinterpreted as dye leakage on FFA. The OCTA exhibited the lowest agreement among readers; even though OCTA has demonstrated good sensitivity and specificity in the identification of the CNV network, no robust signs of neovascular activity have been validated so far. Further research is needed to tackle this issue, optimizing the role of OCTA in the follow-up of neovascular membranes.
We recognize that the present study has several limitations. Patients underwent a mean number of injections of 4 ± 2 in 12 months, which is lower as compared to the existing literature on naive patients with AS-related CNV. The risk of undertreatment in AS is concrete, as the disease is characterized by several neovascular relapses; we speculate that the treatment criteria used in the present study (namely, haemorrhage, dye leakage, fluid) might not be sensitive enough to properly control CNV activity. In this view, the need for new imaging biomarkers suggesting active CNV is of paramount importance. We forecast the inclusion of SHRM in the multimodal assessment of AS patients could provide better clinical and therapeutic outcomes.
Also, the analysis of OCTA can be challenging in patients with AS, especially in the presence of retinal fibrosis and atrophy, which might bias the interpretation of the OCTA slab. For this reason, we limited the investigation of OCTA on the CNV qualitative features, instead of using quantitative approaches.
Conclusions
In this study on patients with AS, we observed that OCT was the most contributing device in detecting eyes with active CNV. SHRM was the most observed feature associated with neovascular activity; SHMR morphologic changes during the follow-up might be helpful in following the response to anti-VEGF treatment. Further studies are needed to assess the use of SHRM with fuzzy or mixed borders among the treatment criteria in clinical trials for CNV secondary to AS.
Summary
What was known before
-
Angioid streaks can be complicated by choroidal neovascularization.
-
Detection of choroidal neovascularization activity is complex in angioid streaks.
What this study adds
-
The agreement among readers on choroidal neovascularization activity was excellent for OCT, good for FFA and ICGA, and poor using OCTA.
-
Structural optical coherence tomography was the most contributing technique for detecting active choroidal neovascularization.
-
Subretinal hyper-reflective material was the most observed feature of active choroidal neovascularization.
References
Clarkson JG, Altman RD. Angioid streaks. Surv Ophthalmol. 1982;26:235–46.
Marchese A, Rabiolo A, Corbelli E, Carnevali A, Cicinelli MV, Giuffre C, et al. Ultra-widefield imaging in patients with angioid streaks secondary to pseudoxanthoma elasticum. Ophthalmol Retin. 2017;1:137–44.
Mansour AM, Shields JA, Annesley WH Jr., el-Baba F, Tasman W, Tomer TL. Macular degeneration in angioid streaks. Ophthalmologica. 1988;197:36–41.
Singerman LJ, Hatem G. Laser treatment of choroidal neovascular membranes in angioid streaks. Retina. 1981;1:75–83.
Battaglia Parodi M, Iacono P, La Spina C, Berchicci L, Scotti F, Leys A, et al. Intravitreal bevacizumab for nonsubfoveal choroidal neovascularization associated with angioid streaks. Am J Ophthalmol. 2014;157:374–7.
Pece A, Avanza P, Introini U, Brancato R. Indocyanine green angiography in angioid streaks. Acta Ophthalmol Scand. 1997;75:261–5.
Pokroy R, Mimouni M, Barayev E, Segev F, Geffen N, Nemet AY, et al. Prognostic value of subretinal hyperreflective material in neovascular age-related macular degeneration treated with bevacizumab. Retina. 2018;38:1485–91.
Willoughby AS, Ying GS, Toth CA, Maguire MG, Burns RE, Grunwald JE, et al. Subretinal hyperreflective material in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2015;122:1846–53.
Casalino G, Bandello F, Chakravarthy U. Changes in neovascular lesion hyperreflectivity after anti-VEGF treatment in age-related macular degeneration: an integrated multimodal imaging analysis. Investig Ophthalmol Vis Sci. 2016;57:288–298.
Giani A, Luiselli C, Esmaili DD, Salvetti P, Cigada M, Miller JW, et al. Spectral-domain optical coherence tomography as an indicator of fluorescein angiography leakage from choroidal neovascularization. Investig Ophthalmol Vis Sci. 2011;52:5579–86.
Coscas F, Lupidi M, Boulet JF, Sellam A, Cabral D, Serra R, et al. Optical coherence tomography angiography in exudative age-related macular degeneration: a predictive model for treatment decisions. Br J Ophthalmol. 2019;103:1342–6.
Corbelli E, Carnevali A, Marchese A, Cicinelli MV, Querques L, Sacconi R, et al. Optical coherence tomography angiography features of angioid streaks. Retina. 2018;38:2128–36.
Battaglia Parodi M, Romano F, Marchese A, Arrigo A, Llorenc V, Cicinelli MV, et al. Anti-VEGF treatment for choroidal neovascularization complicating pattern dystrophy-like deposit associated with pseudoxanthoma elasticum. Graefes Arch Clin Exp Ophthalmol. 2019;257:273–8.
Zweifel SA, Imamura Y, Freund KB, Spaide RF. Multimodal fundus imaging of pseudoxanthoma elasticum. Retina. 2011;31:482–91.
Marchese A, Parravano M, Rabiolo A, Carnevali A, Corbelli E, Cicinelli MV, et al. Optical coherence tomography analysis of evolution of Bruch’s membrane features in angioid streaks. Eye. 2017;31:1600–5.
Ellabban AA, Hangai M, Yamashiro K, Nakagawa S, Tsujikawa A, Yoshimura N. Tomographic fundus features in pseudoxanthoma elasticum: comparison with neovascular age-related macular degeneration in Japanese patients. Eye. 2012;26:1086–94.
Marchese A, Romano F, Cicinelli MV, Bandello F, Battaglia Parodi M. Chorioretinal punched-out lesions in pseudoxanthoma elasticum. Retina. 2018;38:e43–4.
Gliem M, Birtel J, Muller PL, Hendig D, Faust I, Herrmann P, et al. Acute retinopathy in pseudoxanthoma elasticum. JAMA Ophthalmol. 2019;137:1165–73.
Romano F, Mercuri S, Arrigo A, Marchese A, Cicinelli MV, Albertini GC, et al. Identification of hyperreflective foci in angioid streaks. Eye. 2019;33:1916–23.
Parodi MB, Arrigo A, Romano F, Aragona E, Marchese A, Cicinelli MV, et al. Hyperreflective foci number correlates with choroidal neovascularization activity in angioid streaks. Investig Ophthalmol Vis Sci. 2018;59:3314–9.
McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22:276–82.
Lai TYY, Staurenghi G, Lanzetta P, Holz FG, Melissa Liew SH, Desset-Brethes S, et al. Efficacy and safety of ranibizumab for the treatment of choroidal neovascularization due to uncommon cause: twelve-month results of the MINERVA study. Retina. 2018;38:1464–77.
Parodi MB, Iacono P, Bandello F. Juxtafoveal choroidal neovascularization secondary to persistent placoid maculopathy treated with intravitreal bevacizumab. Ocul Immunol Inflamm. 2010;18:399–401.
Gliem M, Birtel J, Herrmann P, Fimmers R, Berger M, Coch C, et al. Aflibercept for choroidal neovascularizations secondary to pseudoxanthoma elasticum: a prospective study. Graefes Arch Clin Exp Ophthalmol. 2020;258:311–8.
Parodi MB, Cicinelli MV, Marchese A, Giuffre C, Viola F, Staurenghi G, et al. Intravitreal aflibercept for management of choroidal neovascularization secondary to angioid streaks: The Italian EYLEA-STRIE study. Eur J Ophthalmol. 2020:1120672120928305.
Casalino G, Stevenson MR, Bandello F, Chakravarthy U. Tomographic biomarkers predicting progression to fibrosis in treated neovascular age-related macular degeneration: a multimodal imaging study. Ophthalmol Retin. 2018;2:451–61.
Author information
Authors and Affiliations
Contributions
AM, CG, MVC and MBP contributed to the design and conduction of the study, the correction of the draft, and the final revision. AM and MVC collected and analyzed the data. AM wrote the first draft and edited the table and figures. AA and FB revised the final version of the manuscript. All authors read and approved the final manuscript. All authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
FB has the following disclosures: Allergan (S), Alimera (S), Bayer (S), Farmila-Thea (S), Schering Pharma (S), sanofi-Aventis (S), Novagali (S), Pharma (S), Hoffmann-La Roche (S), Genetech (S), Novartis (S). MBP has the following disclosures: Bausch & Lomb Inc. (C). The other authors have nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marchese, A., Giuffrè, C., Cicinelli, M.V. et al. The identification of activity of choroidal neovascularization complicating angioid streaks. Eye 36, 1027–1033 (2022). https://doi.org/10.1038/s41433-021-01555-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01555-5
- Springer Nature Limited