Abstract
Purpose
To investigate clinical features and outcomes of conjunctival melanoma classified by tumour origin.
Methods
Retrospective review of conjunctival melanoma patients at a single ocular oncology centre between April 18, 1974 and September 9, 2019. Lesions were divided into three tumour origin groups (primary acquired melanosis [PAM], nevus, and de novo) and clinical features and outcomes were compared.
Results
There were 629 patients with conjunctival melanoma that arose from PAM (n = 476, 76%), nevus (n = 59, 9%), or de novo (n = 94, 15%). A comparison (PAM vs. nevus vs. de novo) revealed patients with tumours arising from PAM presented with older mean age (62 vs. 52 vs. 55 years, p < 0.001), worse initial logMAR visual acuity (Snellen equivalent 20/30 vs. 20/25 vs. 20/25, p = 0.03), and greater clock hour involvement (4.8 vs. 4.0 vs. 3.2, p < 0.001). Tumours arising from nevus had lower frequency of fornix (31% vs. 9% vs. 24%, p = 0.02) and tarsal involvement (29% vs. 9% vs. 26%, p = 0.046) and more frequent classification as AJCC category T1 (60% vs. 89% vs. 62%, p = 0.01). After follow-up of (57.2 vs. 68.2 vs. 51.7 months, p = 0.35), tumours arising from PAM had worse mean final visual acuity (20/50 vs. 20/40 vs. 20/40, p = 0.02) and greater frequency of visual acuity loss ≥3 lines (25% vs. 15% vs. 10%, p = 0.02). Kaplan–Meier estimates for 5-year risk showed no difference by tumour origin for visual acuity loss ≥3 lines, local tumour recurrence, exenteration, metastasis, or death.
Conclusions
Conjunctival melanoma most often arose from PAM, and tumour origin did not affect clinical outcomes.
Similar content being viewed by others
Introduction
Conjunctival melanoma is a rare malignancy of the ocular surface. Although it only accounts for 2% of ocular tumours, conjunctival melanoma can be devastating due to its propensity to recur after excision, metastasize throughout the body, and ultimately lead to death if not detected early and properly managed [1,2,3]. Despite multiple treatment options, the incidence of conjunctival melanoma continues to increase in the United States and, like cutaneous melanoma, increased sun exposure could be a contributing factor [4,5,6]. The increasing incidence and relatively high mortality of 13% at 10 years warrants attention to this deadly disease, particularly in the clinical evaluation of melanoma and its precursors [3].
Conjunctival melanoma is the proliferation of aberrant melanocytes, which can arise from three distinct entities [7]. The most common precursor is primary acquired melanosis (PAM) (74%), but melanoma can also arise from a pre-existing nevus (7%) or de novo (19%) [8]. Melanoma derived from these precursors have different genetic profiles, such as a higher prevalence of BRAF mutation in melanoma arising from nevus [9]. These genetic profiles could be responsible for differences in tumour characteristics as well as tumour behaviour. In 2011, Shields et al. reported 382 conjunctival melanoma patients with mean follow-up of 4.3 years and found worse survival in patients whose melanoma arose de novo, suggesting tumour origin could be important for prediction of outcomes such as recurrence and overall patient prognosis [8]. Herein, we investigate clinical features and outcomes of conjunctival melanoma based on tumour origin in 629 patients over mean follow-up of nearly 5 years.
Materials and methods
Medical records were retrospectively reviewed for patients with both clinical and confirmed histopathologic conjunctival melanoma managed on the Ocular Oncology Service at Wills Eye Hospital, Thomas Jefferson University, Philadelphia, between April 18, 1974, and September 9, 2019. Patients whose lesions were completely treated elsewhere and were referred for either a second opinion or monitoring for recurrence were also included in the study. Suspected melanoma patients who had only PAM (premalignant lesion, melanoma in situ) were excluded. Tumours were classified based on the origin of melanoma (PAM, nevus, or de novo), which was determined by a trained ocular pathologist after resection of the tumour. This study received Institutional Review Board approval from Wills Eye Hospital, adhered to the tenets of the Declaration of Helsinki, and complied with the Health Insurance Portability and Accountability Act. Informed consent was obtained from all patients.
Patients were all evaluated by trained ocular oncologists (CLS, SEL, JAS) using external and slit lamp bio-microscopy. Examination findings were documented on large conjunctival drawings, and external photographs were taken at each visit. Demographic data were recorded, including age (in years), race (Caucasian, African American, Hispanic, Asian Indian, Asian Oriental, others/unknown), sex (male, female), and the involved eye (right, left). Ocular history included a past diagnosis of conjunctival complexion associated melanosis, melanocytosis, conjunctival secondary acquired melanosis, and a prior history of conjunctival or eyelid surgery. Smoking history was also noted.
Clinical features at presentation included best-corrected visual acuity, largest tumour basal diameter (millimetres), tumour thickness (millimetres), number of quadrants involved (1–4), number of clock hours involved (0–12), location of tumour (bulbar conjunctiva, limbus, cornea, plica, caruncle, fornix, tarsal conjunctiva, eyelid, orbit), American Joint Committee on Cancer classification (8th edition), presence of feeder and intrinsic vessels, and tumour colour (no pigmentation, complete pigmentation, partial pigmentation).
Conjunctival melanoma was primarily managed by complete surgical resection. This included superficial keratectomy for the corneal component, partial lamellar scleroconjunctivectomy, wide surgical resection for the conjunctival component using a no-touch technique, and supplemental double freeze-thaw cryotherapy to adjacent conjunctival margins. Associated PAM was treated with cryotherapy, excision, or both. Other treatment options included observation, medical treatment (cryotherapy, adjuvant mitomycin C, interferon α-2b), radiation therapy (external beam radiation therapy, plaque radiotherapy), or surgical treatment (enucleation, exenteration). The types of primary, secondary, and tertiary treatments, as well as the total number of surgical, medical, and radiation treatments were recorded. Primary treatment was defined as the first treatment of the malignancy by an ophthalmologist. If the first treatment was performed at an outside institution, records were obtained from the provider to document initial management. Visual acuity as well as treatment outcomes were recorded at date of last follow-up. Treatment outcomes included visual acuity loss ≥3 lines, local tumour control, tumour recurrence, enucleation, exenteration, metastasis, site of metastasis (locoregional lymph nodes, systemic), and death. Tumour control was defined as no clinical or histopathologic evidence of tumour. Recurrence was defined as the presence of new melanoma at the same site of previous melanoma or at any site on the eye or adnexa. Spread to locoregional lymph nodes was assessed by palpation of preauricular, submandibular, and cervical lymph nodes at each visit. Lymph node metastasis was confirmed histopathologically after sentinel lymph node biopsy. Distant metastasis was assessed by history at each visit and physical examination once a year. Distant metastasis was confirmed by PET scan or histopathology after biopsy was obtained.
Statistical analysis was performed using SPSS Statistics Software (version 23, SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean (median, range). The one-sample Kolmogorov–Smirnov test was used to assess for normality. Comparison between the three tumour origin categories (PAM vs. nevus vs. de novo) was performed using the one-way ANOVA test for continuous variables with normal distribution and Kruskal–Wallis H test for continuous variables without normal distribution. Comparison of categorical variables was performed using the Chi-square test and Fisher’s exact test when indicated. Kaplan–Meier analysis was performed to determine cumulative probability of all outcomes, including vision loss, tumour recurrence, enucleation, exenteration, metastasis, and death. The log-rank test was performed to assess differences in survival distribution between the tumour origin categories. Hazard ratio and 95% confidence interval was calculated for the tumour origin categories at each time point of interest using the Cox proportional hazards model. A p < 0.05 was considered statistically significant.
Results
There were 629 consecutive patients with conjunctival melanoma managed on the Oncology Service at Wills Eye Hospital included in the study. Tumours were classified based on the origin of the melanoma including 476 (76%) from PAM, 59 (9%) from pre-existing nevus, and 94 (15%) de novo (Fig. 1). Demographic features are listed in Table 1. A comparison (PAM vs. nevus vs. de novo) revealed that conjunctival melanoma arising from PAM presented at an older mean patient age (62.3 vs. 52.3 vs. 55.4 years, p < 0.001) and with greater number of prior conjunctival surgeries (1.9 vs. 1.7 vs. 1.5, p = 0.01). Conjunctival melanoma arising de novo had a greater frequency of prior conjunctival surgery (74% vs. 71% vs. 88%, p = 0.01).
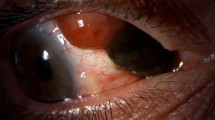
A Conjunctival melanoma originating from PAM on the bulbar conjunctiva with limbal involvement. B Conjunctival melanoma originating from a pre-existing nevus on the bulbar conjunctiva with prominent feeder vessels temporally. C Conjunctival melanoma originating de novo on the bulbar conjunctiva with limbal involvement and a dilated feeder vessel superiorly.
Clinical features are listed in Table 2. A comparison (PAM vs. nevus vs. de novo) revealed patients with tumours arising from PAM had worse logMAR visual acuity at presentation (Snellen equivalent 20/30 vs. 20/25 vs. 20/25, p = 0.03) and greater clock hour involvement (4.8 vs. 4.0 vs. 3.2, p < 0.001). Patients with tumours arising from nevus had greater tumour thickness (2.6 vs. 3.2 vs. 2.9 mm, p = 0.04), less forniceal involvement (31% vs. 9% vs. 24%, p = 0.02) and less tarsal involvement (29% vs. 9% vs. 26%, p = 0.046).
Treatment modalities are listed in Table 3. A comparison (PAM vs. nevus vs. de novo) revealed that patients whose tumour arose from PAM required fewer treatments (3.2 vs. 5.0 vs. 5.0, p < 0.001) and those from PAM or nevus needed substantially less radiation treatment (10% vs. 0% vs. 32%, p < 0.001).
Treatment outcomes are listed in Table 4. Comparison revealed that patients whose tumour arose from PAM had worse logMAR visual acuity at date last seen (Snellen equivalent 20/50 vs. 20/40 vs. 20/40, p = 0.02) and greater frequency of visual acuity loss ≥3 lines (25% vs. 15% vs. 10%, p = 0.01).
Kaplan–Meier results are listed in Fig. 2. Comparison (PAM vs. nevus vs. de novo) revealed no difference in the 5-year estimated risk of visual acuity loss ≥3 lines (27% vs. 16% vs. 12%, p = 0.06) (Fig. 2A), melanoma recurrence (40% vs. 28% vs. 42%, p = 0.46) (Fig. 2B), enucleation (1% vs. 2% vs. 0%, p = 0.54) (Fig. 2C), exenteration (13% vs. 9% vs. 11%, p = 0.74) (Fig. 2D), metastasis (21% vs. 16% vs. 26%, p = 0.37) (Fig. 2E), or melanoma-related death (7% vs. 3% vs. 13%, p = 0.67) (Fig. 2F).
Kaplan–Meier estimated 5-year risk revealed no difference between groups [PAM (green line) vs. nevus (red line) vs. de novo (blue line)] for (A) visual acuity loss (27% vs. 16% vs. 12%, p = 0.06), (B) recurrence/new tumour (40% vs. 28% vs. 42%, p = 0.46), (C) enucleation (1% vs. 2% vs. 0%, p = 0.54), (D) exenteration (13% vs. 9% vs. 11%, p = 0.74), (E) melanoma-related metastasis (21% vs. 16% vs. 26%, p = 0.37), or (F) melanoma-related death (7% vs. 3% vs. 13%, p = 0.67).
Discussion
In their 2011 report of conjunctival melanoma, Shields et al. wrote “conjunctival melanoma is a rare malignancy but its potential for metastasis and death can be profound.” [8] This claim is substantiated by high rates of melanoma-related metastasis and melanoma death at 26% and 13%, respectively, after 10 years follow-up [3]. A high recurrence rate of 51% at 10 years and an increase in incidence from 0.19 to 0.43 (per million, age-adjusted) between 1973 and 1999 are both similarly germane [3, 6]. Identification of melanoma precursor lesions is essential to mitigate and prevent these devastating outcomes.
In 2011, Shields et al. compiled data from 382 patients with conjunctival melanoma and found that melanoma arose from PAM in 284 patients (74%), nevus in 26 patients (7%), or de novo in 72 patients (19%) [8]. The authors noted a younger age of presentation in patients with melanoma arising from nevus compared with those arising from PAM or de novo. The authors also observed better survival in patients with melanoma arising from PAM or nevus compared with those arising de novo. This differs from the findings of Paridaens et al. who reported on 256 patients with a mean of 9 years follow-up [10]. That study, conducted in 1994, found that tumour thickness was an accurate indicator for conjunctival melanoma prognosis, while sex, age, and tumour origin were not [10]. Similarly, in 2015, Larsen et al. studied 129 patients with conjunctival melanoma and mean follow-up of 6.1 years and did not identify any association between tumour origin and recurrence, metastasis, or death [9].
In the current study of 629 patients with nearly 5 years follow-up, we found that conjunctival melanoma arose from PAM (76%), pre-existing nevus (9%), and de novo (15%), consistent with previous reports [8]. Patients whose melanoma arose from nevus were youngest while those whose melanoma arose from PAM were oldest, similar to a previous report [8].
At presentation, tumours arising from nevus were thicker and more often had feeder vessels compared with tumours arising from PAM or de novo. This association may be due to higher metabolic activity and increased glucose utilization, although this theory has not been directly tested [11, 12]. Furthermore, this relationship could be due to genetic aberrations affecting cellular proliferation, as more BRAF mutations are found in melanoma arising from nevus (67%) compared with PAM (36%) [9]. Further studies are needed to determine if patients with melanoma arising from nevus would benefit from supplemental BRAF inhibitor therapy.
Melanoma arising from PAM had the greatest clock hour involvement compared with nevus or de novo, highlighting the extensive nature of PAM and its propensity for high-risk locations. In addition, patients with melanoma arising from PAM had a greater number of prior conjunctival surgeries. Given the potential for incomplete removal, these prior surgeries create an opportunity for tumour seeding [3], which could explain higher recurrence rates in melanoma arising from PAM found in other studies [9]. We also observed more recurrences in patients with melanoma arising from PAM, but this did not reach statistical significance.
Patients with melanoma arising from PAM had worse visual acuity at date last seen and had greater frequency of visual acuity loss ≥3 lines compared to the other groups. While this could be due to the extensive nature of PAM and the larger resections needed to eradicate the lesion [13], careful interpretation of this result is warranted as this difference is most likely due to worse visual acuity at date first seen and older patient age in this group.
Regarding other outcomes, we did not observe an association between tumour origin and exenteration, metastasis, or death. Previous studies similarly did not find tumour origin to be predictive of death or metastasis [9, 10] The prior finding that melanoma arising de novo carried an increased risk of death and metastasis in 382 patients could be due to varying sample size among these studies [8].
Limitations of this study include the retrospective study design. In addition, some patients had limited follow-up, yet there was no difference in follow-up time between the three groups. Furthermore, these data represent patients from a tertiary referral centre and may not be representative of the general population. Similarly, some patients were partially managed elsewhere, which could contribute to referral bias. Study strengths include the large sample size and mean follow-up for the entire cohort of nearly 5 years.
In conclusion, our analysis of conjunctival melanoma and tumour origin found that conjunctival melanoma most often arose from PAM (76%), and these melanomas were detected at an older age and had more extensive clock hour involvement. Tumours arising from nevus were detected at a younger age and were thicker with more feeder vessels. Although tumour origin did not impact 5-year outcomes of visual acuity loss, tumour recurrence, enucleation, exenteration, metastasis, or death, attention to these precursor lesions is crucial given the potential to develop into malignant melanoma.
Summary
What was known before
-
Conjunctival melanoma can arise from three precursor lesions: primary acquired melanosis (PAM), a pre-existing nevus, or de novo. Conjunctival melanoma can be devastating due to its propensity to recur after excision, metastasize throughout the body, and ultimately lead to death if not detected early and properly managed.
What this study adds
-
This study investigates clinical features and outcomes based on tumour origin in 629 patients, the largest cohort of conjunctival melanoma to date. Conjunctival melanoma most often arose from PAM and tend to be detected at an older age. Whereas conjunctival melanoma arising from nevus are thicker and tend to be detected at a younger age. Tumour origin did not impact 5-year outcomes of visual acuity loss, tumour recurrence, enucleation, exenteration, metastasis, or death.
References
McCartney AC. Pathology of ocular melanomas. Br Med Bull. 1995;51:678–93.
Seregard S. Conjunctival melanoma. Surv Ophthalmol. 1998;42:321–50.
Shields CL, Shields JA, Gündüz K, Cater J, Mercado GV, Gross N, et al. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Arch Ophthalmol. 2000;118:1497–507.
Tuomaala S, Eskelin S, Tarkkanen A, Kivelä T. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399–408.
Triay E, Bergman L, Nilsson B, All-Ericsson C, Seregard S. Time trends in the incidence of conjunctival melanoma in Sweden. Br J Ophthalmol. 2009;93:1524–8.
Yu G-P, Hu D-N, McCormick S, Finger PT. Conjunctival melanoma: is it increasing in the United States? Am J Ophthalmol. 2003;135:800–6.
Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Indian J Ophthalmol. 2019;67:1930–48.
Shields CL, Markowitz JS, Belinsky I, Schwartzstein H, George NS, Lally SE, et al. Conjunctival melanoma: outcomes based on tumor origin in 382 consecutive cases. Ophthalmology. 2011;118:389–95.e952.
Larsen AC, Dahmcke CM, Dahl C, Siersma VD, Toft PB, Coupland SE, et al. A retrospective review of conjunctival melanoma presentation, treatment, and outcome and an investigation of features associated with BRAF mutations. JAMA Ophthalmol. 2015;133:1295–303.
Paridaens AD, Minassian DC, McCartney AC, Hungerford JL. Prognostic factors in primary malignant melanoma of the conjunctiva: a clinicopathological study of 256 cases. Br J Ophthalmol. 1994;78:252.
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14.
Vazquez A, Kamphorst JJ, Markert EK, Schug ZT, Tardito S, Gottlieb E. Cancer metabolism at a glance. J Cell Sci. 2016;129:3367–73.
Shields JA, Shields CL, Mashayekhi A, Marr BP, Renavides R, Thangappan A, et al. Primary acquired melanosis of the conjunctiva: risks for progression to melanoma in 311 eyes. The 2006 Lorenz E. Zimmerman Lecture. Ophthalmology. 2008;115:511–9.e2.
Funding
Support provided by the Eye Tumour Research Foundation, Philadelphia, PA (CLS, JAS). The funders had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review or approval of the manuscript. Carol Shields, M.D. has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. No conflicting relationship exists for any author.
Author information
Authors and Affiliations
Contributions
CLS was responsible for conception of the work. RRP, AY, LAD, SV, ALP, and CLS partook in the design of the work. RRP, AY, SV, ALP were responsible for data collection. RRP, AY, SV, ALP were responsible for data analysis and interpretation. RRP, AY, SV, ALP drafted the original article. LAD, SEL, JAS, and CLS were responsible for subsequent critical revisions of the article. RRP, AY, LAD, SV, ALP, SEL, JAS, and CLS all approved the final version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pacheco, R.R., Yaghy, A., Dalvin, L.A. et al. Conjunctival melanoma: outcomes based on tumour origin in 629 patients at a single ocular oncology centre. Eye 36, 603–611 (2022). https://doi.org/10.1038/s41433-021-01508-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01508-y
- Springer Nature Limited
This article is cited by
-
Factors affecting recurrence and metastasis in conjunctival melanoma
International Ophthalmology (2023)
-
IGF-1R is a molecular determinant for response to p53 reactivation therapy in conjunctival melanoma
Oncogene (2022)