Abstract
Purpose
This study was undertaken to investigate the neurovascular changes in the retina of prediabetic subjects.
Methods
Subjects enroled in a prospective study were separated into prediabetic and normal control groups based on their glycosylated haemoglobin (HbA1C) levels, fasting and postprandial blood sugar levels and glucose tolerance test. All the subjects underwent detailed ophthalmic evaluation, which included fundus examination, fundus photography, optical coherence tomography angiography (OCTA), and multifocal electroretinogram (mfERG). Comparisons were done between the groups using the Wilcoxon signed rank test.
Results
The median age was 48 years for the normal controls (n = 40), and 49.5 years for prediabetic subjects (n = 45) (p = 0.306). There was no difference in the vision, contrast sensitivity, thickness of the ganglion cell complex or the foveal avascular zone parameters between the groups. But the central foveal thickness and subfoveal choroidal thickness were significantly reduced in prediabetics (p < 0.01). The mfERG showed significant differences in the amplitude. The average amplitude was 35 ± 12 nv/deg2 in the normals and 29 ± 11 nv/deg2 in the prediabetics (p = 0.003). A weak positive correlation was noted between the mfERG and vascular parameters in the prediabetic group.
Conclusions
The prediabetic stage reveals earliest functional neuronal changes in the retina. The neuronal function seems to be affected much earlier than clinically appreciable structural changes in the ganglion cell complex and precedes vascular changes in the retina.
Similar content being viewed by others
Introduction
A paradigm shift is occurring in our understanding of the diabetic eye disease. Emerging evidence points to early neuronal damage in the retina even before the onset of clinically visible retinopathy changes [1]. These early changes are progressive and independent of the glycosylated haemoglobin level. A new term called retinal diabetic neuropathy or diabetic retinal neurodegeneration has been coined to denote these changes in the retina of diabetic patients [1, 2]. At the same time, a state of hyperglycaemia that has not yet reached the level of diabetes is increasingly being recognized as a stage of prediabetes. This stage is modifiable with lifestyle changes. It is postulated that the onset of diabetic changes occurs in this prediabetic stage itself. However, the literature is lacking in the information about the earliest changes seen in the retina in this stage. This study was undertaken to investigate and document the structural and functional changes seen in the retina in the prediabetic stage.
Methods
A prospective study was conducted between November 2017 and August 2018. The study was approved by the institutional review boards and adhered to the tenets of Declaration of Helsinki. After a written informed consent, the subjects between the ages 35 and 60 years were enroled in the study. A detailed medical and ocular history was taken for all subjects. All subjects underwent detailed ophthalmic evaluation including a dilated fundus examination by a retinal specialist (DR). They also underwent estimation of fasting blood sugar levels, postprandial blood sugar, glycosylated haemoglobin (HbA1C), glucose tolerance test, and routine urine examination. We also measured the blood pressure and the body mass index for all the subjects. The subjects were grouped into prediabetic group if they had HbA1C level between 5.7 and 6.4% or 39 and 46 mmol/mol (according to the National Glycohemoglobin Standardization Program certified protocol), fasting plasma glucose (FPG) levels between 5.5 and 6.9 mmol/l (100–125 mg/dl) indicating impaired fasting glucose. An oral glucose tolerance test (OGTT) was done. The prediabetic patients had levels between 7.8 and 11.0 mmol/l (140–199 mg/dl) at 2 h indicating impaired glucose tolerance. The control subjects had HbA1C level below 5.7% or 39 mmol/mol and FPG ≤ 5.5 mmol/l (100 mg/dl). Any of the subjects with HbA1C level ≥ 6.5% or 48 mmol/mol, FPG ≥ 6.9 mmol/l (125 mg/dl) and OGTT ≥ 11.1 mmol/l (200 mg/dl) at 2 h were diagnosed to have diabetes mellitus and were excluded from the study. Any subjects with history of dyslipidemia, chronic renal disorder, uncontrolled hypertension, ischaemic heart disease, inflammatory bowel disease, asthma, autoimmune disorders, hepatitis, history of chronic smoking were excluded from the study. Also subjects with neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease were excluded. Pregnant and lactating women were excluded. Subjects with ocular media opacities, refractive error more than ±6 dioptres, reduced vision, glaucoma, retinal pathology, and patients with any history of intravitreal injection, laser, or major ocular surgery in the past were excluded from the study.
Data acquisition with optical coherence tomography angiography
All subjects underwent optical coherence tomography angiography (OCTA) after dilation with tropicamide eye drops, on the Zeiss Angioplex 5000 machine (Carl Zeiss Meditec, Inc., Dublin, CA, USA). The machine uses wavelength of 840 nm and does 68,000 A scans per second. The scans are repeated several times consecutively in the same location and the comparison of contrast change over time is used to image vascular flow. Noninvasive, high quality images of 3 × 3, 6 × 6, 8 × 8 mm square cube can be generated using intensity based frequency filtering technique. Accuracy and sensitivity are improved by optical microangiography algorithm (OMAG) and tracking is enabled using FastTrac™ retinal tracking technology. Vessel density is defined as the total length of perfused vasculature per unit area in a region of measurement and perfusion density is defined as the total area of perfused vasculature per unit area in a region of measurement. Automated segmentation is done by the in-built software. The superficial vascular plexus is between the internal limiting membrane (ILM) and the inner plexiform layer (IPL). Perfusion indices and foveal avascular zone (FAZ) measurements for the superficial retinal vascular plexus are provided automatically for the 3 × 3 and 6 × 6 angio scans by the built-in software. All the measurements were taken by a single operator (DD). 3 × 3 and 6 × 6 mm square cube angio scans were taken centred on the fovea. The regions of the tissue were subdivided according to Early Treatment of Diabetic Retinopathy Study (ETDRS) subfields. Measurements provided in both tabular form and as density maps (ETDRS grid) through the angioplex metrics toolbox were used for analysis. Scans with poor signal strength (<5) and motion artefacts were excluded from analysis. Only the centre and inner rings in the ETDRS grid were included from the 6 × 6 scans to be compared with 3 × 3 cube. The ganglion cell complex (GCC) thickness was measured from the ILM to the posterior border of the IPL, using macular cube protocol in the 6 × 6 mm scans. The GCC thickness was measured in zones corresponding to the ETDRS zones and correlated with the mfERG implicit times and amplitudes in the corresponding zones.
Multifocal electroretinogram (mfERG)
After pupillary dilation, the mfERG was recorded using the visual evoked response imaging system (VERIS system) (Electro Diagnostic, San Mateo, CA, USA). A bipolar contact lens electrode was placed on the cornea after instilling topical anaesthetic eye drops and a ground electrode was fixed on the earlobe. Lubricating eye drops, such as hydroxypropyl methyl cellulose, were used as a coupling agent. The subjects were instructed to fixate at the red cross in the centre of the projected hexagons. The measurement was done in dim light with the fellow eye covered. The right eye was tested first. On average each eye took 4 min for the measurement and tests were repeated for reliability. All the test results included in the analysis were of good quality and sufficient reliability. The recording process involved stimulation of the retina with an array of 103 hexagonal flickering elements, displayed at a 60-Hz frame rate centred on the fovea covering a visual field of 30°. The luminance of each hexagon was independently alternated between black (2 candela/m2 of luminance) and white (200 candela/m2 of luminance) in a pseudorandom binary m-sequence. The fixation was monitored by the operator through an in-built camera with infra-red illumination and also by the fundus monitoring system of the projector. The responses were recorded from six rings in the macular area, the first ring corresponds to the fovea, the 2nd and 3rd rings correspond to the parafovea and the 4th, 5th, and 6th rings correspond to the perifoveal area. The mfERG report contains the implicit time and amplitude of the retinal locations in these rings. The recording and interpretation were done according to the International Society for Clinical Electrophysiology of Vision guidelines for basic mfERG [3].
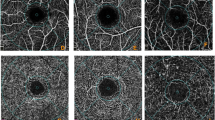
The mfERG reports were exported as PDF file from the machine. For comparison with the ETDRS zones in the OCTA, the mfERG hexagon was divided into similar five zones- central, superior, inferior, nasal, and temporal. Central zone included the central 7 points, nasal and temporal had 30 points while the superior and inferior had 18 points each. (Fig. 1)
The left image is a 3 × 3 mm scan and the central image is a 6 × 6 mm scan, both show overlay of the Early Treatment Diabetic Retinopathy Study (ETDRS) zones dividing the scan image into sectors. The right image shows the division of arrays of the multifocal electroretinography similar to the ETDRS zones. The lower half shows the comparative analysis of the implicit times (left graph) and amplitudes (right graph) of the multifocal electroretinography rings in control and prediabetic subjects.
Statistical analysis
Data were coded, entered, and analyzed with the statistical package SPSS version 20 (IBM Corp, Armonk, NY). All the data were tested for normality using the Kolmogorov Smirnov test. Since the data were not normally distributed, we summarized it using median and inter quartile range (IQR) for quantitative variables. Data from both eyes of subjects were used. Comparisons between groups were done using Wilcoxon signed rank test. A repeated measures analysis was done to correct for within- subject design. Correlations between quantitative variables such as the vascular parameters and mfERG implicit time and amplitudes were done using Spearman’s correlation coefficient. p value of <0.05 was considered as statistically significant.
Results
This study included 45 eyes of 23 prediabetic subjects. One eye of one patient was excluded on account of amblyopia causing poor vision. Forty eyes of 20 age matched healthy controls were also included. The male: female distribution was 12:11 in the prediabetic group and 10:10 in the control group. The age ranged from 39 to 63 years in the prediabetic group and 36 to 58 years in controls. The median age with IQR was 49.50 (7) years and 48(12) years in the prediabetic and control groups respectively (p = 0.08). There was significant difference in the HbA1C between the 2 groups (median values of 6.08% versus 5.45%, in prediabetic and control groups, p < 0.01). There was no difference in the vision, contrast sensitivity, thickness of the GCC or the FAZ parameters between the groups. However, the central foveal thickness was significantly less in the prediabetic group (p < 0.01). The subfoveal choroidal thickness was also significantly less in the prediabetic group (p < 0.01). The mfERG revealed significant difference in the average amplitude (p = 0.003) but the average implicit time did not show significant difference (p = 0.39). Table 1 lists the demographic details and the mfERG parameters. Figure 1 shows the comparative differences in the implicit times and amplitudes of mfERG in both the groups. Quadrant wise comparison showed significant differences in the mfERG amplitudes in all the quadrants with the nasal and inferior quadrants being highly significant (Table 2). The GCC and the retinal nerve fibre layer (RNFL) thickness, however, did not show significant difference in the two groups.
The correlation analysis did not show any significant correlation between implicit time and GCC thickness of the four quadrants in both the groups. A weak positive correlation between the mfERG amplitude and GCC thickness was noted in the control group. The values were, in the temporal quadrant (r = 0.343, p = 0.03), inferior quadrant (r = 0.316, p = 0.04), superior quadrant (r = 0.178, p = 0.27), and nasal quadrant (r = 0.357, p = 0.02). In the prediabetic group, there was weak positive correlation found between mfERG amplitude and GCC thickness, but it was significant for the inferior quadrant alone (r = 0.299, p = 0.03). The values for the other quadrants were temporal quadrant (r = 0.160, p = 0.27), nasal quadrant (r = 0.127, p = 0.383), and superior quadrant (r = 0.020, p = 0.890). Table 3 lists the vascular parameters of the superficial plexus in 3 × 3 mm and 6 × 6 mm scans. The vascular parameters did not vary between the two groups, in either scan size.
We attempted to study the correlation between the vascular and neuronal factors. Tables 4 and 5 show the correlation between vascular and neuronal factors in both the groups. In the normal control group, the FAZ parameters and vessel density parameters had weak negative but statistically significant correlation with implicit time of mfERG rings. In the prediabetic group, the FAZ parameters did not show significant correlation with the neuronal parameters in both 3 and 6 mm scans. But, in the 3 mm scan perfusion density was negatively correlated with implicit time of the 2nd ring (r value −0.328, p value 0.02). The vessel density also showed significant but weak positive correlation with mfERG implicit times and amplitudes.
Discussion
Prediabetes is a term given to a condition wherein the hyperglycaemia has not yet reached the levels seen in diabetes. The sedentary lifestyle and the lack of physical exercise coupled with unhealthy eating habits has seen a rise in the prevalence of prediabetes. The number of individuals identified as having prediabetes is expected to rise to 400 million worldwide by 2030 [4]. Hyperglycaemia triggers various metabolic processes that include protein and lipid glycosylation and the production of oxidative species. These products are thought to cause nerve damage. In experimental animals, after inducing diabetes, neuroretinal cell apoptosis can be seen as early as 1 month [5]. The retinal ganglion and amacrine cells are the first neurons to be affected by diabetes-induced apoptosis [6]. This was confirmed in a study by Bearse et al. that found abnormalities of multifocal oscillatory potentials due to early damage to amacrine cells in eyes of diabetic patients without any diabetic retinopathy (DR) [7]. The neurodegeneration leads to inner retinal thinning with reactive gliosis. Functionally, it manifests as reduced contrast sensitivity, loss of dark adaptation, abnormalities of colour vision, microperimetry, and changes in the ERG such as increased implicit times [5]. The neurodegenerative changes may progress earlier even when the vascular changes are seen to be stable. Lim et al. have noted progressive peripapillary nerve fibre loss in patients with DR even without concurrent progression of the vascular changes [6].
The mfERG is a highly sensitive and reliable method to evaluate retinal neuronal function. The baseline data collected from the European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR) participants showed alterations of the mfERG in almost 60% of patients with type 2 diabetes who did not have diabetic retinopathy (ETDRS level 20) [8]. Several other studies reported similar findings [9,10,11]. These findings suggest that functional impairment followed by structural changes due to neurodegeneration occur much earlier in the diabetic retina. In fact, neurodegeneration may play a role in the pathogenesis of DR. It has been reported that an increase of the implicit time in mfERG is a predictor for the development of visible vascular abnormalities over 1-year [9, 10] and 3-year periods [12].
In this study, the mfERG amplitude showed significant difference but not the implicit time. Similar findings were reported by the EUROCONDOR study wherein the mfERG P1 amplitude was seen to be more sensitive than the P1 implicit time [8]. The amplitudes were significantly lower in patients with moderate level of DR. In 58% of patients, mfERG abnormalities were present in the absence of visible retinopathy. However, Safi et al. found the implicit time to be more commonly affected than the amplitude owing to intersubject variability and also possibly due to the fact that more extensive damage is needed to reflect in the amplitude changes [5]. The highly significant mfERG changes in the nasal and inferior retina in this study, might possibly be due to more predilection of the nasal and inferior retina for ischaemic changes [5]. A decrease in amplitudes from centre to periphery seen in this study has been noted before by others as well. This corresponds with the reduction in the cone density away from the fovea [13, 14].
There is little information regarding the relationship between mfERG and SDOCT and the presence of early microvascular impairment. In the present study, the mfERG abnormalities were noted even in the absence of any changes in the thickness of the GCC. Although the mfERG amplitudes showed significant difference, no difference was found in the structural changes such as GCC and RNFL thickness indicating that functional changes occur much before the structural changes are visible. However, a weak positive correlation was noted between the GCC thickness and the mfERG amplitude in both controls as well as prediabetic subjects.
Since the mfERG is a highly sensitive test it can detect functional impairment much earlier before measurable structural degenerative changes in GCC or RNFL. However, the central foveal thickness was significantly reduced. This can be explained by the fact that the mfERG measures the cone and bipolar cell function which may not get reflected in the inner retina thickness changes but can definitely be seen at the fovea since it has the highest concentration of cones. Hence this discordance is not entirely surprising and has been noted by Santos et al. also [8]. Alves et al. reported thinning of the inner retinal layers, without overt vascular and inflammatory alterations in a prediabetic rat model [15]. And Sahin et al. have described thinning of the inner macular GCC layer in prediabetic subjects [16]. Early neuroretinal cell apoptosis within 1 month of inducing diabetes in experimental animals has been noted [5]. Lim et al. have demonstrated thinning of neuroretinal layers at the rate of 0.53 microns per year in diabetic patients without retinopathy [6].
The EUROCONDOR have postulated two different phenotypes of DR, one a primarily microvascular phenotype and the other primarily neurodegenerative [8]. They noted 61% of patients with no visible DR changes having mfERG and structural SDOCT abnormalities which they classified into neurodegenerative phenotype. And further 32% of patients with visible DR changes who did not have any abnormalities on mfERG or SDOCT as microvascular phenotype.
Ischaemic changes and decreased neurovascular flow have also been reported to be associated with areas of neuropathy [5]. We found that the vascular parameters such as the VD and the PD did not show significant differences but the subfoveal choroidal thickness was significantly reduced in the prediabetic group. This might be an indication of early vascular insufficiency which can potentially lead to secretion of vascular endothelial growth factor, eventually precipitating DR changes. The VD showed a weak positive but significant correlation with implicit time and amplitudes. No FAZ changes were observed in this study. Some researchers have found abnormalities of FAZ, decreased density of superficial and deep capillary plexuses and reduced blood flow in diabetic retina without retinopathy changes [17, 18] while others did not find any FAZ changes [19].
There are conflicting reports about whether the HbA1c levels affect the mfERG values. This study did not find any correlation between the HbA1c levels and mfERG parameters. A report by the EUROCONDOR project as well did not find any relationship between glycaemic control and mfERG, but patients with poor glycaemic control (HbA1c > 10%) were excluded from the study [8]. This relationship was explored in detail in an interesting study by Klemp et al., wherein the authors found that in fact acute euglycemia prolonged the mfERG more than acute hyperglycaemia. The delay in the implicit time was seen to be proportional to the patients’ habitual hyperglycaemia. They also observed that over a period of time adaptational response occurs and the implicit time gets normalized despite chronic hyperglycaemia [20].
Prediabetes is considered a lifestyle disease and most people with prediabetes also have signs of impaired metabolism which include insulin resistance, dyslipidemia, hypertension and obesity. These individuals are considered to be at relatively higher risk for the future development of diabetes and cardiovascular events [21]. However, prediabetes is modifiable by adopting changes in the lifestyle, exercise and proper diet. Progression to diabetes can thus be prevented or delayed. It would be interesting to see the effect of these modifying factors on the retinal neuronal and microvascular changes.
Conclusions
Currently established therapies for DR are effective for stabilizing the disease process. However, despite early intervention for visible clinical changes, patients may remain visually impaired. It is apparent from the results of this study that neurodegenerative changes occur much earlier right from the stage of prediabetes. Based on this it can be hypothesized that instituting early measures for neuroprotection might prevent vision loss in diabetic patients. These measures might possibly prevent the progression of vascular changes as well.
Summary
What was known before
-
Diabetic retinal neurodegeneration—a new term to denote the neuronal changes occurring in the retina in diabetes.
-
Hyperglycemeia triggers neuronal apoptosis—this can be seen within 1 month.
-
Progressive retinal nerve fibre layer loss—in diabetic retinopathy, this loss can progress even without concurrent progression of vascular changes.
What this study adds
-
Retinal changes at prediabetic stage—early neurovascular changes are seen very early in the retina at prediabetic stage.
-
Neuronal dysfunction precedes structural changes—early neuronal functional changes are seen in the absence of structural changes.
-
Vascular changes follow neurodegenerative changes—neuronal function is affected earlier than vascular changes.
Data availability
Most of the data are given in the paper. Data are freely available for perusal on request.
References
Sohn EH, van Dijk HW, Jiao C, Kok PH, Jeong W, Demirkaya N, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci USA. 2016;113:E2655–64. https://doi.org/10.1073/pnas.1522014113.
Sohn EH, Han IC, Abramoff MD. Diabetic retinal neurodegeneration-should we redefine retinopathy from diabetes? JAMA Ophthalmol. 2019. https://doi.org/10.1001/jamaophthalmol.2019.2536.
Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS.International Society For Clinical Electrophysiology of Vision et al. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc Ophthalmol.2012;124:1–13. https://doi.org/10.1007/s10633-011-9296-8.
Zimmet P, Shaw J, Alberti KG. Preventing type 2 diabetes and the dysmetabolic syndrome in the real world: a realistic view. Diabet Med. 2003;20:693–702.
Safi H, Safi S, Hafezi-Moghadam A, Ahmadieh H. Early detection of diabetic retinopathy. Surv Ophthalmol. 2018;63:601–8.
Lim HB, Shin YI, Lee MW, Park GS, Kim JY. Longitudinal changes in the peripapillary retinal nerve fiber layer thickness of patients with type 2 diabetes. JAMA Ophthalmol. 2019. https://doi.org/10.1001/jamaophthalmol.2019.2537.
Bearse MA Jr, Han Y, Schneck ME, Barez S, Jacobsen C, Adams AJ. Local multifocal oscillatory potential abnormalities in diabetes and early diabetic retinopathy. Investig Ophthalmol Vis Sci. 2004;45:3259–65.
Santos AR, Ribeiro L, Bandello F, Lattanzio R, Egan C, Frydkjaer-Olsen U.European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR) et al. Functional and structural findings of neurodegeneration in early stages of diabetic retinopathy: cross-sectional analyses of baseline data of the EUROCONDOR Project. Diabetes. 2017;66:2503–10. https://doi.org/10.2337/db16-1453.
Harrison WW, Bearse MA Jr, Ng JS, Jewell NP, Barez S, Burger D, et al. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Investig Ophthalmol Vis Sci. 2011;52:772–7. https://doi.org/10.1167/iovs.10-5931
Han Y, Schneck ME, Bearse MA Jr, Barez S, Jacobsen CH, Jewell NP, et al. Formulation and evaluation of a predictive model to identify the sites of future diabetic retinopathy. Investig Ophthalmol Vis Sci. 2004;45:4106–12.
Bearse MA Jr, Adams AJ, Han Y, Schneck ME, Ng J, Bronson-Castain K, et al. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 2006;25:425–48.
Ng JS, Bearse MA Jr, Schneck ME, Barez S, Adams AJ. Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Investig Ophthalmol Vis Sci. 2008;49:1622–8.
Nagatomo A, Nao-i N, Maruiwa F, Arai M, Sawada A. Multifocal electroretinograms in normal subjects. Jpn J Ophthalmol. 1998;42:129–35.
Azad R, Ghatak U, Sharma YR, Chandra P. Multifocal electroretinogram in normal emmetropic subjects: correlation with optical coherence tomography. Indian J Ophthalmol. 2012;60:49–52.
Alves MRP, Boia R, Campos EJ, Martins J, Nunes S, Madeira MH, et al. Subtle thinning of retinal layers without overt vascular and inflammatory alterations in a rat model of prediabetes. Mol Vis. 2018;24:353–66.
Şahin M, Şahin A, Kılınç F, Karaalp Ü, Yüksel H, Özkurt ZG, et al. Early detection of macular and peripapillary changes with spectralis optical coherence tomography in patients with prediabetes. Arch Physiol Biochem. 2018;124:75–79. https://doi.org/10.1080/13813455.2017.1361450.
de Carlo TE, Chin AT, Bonini Filho MA, Adhi M, Branchini L, Salz DA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina 2015;35:2364–70. https://doi.org/10.1097/IAE.0000000000000882
Dimitrova G, Chihara E, Takahashi H, Amano H, Okazaki K. Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Investig Ophthalmol Vis Sci. 2017;58:190–6. https://doi.org/10.1167/iovs.16-20531
Carnevali A, Sacconi R, Corbelli E, Tomasso L, Querques L, Zerbini G, et al. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017;54:695–702. https://doi.org/10.1007/s00592-017-0996-8
Klemp K, Sander B, Brockhoff PB, Vaag A, Lund-Andersen H, Larsen M. The multifocal ERG in diabetic patients without retinopathy during euglycemic clamping. Invest Ophthalmol Vis Sci. 2005;46:2620–6.
Zaleska-Żmijewska A, Piątkiewicz P, Śmigielska B, Sokołowska-Oracz A, Wawrzyniak ZM, Romaniuk D, et al. Retinal photoreceptors and microvascular changes in prediabetes measured with adaptive optics (rtx1™): a case-control study. J Diabetes Res. 2017; 4174292. https://doi.org/10.1155/2017/4174292.
Funding
This study was funded by a grant from the Novartis Healthcare Private Ltd. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception, design of the study and analysis, and interpretation of data. DR, KK, ST, UD, and AN—contributed significantly to the conception, design, analysis, interpretation of the data and review of the draft. DR, RN, DD, and NP—collected the data, coded it, analyzed and interpreted it. They also contributed to the writing of the draft and critically reviewing it.
Corresponding authors
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Medical Research Foundation, Sankara Nethralaya. Study No. 527-2016-P dated 27th May, 2016.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent Written informed consent was given by all the participants. Consent for publication of data was given by all the participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ratra, D., Nagarajan, R., Dalan, D. et al. Early structural and functional neurovascular changes in the retina in the prediabetic stage. Eye 35, 858–867 (2021). https://doi.org/10.1038/s41433-020-0984-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-0984-z
- Springer Nature Limited
This article is cited by
-
Contrast sensitivity function under three light conditions in patients with type 1 diabetes mellitus without retinopathy: a cross-sectional, case–control study
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Macular and peripapillary retinal nerve fiber layer thinning in eyes with prediabetes in the elderly population: OTASSHA study
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Advances in cell therapies using stem cells/progenitors as a novel approach for neurovascular repair of the diabetic retina
Stem Cell Research & Therapy (2022)
-
Early retinal functional alteration in relation to diabetes duration in patients with type 2 diabetes without diabetic retinopathy
Scientific Reports (2022)