Abstract
Purpose
To evaluate outcomes of Boston keratoprosthesis type 1 (K-Pro) surgery in a cohort of high-risk patients at Moorfields Eye Hospital. Our patients were referred to us at the end-point of their ocular disease.
Methods
A retrospective review of all K-Pro surgery performed between March 2011 and July 2015 with a minimum follow-up of 12 months.
Results
39 eyes of 38 patients were included. Mean follow-up was 28.4 months (range: 12–56). The main indication for surgery was bullous keratopathy from multiple failed grafts (56%). 26 cases (72.2%) had known posterior segment disease pre-operatively. Mean BCVA for the entire cohort (n = 39) initially improved from HM vision to 1/60 before returning to CF vision by 6 months and was maintained for the duration of follow-up. By final follow-up (n = 39), 46% had improved vision (1 line improvement in 10%; 2 lines or more in 36%) and 31% maintained pre-operative visual acuity. Anterior segment pathology was not an independent variable in visual outcome. However, absence of posterior segment disease was significant and performed best, improving from HM to 6/15 and maintaining that vision in the longer term. There were 13 (33%) cases of progressive glaucomatous optic neuropathy, 10 (26%) retinal detachments, 8 (21%) retroprosthetic membranes, 3 (8%) infective keratitis and 2 (5%) vitritis of which 1 progressed to endophthalmitis. In all, 3 (8%) had NPL vision and 4 (10%) required removal of the K-Pro.
Conclusions
Implantation of the Boston K-Pro can lead to improved vision, with the main limiting factor being posterior segment pathology.
Similar content being viewed by others
Introduction
When successful, corneal transplantation provides excellent visual rehabilitation, making it the treatment of choice in the surgical management of corneal blindness [1]. Its very success has made it the most common form of solid organ transplantation in the world [1, 2]. Nevertheless, there are still a cohort of patients in whom the outcomes are not as good as would be desired. These “high-risk” patients include those with corneal vascularization, a history of multiple corneal graft rejection and failure, herpes simplex keratitis and aniridia [3, 4]. Alternatives to corneal transplantation include the Boston keratoprosthesis (K-Pro); however, in the United Kingdom (UK), it is still considered an option of last resort and its use is reserved for very complex and advanced pathology.
This report is the first to present outcomes of K-Pro surgery in the UK. All our patients had been referred to us as the final opinion on the end point for their ocular disease. These outcomes reflect both the severity as well as the advanced nature of their underlying disease.
Methods
All patients who had K-Pro surgery (Boston keratoprosthesis type 1 with a separate PMMA or titanium locking ring) performed at Moorfields Eye Hospital, London, between March 2011 and July 2015, with a minimum 12-month follow-up, were included in our retrospective review. Hospital approval was obtained and the study adhered to the tenants of the Declaration of Helsinki. Keratoprosthesis surgery was performed as per manufacturer recommendations. If the patient had a crystalline lens at time of surgery, open sky extracapsular cataract extraction was performed followed by implantation of a zero power intraocular lens and an aphakic K-Pro. Aphakic and pseudophakic patients were left as is, and an appropriate aphakic or pseudophakic K-Pro inserted. Best corrected visual acuity (BCVA) prior to surgery was recorded as well as the underlying diagnosis, number of previous corneal grafts, previous glaucoma surgery and ocular comorbidities. BCVA at 6 weeks, 6 months and 1 year post operation, as well as the most recent follow-up were recorded. Snellen acuities were converted to logMAR. All ocular complications were noted. Intraocular pressure (IOP) was monitored via digital palpation and serial monitoring of the optic nerve head.
During the series our glaucoma surgical strategy changed. In the first 21 K-Pro cases, only patients with pre-existing glaucoma underwent aqueous shunt implantation (Baerveldt 350) prior to, or at the time of K-Pro surgery if IOP control was deemed to be inadequate. For all others, if IOP control was inadequate after K-Pro implantation, then escalating IOP-lowering treatment was instituted; initially medical treatment and if this failed, Baerveldt aqueous shunt implantation. In 2013, we began an integrated K-Pro clinic in conjunction with a dedicated glaucoma specialist. The glaucoma management strategy changed in that all patients in whom there was an established diagnosis of glaucoma, history of steroid response or of ocular hypertension underwent Baerveldt 350 implantation either before or at the time of K-Pro implantation if a functioning tube was not already in situ. Patients with no history of established glaucoma, ocular hypertension or steroid response had a Baerveldt 350 plate sutured to the sclera without the tube portion connected into the eye at the time of K-Pro implantation. The intention of this approach was to connect the tube as a secondary procedure if there was subsequent (de novo) IOP elevation that could not be controlled medically. Our decision to use Baerveldt 350 aqueous shunts reflects regional practice whereby Baerveldts are the predominant Glaucoma Drainage Device in use in the UK and Australia.
All patients were fitted with a large diameter bandage contact lens that was changed every 2 months. The initial post-op regimen was g. dexamethasone 0.1% x4–8 per day, g. moxifloxacin QID for 1–2 weeks and g. vancomycin 5% daily in addition to any pre-existing glaucoma medications. The vancomycin was continued for the lifetime of the K-Pro. The steroid dose was altered according to the patient’s clinical condition.
A distinction was made between patients who had pre-operative posterior segment disease (diagnosis of glaucoma or retinal diseases) and those who did not. The Mann–Whitney U-Test for non-parametric testing was used to assess the significance of the difference in mean BCVA between the groups at the 12 month and final reviews.
Results
A total of 43 eyes from 42 patients were identified in the initial analyses. Of these, four cases had incomplete notes (follow-up outside Moorfields Eye Hospital) and were excluded. The remaining 39 cases were included in the final analysis and Table 1 display patient demographics and ocular comorbidities. Of note, 62% of cases had a pre-existing diagnosis of glaucoma of which most (15 of 24 cases) had pre-existing tube surgery. 95% of cases had a history of failed corneal transplant surgery, with one case having 8 previous corneal grafts.
Table 2 displays the concomitant procedures performed at the time of K-Pro surgery. Glaucoma surgery accounted for over 20% of combined procedures with most being the pre-placement of the tube plate without connection of the tube in the eye. To date only one Baerveldt tube has needed to be connected into the eye as a secondary procedure.
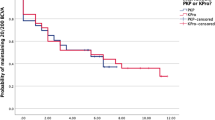
All patients had follow-up for a minimum of 12 months and all data was included in analysis. Within the first 12 months, in all cases vision improved after K-Pro surgery. For the entire cohort (Fig. 1a), mean BCVA improved from hand movements (HM) to 3/60 by 6 weeks post operation. However, this declined to 1/60 by 12 months, with the main reason being either progressive glaucoma or retinal detachments involving the macula. For those with known pre-existing posterior segment disease mean BCVA initially improved from HM to 1/60 at 6 weeks, before gradually falling to count fingers (CF) by 6 months. This vision was maintained to 12 months and beyond. By contrast, those without posterior segment disease achieved significantly better outcomes (p < 0.0001). Mean BCVA improved from HM to 6/15 at 6 weeks post operation and was maintained to 12 months and beyond unless de novo glaucoma (n = 2) or retinal detachment (n = 1) was encountered.
Visual outcomes from keratoprosthesis surgery. a For those with no pre-operative posterior segment pathology (n = 10), mean vision improves from hand movements vision to 6/15. This is maintained unless patients develop new incidence of glaucoma (n = 2) or retinal detachments (n = 1), in which case vision is reduced. In our cohort, these three new posterior segment pathologies decreased mean best corrected visual acuity to 6/60. b Mean best corrected visual acuity (BCVA) by primary diagnosis. All patients initially improved vision but there were no differences in final visual outcome by primary diagnosis
By final follow-up (mean 28.4 months), only 46% had an improvement in vision (1 line improvement in BCVA in 10%, 2 line or more improvement in 36%), 31% maintained their pre-operative BCVA and 23% had removal of their K-Pro or deteriorated in BCVA (Fig. 2). There was no significant difference in outcomes by underlying diagnosis (Fig. 1b).
Table 3 shows complications from K-Pro surgery. As suggested above, the commonest complication was glaucoma progression based on change in optic disc appearance, which occurred in 33% of cases. Retinal detachments were also a major issue occurring in 26% of cases. 20% developed a retroprosthetic membrane with most being amenable to Nd;YAG membranectomy. There were three (8%) cases of microbial keratitis in two patients, all resulting in the planned removal of the K-Pro. The main risk factor for keratitis was a dry ocular surface. There were two (5%) cases of vitritis; one due to progression of a fungal keratitis and the other due to erosion of the conjunctiva over a glaucoma tube. In both cases the final visual outcome was poor. Supplementary Figure 1 shows the Kaplan–Meier survival curve where removal of the K-Pro was considered failure. After 14 months duration there were no further cases of K-Pro removal for the entire follow-up period.
Table 3 also displays the number of post-operative procedures performed after K-Pro surgery. Surprisingly, the commonest procedure was vitrectomy (36%), which was needed for patients who developed retinal detachments, vitreous haemorrhages and very high intraocular pressures secondary to aqueous misdirection. Once again, glaucoma was a key challenge after K-Pro surgery and 31% required either revision or insertion of a glaucoma drainage device.
Discussion
Patients in our cohort typically had maximal medical and surgical management, up to the point that K-Pro surgery was the only available option to improve vision. Further, vision in the fellow eye was generally poor; the mean BCVA was 1/60 and 15 (39%) patients had no perception of light (NPL). The poorer outcomes seen in our cohort reflects the severity of the underlying diagnosis. K-Pro surgery for many represents a temporary improvement in vision before the natural history of the disease causes vision deterioration. This was clearly understood by our patients and it was appreciated that any potential improvement in vision, even if temporary, was considered a worthwhile outcome.
Best outcomes were seen in patients who had no posterior segment disease (i.e., glaucoma, retinal disease or detachments), where vision was maintained at 6/15, in line with the international literature [5,6,7,8,9]. The anterior segment pathology was not an independent variable; however, complex anterior segment disease often had concomitant glaucoma, which affected final outcomes. Thus our best cases were seen in those who only had multiple failed grafts and no other disease.
Perhaps most importantly, it is critical to recognise the types of patients who do not perform well with K-Pro surgery. Three (8%) cases (from two patients) developed microbial keratitis leading to removal of the K-Pro. Both patients had poor ocular surfaces with dry eyes (one herpes simplex keratitis and one Stevens-Johnson syndrome) where there was either a persistent epithelial defect or the bandage contact lens fell out immediately prior to the keratitis. Herpetic eye disease and persistent epithelial defects are suggested risk factors for keratitis in K-Pros and our experience is in keeping with this [10,11,12].
Three (8%) patients developed spontaneous de novo retinal detachments. All had significant proliferative vitreoretinopathy [13]. Retinal detachments in the context of K-Pros are mostly either tractional or rhegmatogenous in origin and tend to have poorer outcomes [14,15,16]. Often patients do not notice any symptoms until the detachment is extensive and severe, despite close follow-up. One approach to decrease complications is to perform a vitrectomy at the time of initial K-Pro surgery; however, studies suggest that this does not actually reduce the risk of retinal detachments [17]. We suggest that all patients have an in office B-scan ultrasound looking for early retinal detachments at each follow-up visit.
Glaucoma is the perennial bane of K-Pro surgery and this was no exception in our cohort. Three (8%) patients had NPL vision as a result of glaucoma, all through varied and unexpected mechanisms. In one there was unrealised advanced glaucoma prior to K-Pro surgery that progressed despite a reasonable IOP. A second patient had intractable chronic high IOP (~40 mm Hg) in the post-operative period. Finally, a third patient developed aqueous misdirection with an extremely high IOP that resulted in a central retinal artery occlusion. Our experience has taught us to anticipate high IOPs in all patients and to plan for this. We now pre-place Baerveldt tube plates in all eyes that do not have glaucoma/ocular hypertension or a history of steroid response. If the IOP were to rise, we can insert the tube in the eye as a secondary procedure to control the IOP while minimising the risk of hypotony as the bleb over the tube plate would have had time to mature. Thus far, only one eye has needed this procedure. Eyes with pre-existing glaucoma, ocular hypertension or known steroid response undergo full Baerveldt 350 implantation either prior to or at the time of K-Pro implantation. We chose the Baerveldt tube over the Ahmed valve tube as studies demonstrate that it achieves greater IOP reduction in the long term [18, 19]. To ameliorate the hypotensive phase described in Baerveldt tube surgery, a supramid is inserted into the tube and a 10.0 nylon suture used to ligate the tube at point of surgery. In the event of high IOPs in the initial post-operative period, the intraluminal length of the supramid can be adjusted or the 10.0 nylon can be suture lysed with an Argon laser to allow flow through the tube.
Based on our experience, we would suggest that a Boston keratoprosthesis type 1 implant is suitable for patients with any type of anterior segment pathology, as long as there is no posterior segment disease and the eye produces adequate tears (minimum Schirmer 1 of 5 mm). However, most patients who are referred to us do not fit this guideline and so must be considered on a case-by-case basis. Once stable post-operatively, we would suggest a regular follow-up interval of two months where the large diameter bandage contact lens can be changed, a B-scan performed assessing the retina, and digital assessment of IOP coupled with optic disc examination for glaucoma development and progression.
Summary
What was known before
-
Keratoprosthesis surgery is an option for high-risk corneal grafts, which in the UK is generally reserved for very complex pathology.
-
There have been no previous keratoprosthesis outcomes data published from the United Kingdom.
What this study adds
-
Visual outcome was dependent on posterior segment disease (glaucoma, previous retinal detachment) rather than anterior segment disease (underlying pathology that necessitated keratoprosthesis surgery).
-
Those without posterior segment disease attained a mean visual acuity of 6/15, which was maintained for the duration of follow-up.
-
In contrast, those with posterior segment disease initially improved, but mean visual acuity declined to Count Fingers over a mean of 28 months. In general, approximately a third improved vision, a third maintained vision and a third deteriorated in vision at final follow-up.
-
Glaucoma progression and retinal detachment were key challenges in our cohort.
References
Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379(9827):1749–61.
Yu T, Rajendran V, Griffith M, Forrester JV, Kuffova L. High-risk corneal allografts: a therapeutic challenge. World J Transplant. 2016;6(1):10–27.
Coster DJ, Williams KA. Management of high-risk corneal grafts. Eye. 2003;17(8):996–1002.
Chew HF, Ayres BD, Hammersmith KM, et al. Boston keratoprosthesis outcomes and complications. Cornea. 2009;28(9):989–96.
Zerbe BL, Belin MW, Ciolino JB, Boston Type 1 Keratoprosthesis Study Group. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology. 2006;113(10):1779 e1771–1777.
Greiner MA, Li JY, Mannis MJ. Longer-term vision outcomes and complications with the Boston type 1 keratoprosthesis at the University of California, Davis. Ophthalmology. 2011;118(8):1543–50.
Aldave AJ, Sangwan VS, Basu S, et al. International results with the Boston type I keratoprosthesis. Ophthalmology. 2012;119(8):1530–8.
Ahmad S, Mathews PM, Lindsley K, et al. Boston type 1 keratoprosthesis versus repeat donor keratoplasty for corneal graft failure: a systematic review and meta-analysis. Ophthalmology. 2016;123(1):165–77.
Al Arfaj K. Boston keratoprosthesis - clinical outcomes with wider geographic use and expanding indications - a systematic review. Saudi J Ophthalmol: Off J Saudi Ophthalmol Soc. 2015;29(3):212–21.
Chan CC, Holland EJ. Infectious keratitis after Boston type 1 keratoprosthesis implantation. Cornea. 2012;31(10):1128–34.
Chan CC, LoVerde L, Qiang J, Nordlund ML, Holland EJ. Incidence, risk factors, and surgical management of Boston type 1 keratoprothesis corneal melts, leaks, and extrusions. Cornea. 2016;35(8):1049–56.
Kim MJ, Yu F, Aldave AJ. Microbial keratitis after Boston type I keratoprosthesis implantation: incidence, organisms, risk factors, and outcomes. Ophthalmology. 2013;120(11):2209–16.
Petrou P, Banerjee PJ, Wilkins MR, et al. Characteristics and vitreoretinal management of retinal detachment in eyes with Boston keratoprosthesis. Br J Ophthalmol. 2017;101(5):629–33.
Modjtahedi BS, Eliott D. Vitreoretinal complications of the Boston Keratoprosthesis. Semin Ophthalmol. 2014;29(5-6):338–48.
Klufas MA, Yannuzzi NA, D’Amico DJ, Kiss S. Vitreoretinal aspects of permanent keratoprosthesis. Surv Ophthalmol. 2015;60(3):216–28.
Ray S, Khan BF, Dohlman CH, D’Amico DJ. Management of vitreoretinal complications in eyes with permanent keratoprosthesis. Arch Ophthalmol. 2002;120(5):559–66.
Perez VL, Leung EH, Berrocal AM, et al. Impact of total pars plana vitrectomy on postoperative complications in aphakic, snap-on, type 1 Boston keratoprosthesis. Ophthalmology 2017;124:1504–9.
Christakis PG, Kalenak JW, Tsai JC, et al. The ahmed versus baerveldt study: five-year treatment outcomes. Ophthalmology. 2016;123(10):2093–102.
Budenz DL, Barton K, Gedde SJ, et al. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015;122(2):308–16.
Acknowledgements
NS and MW acknowledge a proportion of their financial support from the UK Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Samarawickrama, C., Strouthidis, N. & Wilkins, M.R. Boston keratoprosthesis type 1: outcomes of the first 38 cases performed at Moorfields Eye Hospital. Eye 32, 1087–1092 (2018). https://doi.org/10.1038/s41433-018-0016-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-018-0016-4
- Springer Nature Limited
This article is cited by
-
Wide-field vitreoretinal surgery in eyes with Boston type 1 keratoprosthesis
International Ophthalmology (2022)
-
Versorgung von vaskularisierten Hochrisikoaugen mittels Boston-Keratoprothese
Der Ophthalmologe (2021)