Abstract
Objectives
In population studies, iodine intake estimation relies on median urinary iodine concentration (UIC). However, interpreting UIC measurements can be challenging.
Methods
In our study, we included 772 adult participants from three groups: nationally representative gender-mixed, women of reproductive age, and pregnant women. We measured UIC and urinary creatinine (U-Cr) to calculate the iodine-to-creatinine ratio (I/Cr). U-Cr cut-off value of 0.226 g/L was used for differentiation between diluted and undiluted urine samples. After excluding samples below this cut-off, new median UIC and I/Cr ratios were calculated. We additionally evaluated the influence of urine sample collection time on UIC.
Results
Median UICs were 91.8 µg/L for nationally representative group, 58.3 µg/L for women of reproductive age, and 74.9 µg/L for pregnant women, while I/Cr ratios were 91.7, 102.0, and 159.2 µg/g, respectively. After implementing U-Cr cut-off and excluding all data where U-Cr was below cut-off, new median values were 93.4, 76.3, and 95.4 µg/L for UICs, and 88.6, 88.8, and 128.7 µg/g for I/Cr ratios, respectively. In women of reproductive age, median UIC was significantly lower in urine samples collected after 9:30 and after 12:00 as compared to samples collected before 9.30 (53.4, 57.8, and 97.3 μg/L, respectively).
Conclusions
UIC results should be interpreted with caution, considering urine dilution and sample collection timing. U-Cr measurement should be included in population-based iodine intake studies, with corrections applied especially for pregnant women and younger adults, for whom morning is best for single-spot samples.
Similar content being viewed by others
Introduction
Iodine is an essential component of thyroid hormones, which regulate a variety of important physiological processes. Dietary iodine intake influences the prevalence of thyroid disorders. Excessive iodine intake is associated with an increased prevalence of autoimmune thyroid disorders, especially in previously iodine-deficient regions [1, 2]. Conversely, insufficient iodine intake increases the risk of iodine-deficiency disorders like goiter and thyroid autonomy, which are common worldwide health problems. To address this, the World Health Organization (WHO) recommends universal salt iodization, the easiest and cheapest way to ensure adequate iodine supply [1].
According to the WHO, the recommended daily iodine intake is 150 µg in adults and 250 µg in pregnant and lactating women [1]. The most important method for assessing and monitoring iodine supply in a population is a measurement of urinary iodine concentration (UIC). This is because, in healthy iodine-sufficient subjects, the majority (~90%) of dietary iodine is renally excreted, while the rest is absorbed by the thyroid [3]. According to WHO guidelines, UIC below 20 µg/L reflects severe iodine deficiency, UIC 20–49 µg/L moderate iodine deficiency, and UIC 50–99 µg/L mild iodine deficiency [1].
UIC can be measured through a 24-h urine collection or a single-spot urine sample [4]. There are advantages and drawbacks of different sampling methods. A 24-h urine collection is the most accurate method and suitable for individual measurements but is time-consuming and carries a risk of incomplete sampling [5]. Conversely, single-spot urine sampling is easier but may result in diurnal UIC variations and is not directly comparable to 24-h urinary iodine excretion [6]. Recommendations for single-spot urine sampling do not specify the time of the sample collection [1].
An alternative method to estimate iodine intake is using urinary iodine-to-creatinine (I/Cr) ratio. Creatinine serves as a marker of hydration and potential urine dilution [7]. The European Guidelines for Workplace Drug Testing in Urine recommend checking the sample’s validity for possible dilution using creatinine either before or during the screening process, as urine dilution can lead to false negative results in drug testing [8]. While the I/Cr ratio helps to adjust variations in urinary volume, it can be affected by changes in creatinine excretion due to sex, age, diet, and kidney function. Therefore, some authors propose calculating the I/Cr ratio from 24-h urinary iodine with age and sex adjustment [9,10,11].
The aim of our work was to evaluate the results of UIC measurements taking into account urine dilution and the time of single-spot urine sample collection, and to highlight the importance of a precise protocol when using single-spot urine samples on a population level.
Materials and methods
Study design and data collection
The Nutrihealth study was conducted as an upgrade of the Slovenian National Dietary Survey SI.Menu 2017/2018, which was carried out following the European Food Safety Authority (EFSA) Guidance on EU Menu Methodology [12,13,14]. The study included a nationally representative (age/sex/region) sample of adults (18–74 years). The recruitment period was between March 2017 and February 2018. A subsample of adult participants that completed SI.Menu study was invited to participate in the Nutrihealth study. Altogether, 279/280 provided their urine samples. The collection of urine samples was carried out in local healthcare centers between June 2017 and September 2018.
EUthyroid (EU) study was conducted at the Division of Nuclear Medicine, University Medical Centre Ljubljana, between October 2017 and July 2018. Healthy female volunteers were recruited from different parts of the country. Two groups of volunteers, women of reproductive age (WR) and pregnant women (PW) participated in the study. We included females aged 18–49 years, with no known thyroid disease and not taking thyroid medication. The sample group was not representative of an adult national population. Overall, 308 WR and 184 PW voluntarily participated in the study with 308/308 WR and 183/184 PW providing urine samples for further analysis.
Both Nutrihealth and EU studies received approval from the Slovenian National Medical Ethics Committee, and informed consent was obtained from all participants.
All urine samples were collected between 8:00 and 15:00 into a urine container (120 mL, non-sterile, with screw cap, Golias, Slovenia) and then stored in a temperature-controlled freezer at −80° C until the analysis.
To confirm normal thyroid function, venous blood for thyroid stimulating hormone (TSH) determination was collected into serum collective tubes (BD Vacutainer® blood collection tubes) by trained personnel. Blood was coagulating for 30 min and was centrifuged afterward for 10 min at 3000 rpm.
Methods
The researchers analyzing the urine and venous blood samples were unaware of the experimental conditions.
UIC was measured with the spectrophotometric method on a microplate based on the Sandell–Kolthoff (S–K) reaction with ammonium persulfate digestion pretreatment of samples prior to analysis. The analysis is comparable to the gold standard method for iodine determination—inductively coupled plasma mass spectrometry. S–K reaction uses catalytic activity of iodide ion in the reaction of arsenic acid and ceric ammonium sulfate in sulfuric acid solution. Iodide’s presence increases the reaction rate. The reaction rate is directly proportional to iodide ion concentration in a solution [15]. All our reagents were prepared inhouse. The limit of detection is 6.9 µg/L, linearity of the assay is in the range from 6.9 to 400 µg/L. The intra-assay and inter-assay coefficients of variation ranged from 2.3% to 2.5% and from 4.9% to 6.3%, respectively [16].
Urinary creatinine (U-Cr) was measured using the automated system Dimension® clinical chemistry system (Siemens Healthineers). It was determined spectrophotometrically using a modified kinetic Jaffe reaction. Creatinine in urine sample reacts with picric acid in an alkaline solution to form a red chromophore. Measurement is performed in bichromatic rate technique at two different wavelengths, 510 and 600 nm. Measured absorbance at 510 nm is proportional to creatinine concentration in the urine sample. Bilirubin is oxidized and eliminated with potassium ferricyanide. Absorbance at 600 nm is used to eliminate interferences in the sample. The limit of detection is 0.13 g/L, linearity of the assay is in the range from 0.13 to 4.0 g/L. The intra-assay and inter-assay coefficients of variation ranged from 1.0% to 1.1%, and 2.3% to 2.4%, respectively.
The cut-off value for U-Cr was taken from the European Guidelines for Workplace Drug Testing in Urine, where U-Cr cut-off value of 0.226 g/L differentiates between the diluted and undiluted sample [8].
TSH was measured as TSH3-Ultra assay on Advia Centaur XP Immunoassay System (Siemens Healthineers). Reference interval of TSH for the adult population is laboratory-own and was determined by CLSI guidelines [17]. The normal reference TSH range is from 0.59 to 4.23 mIU/L.
Statistical methods
Descriptive statistics was used to calculate group characteristics. The Kolmogorov–Smirnov test was employed to assess the normality of data distribution. None of the data was normally distributed; therefore, all data are presented as medians with a 95% confidence interval (CI), except for age, which is presented as the mean and associated range. Inferential statistics was performed using the Mann–Whitney test and Chi-squared test. Statistical analysis was conducted using MedCalc Statistical Software version 20.014 (MedCalc Software bvba, Ostend, Belgium). Statistical significance for all tests was set as P < 0.05.
Results
A total of 772 participants were included in our study and classified into three groups: (1) Nutrihealth, (2) EU WR, and (3) EU PW. The characteristics of the study population are outlined in Table 1. Overall, two participants failed to provide a urine sample and 31 samples were removed from evaluation since their results were either below or above the detection limit. Participants in the Nutrihealth group were significantly older than EU WR and EU PW participants (P < 0.001 and P < 0.001, respectively) and those in EU WR group were significantly younger than EU PW participants (P < 0.001).
The median UIC value and I/Cr ratio were calculated for each group and compared between groups (Table 2). In Nutrihealth group, the UIC was significantly higher compared to EU WR and EU PW groups (P < 0.001 and P = 0.007, respectively) and significantly lower in EU WR than in EU PW group (P = 0.002). On the contrary, I/Cr ratio was significantly lower in Nutrihealth than in EU WR and EU PW groups (P = 0.003 and P < 0.001, respectively), but significantly lower in EU WR than in EU PW group (P < 0.001). There were no significant differences between UIC value and I/Cr ratio in the Nutrihealth study, whereas in EU WR and EU PW significant differences were observed (P < 0.001 and P < 0.001, respectively).
To exclude too diluted urine samples, a U-Cr cut-off value of 0.226 g/L [9] was implemented and a second evaluation was performed. This yielded 14 invalid samples (5%) in the Nutrihealth group, 63 (22%) in EU WR, and 47 samples (26%) in EU PW group (Table 2). Following the removal of those samples and re-calculation of the median UIC values and I/Cr ratios, the UIC in the Nutrihealth group was significantly higher only compared to EU WR (P = 0.007), but not compared to EU PW group (P = 0.976). The UIC in EU WR group remained significantly lower than in EU PW group (P = 0.009). The I/Cr ratio did not differ between Nutrihealth and EU WR groups (P = 0.437), but was significantly lower in both groups compared to EU PW group (P < 0.001 and P < 0.001, respectively). No statistically significant differences between UIC value and I/Cr ratio were observed in the Nutrihealth study, whereas significant change persisted in the EU study for both groups (Table 2).
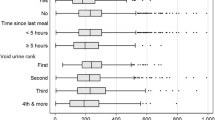
In addition, we evaluated the impact of the median time of urine sample collection on UIC. The groups were further divided into subgroups according to the time of urine sample collection: (1) before 9:30, (2) 9:30–12:00, and (3) after 12:00. These timings were selected based on the results of the study on variability of U-Cr in healthy individuals [18]. The differences in median times of urine collection for each group were as follows: for the Nutrihealth study 10:34 (25th–75th percentile: 9:25–11:19), for EU WR group 11:23 (25th–75th percentile: 10:06–13:04), and for EU PW group 11:13 (25th–75th percentile: 10:15–12:14), and were statistically significant (P < 0.001).
In Table 3, we present the proportions of urine samples obtained at different time intervals. In the Nutrihealth group, a higher proportion of samples was taken at earlier time intervals than in EU WR and EU PW groups (P < 0.001 and P < 0.001, respectively). A larger proportion of samples from EU WR group was obtained earlier than in EU PW group (P = 0.036).
Further comparison of median UIC, U-Cr, and I/Cr in subgroups with different times of sample collection is presented in Table 4. We did not observe statistical differences in median UIC, U-Cr, and I/Cr ratio in the three sampling times in Nutrihealth and EU PW groups. However, statistically significant differences were observed in median UIC, U-Cr, and I/Cr ratio between the three sampling times in EU WR group (Table 4).
Within the gender-mixed Nutrihealth group, we additionally assessed the influence of gender on UIC, but did not confirm a significant difference between females and males (95.9 and 90.4 µg/L, respectively, P = 0.272).
Discussion
Our results revealed a significant influence of urine dilution on UIC due to physiological (age, drinking habits, and pregnancy) differences among individuals and sample collection timing.
UIC and I/Cr ratio are common urinary biomarkers for assessing population-level iodine supply, each with its advantages and disadvantages. UIC is the most widely used biomarker. When measured from single-spot urine, this simple test provides a good estimate of recent iodine intake. However, it can be significantly affected by factors influencing urine dilution such as sample collection time, hydration, diuresis, fasting, exercise, bladder retention time, and pregnancy, characterized by physiological polyuria [18, 19]. Therefore, I/Cr ratio has been used as another urinary iodine biomarker. In our study, we established significantly higher values of I/Cr than UIC in non-pregnant (EU WR) and PW (EU PW) and similar values of I/Cr and UIC in the gender-mixed individuals (Nutrihealth) who were older than EU WR and EU PW. These differences likely result from distinct physiological characteristics of the three groups (age, drinking habits, and pregnancy status).
Regarding sample collection timing, we assessed urine dilution before analysis using U-Cr as a key dilution marker [7, 8]. U-Cr varies with age, sex, diet, and kidney function, leading to population-wide result differences [18]. Our study revealed higher U-Cr values in morning urine samples and lower values in mid-day samples. Indeed, the first-morning urine sample is typically more concentrated than any other urine sample [20, 21]. EU WR and EU PW groups had more late-day samples, resulting in a larger portion of overly dilute samples with U-Cr values below 0.226 g/L (26% and 22%, respectively).
Pregnancy status can influence UIC. Surprisingly, PW included in our study had higher median UIC than non-pregnant WR, despite increased iodine utilization in pregnancy [19]. Adjusting median UIC for PW is therefore recommended. Using the I/Cr ratio [11], the first measured median UIC for PW was 74.9 µg/L, and the median I/Cr ratio was 159.0 µg/g of creatinine. After excluding 26% of urine samples that were below the U-Cr cut-off, the median UIC significantly increased to 95.4 µg/L and, the I/Cr ratio decreased to 128.4 µg/g of creatinine. Similar trends occurred in non-PW included in our study, with 22% of urine samples below the U-Cr cut-off.
In our study, urine sample collection time had varying effects on results depending on pregnancy status. A high percentage of PW and non-pregnant WR collected samples after 9:30 (96% and 89%, respectively). Daytime urine dilution significantly impacted UIC in the EU WR group, indicating that fluid intake can affect UIC measurements. In PW, UIC and U-Cr did not significantly differ during the day. However, caution is needed in interpreting these results due to the limited number (4%) of EU PW participants who provided morning samples. A study with more morning samples in WR or younger adults could be valuable. PW, with their physiological changes affecting UIC, should be excluded from population-based iodine supply evaluations, as also noted in a recent EU study [22].
With respect to gender, we expected to observe similar UIC values across the gender-mixed Nutrihealth group and EU WR group. Surprisingly, WR had significantly lower UIC than participants in the gender-mixed group. Therefore, we further assessed the influence of gender in the Nutrihealth group, but we did not confirm a significant difference in UIC between females and males.
In contrast to WR, the I/Cr ratio in the gender-mixed Nutrihealth group was comparable to UIC. Excluding 5% of samples below the U-Cr cut-off did not impact UIC median values and I/Cr ratio. Likely, Nutrihealth participants had less diluted urine, more collected samples before noon, and were significantly older than WR, who tend to consume more fluids. The National Health and Nutrition Examination Survey from 2009 to 2012, which included 20,293 participants, showed a significantly lower fluid intake in participants aged 60 and over than of the younger age groups [23]. Aging, on the other hand, is characterized by increased urinary dilution and decreased U-Cr levels as a result of muscle mass reduction and reduced kidney function, whereas U-Cr levels remain relatively stable throughout the day. The latter was confirmed in our Nutrihealth group. However, the time of urine collection did yield statistically significant changes in U-Cr in the EU WR group with the lowest U-Cr found in samples obtained after 12:00. This is also addressed in Sallsten and Barregard’s study on variability of U-Cr in healthy individuals with an average age of 34 years in whom significant changes appeared in I/Cr ratios during the day [18]. Overall, our results showed that correction using the I/Cr ratio appears to be less important in older than in younger subjects.
When attempting to align with WHO recommendations in interpreting our UIC measurements, an issue arose. If only the results of the Nutrihealth group were considered, our population would be very close to iodine sufficiency. When the results of the EU WR and EU PW groups were considered, our population would be mildly iodine deficient. Our findings suggest that both the timing of urine sampling and U-Cr determination are very important for population-based studies including younger females. Using UIC alone to define iodine status in a population may be misleading and inaccurate.
The limitation of our study is that we did not obtain any information from the participants about the possible intake of nutritional supplements or medications. Certain substances like iodine, KBr, CuSO4, MgSO4, or nitrites [24, 25] directly interfere with the S–K reaction in UIC analysis, thereby affecting the measurement results. In our research, such results were not excluded from the analysis.
Our study showed that the results of UIC measurements should be interpreted with caution taking into consideration urine dilution as well as the time of urine sample collection. Although it has been shown that ~500 participants should be included to obtain reliable results in iodine intake studies [26], our results show that urine dilution is also a very important factor influencing the median UIC result. Therefore, in addition to a sufficiently large study, the measurement of U-Cr should be included in the research to differentiate between dilute and undilute urine samples. Overly dilute samples should be excluded from analysis in population-based iodine intake studies. Correction with U-Cr should also be applied in studies that include PW who have physiologically permanently diluted urine. Additionally, the time of urine sampling should be considered in younger adults. The most suitable time for single-spot urine sampling in such individuals seems to be before 9:30. Taking our findings into account, we believe that a precise protocol for urine sampling is needed before starting population-based iodine intake studies.
Data availability
The anonymized data analyzed in this study can be obtained from the corresponding author upon reasonable request.
References
WHO. Assessment of iodine deficiency disorders and monitoring their elimination. World Health Organization. 2007. https://www.who.int/publications/i/item/9789241595827.
Zaletel K, Gaberšček S, Pirnat E, Krhin B, Hojker S. Ten-year follow-up of thyroid epidemiology in Slovenia after increase in salt iodization. Croat Med J. 2011;52:615–21.
Zimmerman MB. Iodine deficiency. Endocr Rev. 2009;30:376–408.
Vejbjerg P, Knudsen N, Perrild H, Laurberg P, Andersen S, Rasmussen LB, et al. Estimation of iodine intake from various urinary iodine measurements in population-based studies. Thyroid 2009;19:1281–6.
Bottini PV, Garlipp CR, Lima PRM, Brito IT, Carvalho LMG. Are patients adequately informed about procedures for 24-h urine collection. Clin Chem Lab Med. 2020;58:32–5.
Perrine CG, Cogswell ME, Swanson CA, Sullivan KM, Chen TC, Carriquiry AL, et al. Comparison of population iodine estimates from 24-hour urine and timed-spot urine samples. Thyroid 2014;24:748–57.
Arndt T. Urine-creatinine concentration as a marker of urine dilution: reflection using a cohort of 45,000 samples. Forensic Sci Int. 2009;186:48–51.
Taskinen S, Beck O, Bosch T, Brcak M, Carmichael D, Fucci N, et al. European guidelines for workplace drug testing in urine. Drug Test Anal. 2017;9:853–65.
Konno N, Yuri K, Miura K, Kumagai M, Murakami S. Clinical evaluation of the iodide/creatinine ratio of casual urine samples as an index of daily iodide excretion in a population study. Endocr J. 1993;40:163–9.
Rasmussen LB, Ovesen L, Christiansen E. Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr. 1999;53:401–7.
Knudsen N, Christiansen E, Brandt-Christensen M, Nygaard B, Perrild H. Age- and sex-adjusted iodine/creatinine ratio. A new standard in epidemiological surveys? Evaluation of three estimates of iodine excretion based on causal urine samples and comparison to 24 h values. Eur J Clin Nutr. 2000;54:361–3.
European Food Safety Authority (EFSA). Guidance on the EU Menu methodology. EFSA J 2014;12:3944.
Gregorič M, Blaznik U, Delfar N, Zaletel M, Lavtar D, Seljak BK, et al. Slovenian national food consumption survey in adolescents, adults and elderly. EFSA Support Publ 2019;16:1–28.
Gregorič M, Hristov H, Blaznik U, Koroušić-Seljak B, Delfar N, Pravst I. Dietary intakes of Slovenian adults and elderly: design and results of the National Dietary Study SI.Menu 2017/18. Nutrients. 2022;14:3618.
Machado A, Lima L, Mesquita RBR, Bordalo AA. Improvement of the Sandell-Kolthoff reaction method (ammonium persulfate digestion) for the determination of iodine in urine samples. Clin Chem Lab Med. 2017;55:e206–8.
Oblak A, Arohonka P, Erlund I, Kuzmanovska S, Zaletel K, Gaberšček S. Validation of a spectrophotometric method for urinary iodine determination on microplate based on Sandell–Kolthoff reaction. Lab Med. 2022;53:376–80.
Oblak A, Biček A, Pirnat E, Zaletel K, Gaberšček S. First estimation of reference intervals for thyroid-stimulating hormone and thyroid hormones in Slovenian population. Acta Chim Slov. 2021;68:488–93.
Sallsten G, Barregard L. Variability of urinary creatinine in healthy individuals. Int J Environ Res Public Health. 2021;18:3166–77.
Gaberšček S, Zaletel K. Thyroid physiology and autoimmunity in pregnancy and after delivery. Expert Rev Clin Immunol. 2011;7:697–706.
European Confederation of Laboratory Medicine. European urinalysis guidelines. Scand J Clin Lab Investig. 2000;231:1–86.
Delanghe J, Speeckaert M. Preanalytical requirements of urinalysis. Biochem Med. 2014;24:89–104.
Ittermann T, Albrecht D, Arohonka P, Bilek R, de Castro JJ, Dahl L, et al. Standardized map of iodine status in Europe. Thyroid 2020;30:1346–54.
Rosinger A, Herrick K. Daily water intake among U.S. men and women. NCHS Data Brief. 2016;242:1–8.
Štolc V. Interference of certain ions with the catalytic action of iodine in the Sandell–Kolthoff reaction. Z Anal Chem. 1961;183:262–7.
Miura Y, Kusakari K. Flow injection analysis of nitrite based on spectrophotometric measurements of iodine formed by oxidation of iodide with nitrite. Anal Sci. 1999;15:923–6.
Andersen S, Karmisholt J, Pedersen KM, Laurberg P. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr. 2008;99:813–8.
Acknowledgements
We extend our gratitude to the medical laboratory teams at University Medical Centre Ljubljana, Slovenia, for their technical support. We also appreciate the regional community healthcare centers, hospitals, as well as the medical laboratory teams throughout Slovenia for their assistance in collecting biological samples.
Funding
The Nutrihealth study was part of the research project L3-8213 “Children’s and adults’ nutrition as a protective or health-risk factor”, funded by the Slovenian Research Agency and Ministry of Health of the Republic of Slovenia. The EU study received funding under grant agreement 634453 from the European Union’s Horizon 2020 Research and Innovation Program entitled “Towards the elimination of iodine deficiency and preventable thyroid-related diseases in Europe” with the goal of harmonizing and sustainably improving iodine intake in Europe.
Author information
Authors and Affiliations
Contributions
Study conception and design: AO, MG, IP, SG, and KZ; provision of study materials or participants: AO, MG, UB, JO, AK, KŽ, ŽL, TZ, IP, SG, and KZ; data acquisition: AO, MH, HH, MG, JO, IP, SG, and KZ; data analysis and interpretation: AO, BK, SG, and KZ; project administration: UB, IP, SG, and KZ; manuscript writing: AO (first draft), BK, TZ, SG, and KZ. All authors reviewed the manuscript and gave final approval of the article to be submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The Nutrihealth and EUthyroid studies were approved by the Slovenian National Medical Ethics Committee (Ministry of Health, Republic of Slovenia). Nutrihealth study had identification number 0120-337/2016-4 and was registered at ClinicalTrials.gov (ID: NCT03284840). EUthyroid study had identification number 0120-508/2017-2.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Oblak, A., Hribar, M., Hristov, H. et al. Interpreting urinary iodine concentration: effects of urine dilution and collection timing. Eur J Clin Nutr (2024). https://doi.org/10.1038/s41430-024-01492-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41430-024-01492-y
- Springer Nature Limited