Abstract
Endoscopic submucosal dissection (ESD) benefits patients in the early stages of cancer, but it poses various risks of complication. Strategies involving the application of clinically approved products to cover ulcers caused by ESD can reduce these complications, but the fixed nature of their properties limit the understanding of their effects on ulcer healing. This study was focused on Tetra–PEG gel, an innovative hydrogel with controllable physical properties made from a sulfhydryl–maleimide pair. The use of biocompatible polyethylene glycol (PEG) in Tetra–PEG gel may allow for its application as a biomaterial. The aims of our study were to identify the characteristics of a self-solidifying hydrogel for endoscopic application and to develop a new ulcer coating agent for post-ESD treatment. We developed a specialized double-lumen catheter and determined the optimal application conditions of the hydrogel. We examined the hydrodynamic properties of the gelling solutions and elucidated the pressure drop that occurred during device operation. Finally, by considering previous experimental results, we successfully applied the hydrogel to post-ESD ulcers in porcine stomachs. We believed that by further optimizing hydrogels with effectively controlled properties and by continuing to investigate them through animal experiments, we could expand our understanding of the relationships among material and ulcer healing properties and apply this knowledge to clinical applications.
Similar content being viewed by others
Introduction
In contrast to open surgery, endoscopic treatment of early-stage gastrointestinal cancer can reduce patient burden. Endoscopic submucosal dissection (ESD) was developed in the late 1990s; this procedure made it possible to complete the en bloc resection of large lesions or lesions with ulceration [1]. However, the ESD technique has a higher frequency of complications, such as bleeding and perforation, than the previously developed endoscopic mucosal resection (EMR) method [2, 3].
To minimize these complications, preventive methods have been employed, such as the utilization of Neoveil [4], a polyglycolic acid (PGA) sheet approved in Japan in 1992 for tissue reinforcement, and PuraStat [5], a hemostatic material that received a CE marking in 2014. These methods have been implemented in clinical practice and have demonstrated efficacy in reducing complications. However, most recent research has focused mainly on adapting techniques for reusing clinically approved products, thereby making it difficult to thoroughly understand the influences of material properties on healing. To gain a comprehensive understanding of these results, studies should be performed on well-defined material properties. Although attempts have been made to design new polymeric materials through animal experiments [6,7,8,9], few scholars have focused on materials with many different controllable physical properties.
A technology platform was developed in the 2000s to create hydrogels with predictable and controllable properties using tetrabranched polymers [10]. This platform enabled the rapid design of hydrogels with specific characteristics, such as elasticity, degradation time, and swelling, by mixing equal amounts of two aqueous solutions of tetrabranched polymers that crosslinked on their own. Hydrogels fabricated with this platform are often referred to as tetra-gels [11]. With the use of biocompatible polyethylene glycol (PEG), hydrogels can function as biomaterials, aiding further research in areas such as hemostatic agent development [12], artificial vitreous body evaluation [13], and tissue adhesive spray development for cell immobilization [14].
This hydrogel can physically embed and cover concave areas. In a previous study, we designed a tetra-gel by using a sulfhydryl (SH)–maleimide (MAL) pair, which is known to form thioether (R − S − R′) and/or disulfide (R − S − S − R′) bonds with SH groups in cysteine residues within a submucosa. We tested the hydrogels in a gastric ulceration rat model [15]. We selected this crosslinking pair for its applicability in stomachs because of its inherent ability to react in a low-pH environment [16]. According to our results, the hydrogel can promote ulcer healing; however, the small animal model required the hydrogel to be administered and evaluated without direct visibility, thereby impeding the complete assessment of its impact on gastric ulcers. Consequently, we concluded that it is necessary to observe hydrogel formation and ulcer healing using endoscopy with direct visibility.
The aims of this study were to optimize a self-solidifying hydrogel component using SH–MAL crosslinking pairs for endoscopic delivery and to ultimately develop a novel coating agent for post-ESD ulcers.
Materials and methods
The present study involved the following 4 experiments: 1) design of a double-lumen catheter that could deliver two solutions independently; 2) evaluation of the delivery of different concentrations of PEG (CPEG) in the hydrogel (adjusted to a neutral and biocompatible pH) through the catheter; 3) hydrodynamic analyses of the hydrogel to be dispensed to characterize the deliverability characteristics of the device; and 4) application of the optimally formulated hydrogel to a porcine post-ESD ulcer using an endoscope, followed by evaluation of the effectiveness of the hydrogel as a coating agent via subsequent endoscopic observations and histological assessments.
Design of the hydrogel dispensing device
A double-lumen hydrogel-dispensing device was designed for this study (Fig. 1). The device had an effective length of 2200 mm and an outer diameter of 2.3 mm. We ensured that this length was sufficient to reach the porcine stomach. Additionally, the catheter contained two independent lumens, each with an equal diameter to allow solutions of similar viscosities to pass through at the same flow rate. The lumen size was increased to a maximum size of 0.8 mm to allow the polymeric solutions to flow smoothly.
Hydrogel dispensing device. a Overall view of the catheter. The effective length is 2200 mm, the outer diameter is 2.3 mm, and the inner diameter is 0.8 mm. b Cross-sectional diagram of the catheter. The catheter has a double lumen structure designed for mixing two solutions at the tip. c Attachment part of the syringe that splits into two branches
Although endoscopic double-lumen catheters are available for fibrin glue [17], they are not suitable for tetra-gel. These catheters have different lumen diameters for managing two solutions with different viscosities (fibrinogen and thrombin). In a tetra-gel, two solutions of similar viscosities must be dispensed to ensure the high predictability of the physical properties of the resulting hydrogel [10].
We used the created catheter to dispense hydrogels with different CPEG ranging from 10 to 100 g/L on a sloped surface to assess whether the hydrogels could be dispensed from the catheter tip.
Hydrogel dispensing tests on a sloped surface
Experimental overview
To evaluate the conditions for hydrogel application to post-ESD ulcers, we performed hydrogel-dispensing tests on a sloped surface with a catheter. We conducted experiments at different CPEG values ranging from 10 to 100 g/L to assess whether they remained on the slope without flowing out. A sloped target surface for dispensing was utilized because materials are often dispensed onto uneven and sloped tissue surfaces during endoscopic procedures. A hydrophilic film was used to prevent the gelling solution from forming droplets.
The interior of each catheter lumen was washed with Milli-Q water. PEG–SH and PEG–MAL were dissolved separately in phosphate buffer (PB) to obtain concentrations (CPEG) of 10–100 g/L. To aid visualization, the PEG–SH solution was supplemented with 1 vol% blue ink (e.g., 0.1 mL of ink for 10 mL of solution). Solutions of PEG–SH and PEG–MAL with the same CPEG were separately added to 5 mL syringes. Each syringe filled with a PEG-based solution was connected to a catheter. An acrylic board with an attached hydrophilic film was placed at an angle of approximately 56° from the ground. Subsequently, the PEG–SH (0.5 mL) and PEG–MAL solutions (0.5 mL) were simultaneously dispensed from the device for a duration of 10 s, targeting the acrylic board. This procedure was repeated for various CPEG values while recording videos of the process.
Experiments 1 and 2 mentioned above were performed at the same time.
With subsequent large animal experiments in mind, we conducted hydrogel dispensing tests not only on plastic boards but also on the submucosal surface of the extracted porcine stomach. We injected saline solution into the submucosal layer of the stomach. By using this injection as a guideline, we removed the mucosal surface to expose the submucosal layer.
Experimental preparation
Tetrabranched poly(ethylene glycol) (PEG) with a molecular weight (Mw) of 20 kg/mol was end-functionalized with sulfhydryl (SH) and maleimide (MAL) groups (PEG–SH and PEG–MAL, respectively) and was purchased from SINOPEG Biotech Co., Ltd. (Fujian, China). Water for injection (WFI) was acquired from Hikari Pharmaceutical (Tokyo, Japan). Polyethylene glycol 20000 (PEG 20000) was obtained from Fujifilm Wako Pure Chemical Corporation (Tokyo, Japan). Blue ink was purchased from Waterman Pen Company (Mysterious Blue) (Paris, France). A transparent acrylic board with a thickness of 3 mm and dimensions of 200 mm × 200 mm was acquired from As One (Osaka, Japan). A 9901 P hydrophilic film was obtained from 3 M (MN, USA). A 5 mL Luer Lock syringe was purchased from Terumo (Tokyo Japan). An indigo carmine injector was acquired from Daiichi Sankyo (Tokyo, Japan). An RC15-AC 0.22 μm filter was obtained from Sartorius AG (Göttingen, Germany). The previous 8 materials were used as received. PB (pH 7.4) was prepared at a concentration of 200 mM as reported previously in the literature [18].
The extracted porcine stomach was purchased from Tokyo Shibaura Zouki Co., Ltd.
Hydrodynamic analyses of the hydrogel
To assess the ease of dispensing the hydrogel from the device, we performed hydrodynamic analyses on the hydrogel components. The dispensability of the device was quantitatively evaluated. An MCR 302 rheometer (Anton Paar GmbH, Graz, Austria) was used for rheological measurements.
Feasibility test of hydrogels and their dedicated catheter: an animal study
The hydrogel conditions were adjusted in the preliminary experiments. Therefore, we evaluated whether the hydrogel could be applied to porcine stomach ulcers under the same conditions. Moreover, we assessed the ulcer-protective effects of the hydrogel via histological evaluation.
Hydrogel preparation
The PEG–SH and PEG–MAL solutions were prepared using the same method as that used for the slope dispensing test. We decided to adopt a CPEG of 40 g/L based on the results of the dispensing tests for subsequent monitoring. As a minor adjustment, indigo carmine was added to the PEG–SH solution at a concentration of 3 vol% for visualization, and the PEG solutions were filtered through a 0.22 μm filter before being loaded in the dispensing device.
Animal experiment
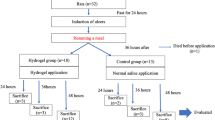
The animal experimental protocols used were approved by the Office for Life Science Research Ethics and Safety of the University of Tokyo. In this experiment, a male pig weighing approximately 30 kg was used. The animal was purchased from Tokyo Laboratory Animal Co.
The animal was fasted for 24 h before performing the procedure. One day after ESD, the animal was fed a normal diet. Drinking water was always allowed, including immediately after ESD.
Endoscopic submucosal dissection and hydrogel application to post-ESD ulcers
An endoscopy system (EPK-i1700; PENTAX Medical, Tokyo, Japan) and endoscope (EG29-i10N; PENTAX Medical, Tokyo, Japan) were used. ESD was performed as previously described in the literature [19]. First, markings were made using a DualKnife J (Olympus Medical). A solution of hyaluronic acid stained with indigo carmine was injected to lift the submucosal layer. Subsequently, mucosal incision and submucosal dissection were performed using DualKnifeJ. Visible blood vessels on the ulcer floor after ESD were coagulated with a radial jaw (Boston Scientific). We performed surgery at two sites on the anterior and posterior walls of the gastric antrum. We created 20 mm ulcers by placing a piece of round cellophane tape with a 20 mm diameter on the stomach surface and making it notable. After four ESD-induced ulcers were created in the stomach, we applied the hydrogel to the posterior wall. The hydrogel was not applied on the anterior wall side and served as a control. The procedure was performed under general anesthesia.
Evaluation points
Endoscopic observation
On postoperative day (POD) 7, the ESD ulcers were observed via endoscopy to check for bleeding, perforation, or other complications.
Sampling and measuring the ulcer reduction rate
After endoscopic observation, the animals were euthanized by a rapid intravenous injection of thiopental and potassium chloride. After cardiopulmonary arrest was confirmed, the animal stomach was removed. We compared the ulcer areas immediately after ESD on POD7 to calculate the ulcer reduction rate. The ulcer size immediately after ESD was measured from the resected specimen, whereas the ulcer size on POD7 was measured from the remaining ulcer based on the extracted stomach. The specimens and a ruler were photographed in the same field of view for comparison, and the areas were measured using ImageJ software. The ulcer reduction rate was calculated by comparing the size of the ulcer on the resected stomach immediately after ESD with that on POD7.
Histological evaluation
The extracted porcine stomachs were thinly sliced and stained with hematoxylin, eosin (HE) and picrosirius red.
HE staining was performed to assess the extent of tissue damage, angiogenesis, granulation, and epithelialization. Picrosirius red staining was performed to determine whether there was a difference in the degree of increased collagen as a result of scarring.
The extent of tissue damage was scored as follows: 1: mucosal epithelium, 2: mucosal lamina propria, 3: submucosal tissue, and 4: submucosal muscle layer.
The degrees of wound healing at the wound site in HE- and picrosirius red-stained specimens were evaluated and scored subjectively on a four-point scale based on either the extent of tissue proliferation or the appearance (0, no findings; 1, insignificant [1–25%]; 2, slight [26–50%]; 3, moderate [51–75%]; 4, high [76–100%]).
To evaluate angiogenesis, the number of vessels per field of view at a 40× magnification was counted. The number of inflammatory cells in a 100 μm2 area of the epithelium was counted and compared between the control and hydrogel groups.
Results and discussion
Design of the hydrogel dispensing device
By using the designed hydrogel-dispensing device, we tested hydrogels with different CPEG values ranging from 10 to 100 g/L. We discovered that regardless of the CPEG value, the two solutions could be continuously dispensed without needing to apply a specific force. Furthermore, the dispensed hydrogel component transformed into a hydrogel within a few seconds (data not shown). Because of the rapid transformation and difficulty of measurement of the specimen, we did not perform a detailed evaluation of the gelation time.
Hydrogel dispensing tests on a sloped surface
The hydrogels were dispensed onto a plastic plate positioned at an angle (Fig. 2) (Supplementary Movies S1–S4). Hydrogel formation occurred when the CPEG value was ≤ 20 g/L after the solution had dripped from the plate. In contrast, hydrogels were formed in the middle of the slope when the CPEG value was ≥ 40 g/L. Hydrogel formation occurred without flowing out of the slope when the CPEG value was ≥ 40 g/L, likely because of the sufficiently short gelation time. The SH–MAL crosslinking pair was used because of its ability to quickly form a hydrogel, even under weakly acidic conditions [16]. To compensate for the decrease in the pH of the gelling solution after it reached the stomach, we used a precautionary measure to adjust the pH to 7.4. In a neutral environment such as the esophagus, a tetra-gel with a crosslinking pair of amino groups and active esters could be applied to post-ESD ulcers [20]. However, we did not use the same material because hydrogel formation occurred very slowly in a low-pH environment [18], making it theoretically unsuitable for application in a stomach. Although hydrogel formation could be accelerated at a low concentration by increasing the pH [18], we chose to fix the pH at 7.4 to minimize uncertainty regarding the living body of the test specimen.
Many different CPEG values were tested to determine the optimal composition. In principle, high concentrations of hydrogels could cause significant swelling and deformation under physiological conditions [21, 22]. Therefore, maintaining a low concentration was considered preferable to prevent unexpected dropouts. We did not directly measure the stiffness or Young’s modulus (E); however, considering prior research on tetra-gels, the E values of the hydrogels with the specified compositions fell within the range of approximately 6–30 kPa, depending on the CPEG, with a slight temperature dependence [23, 24]. Considering that the E values of human stomachs range from approximately 22.5 to 42.5 kPa [25], we could infer that this material was not excessively rigid. Based on the experimental and theoretical results, we concluded that a hydrogel composition of 40 g/L was optimal for further endoscopic evaluation, providing some slope retention at a relatively low concentration.
Additionally, we conducted this experiment on the submucosal layer of the extracted porcine stomach instead of on plastic boards and obtained similar results to those mentioned above. (Supplementary Movies S5–S8).
Quantitative evaluation of dispensability
For a quantitative evaluation of fluid dispensability, it was necessary to measure the pressure drop from the inlet channel to the outlet channel. Fluid dispensability was easier when the pressure drop was small than when it was large. Typically, a pressure drop occurred when the fluid moved through a channel because of friction between the fluid and the channel wall. The Darcy–Weisbach equation empirically connected the pressure drop (Δp) to the fluid viscosity (η), flow velocity, and pipe geometry (Eq. 1):
where λ is the Darcy friction factor, L is the length of the pipe, D is the hydraulic diameter of the pipe, v is the mean flow velocity, and g is the gravitational acceleration [26].
In laminar flow, λ was considered inversely proportional to the Reynolds number (Re), defined as Re = ρvR/η, where ρ is the density, R is the diameter of the pipe, and η is the viscosity of the fluid [27]:
Since we needed viscosity information, we determined the η of aqueous solutions of PEG with the same molecular weights and concentrations as the dispensed solutions using steady-state flow measurements (Fig. 3a). The observed viscosity remained consistent across the various shear rates (data not shown), indicating Newtonian fluid behavior. The concentrations tested (i.e., CPEG = 10–100 g/L) were below the range where entanglement occurred [28]. High viscosities were observed for the solutions with high CPEG values. At the highest CPEG value tested in this study, we obtained a value of approximately 10 mPa·s. The ability of the operator to operate the device without applying a specific force, regardless of the CPEG value, indicated that the developed device was best suited for passing through fluids below 10 mPa·s. Based on the viscosity data obtained, we calculated the Re for each CPEG (Fig. 3b). A high CPEG yielded a low Re, all below 2300. Typically, laminar flow occurred when Re < 2300 [29]. Therefore, we maintained the experiment in the laminar flow regime and considered the application of Eq. 2 appropriate. Finally, using the parameters we derived, we calculated the Δp inherent to the dispensing process based on Eq. 1 (Fig. 3c). In this study, we used the following parameters for each calculation: L is the lumen length of the device (2200 mm), and D is the inner diameter of each lumen (0.8 mm). In each dispensing test, 0.5 mL of each lumen was dispensed over a duration of 10 seconds, equating to an average velocity of 0.05 mL/s; therefore, v was 0.0995 m/s. We found that Δp was dependent on CPEG, and at the optimized concentration (i.e., CPEG = 40 g/L), it was approximately 0.5 Pa. These results indicated that the device design and flow rate conditions that produced Δp ≤ 1.5 Pa would not impose a burden on the operator.
Feasibility tests of hydrogels and their dedicated catheters: an animal study
We conducted an animal study to assess the feasibility of the use of tetra-gels and dedicated catheters. We performed ESD on porcine stomachs and delivered the hydrogels to ulcer beds. On POD7, the statuses of the ESD-induced ulcers were observed endoscopically. Then, the harvested tissues were assessed (Fig. 4). The PEG–SH and PEG–MAL solutions dispensed from the dedicated devices were transformed into hydrogels in the ulcer regions and were retained even after flushing with water (Supplementary Movie S9). To further assess the adherence levels of the hydrogels to ulcerated surfaces, the hydrogels were applied to the stomachs of other porcine specimens. After application, the animals were euthanized, and their stomachs were excised. As a result, it was confirmed that the gels remained at the bases of the ulcers and were not easily removed, even when pulled with tweezers (Supplementary Movie S10). Therefore, we considered that the adhesive properties of the hydrogel were present even in the stomach. Observations on POD7 specimens revealed no significant difference in the macroscopic appearance between the control and hydrogel groups. We measured the ulcer sizes of the collected tissues and compared them with the ulcer sizes immediately after operation (Table 1). In all specimens except those in the second control group, the ulcer sizes were reduced. The ulcer sizes of the specimens in the hydrogel groups tended to shrink. The adhesion of the hydrogel observed in the early stages was considered to occur due to the physical embedding of the gels tightly between the fine folds on the ulcer bases and due to the formation of disulfide and/or thioether bonds between the SH–MAL groups of the hydrogels and the abundant cysteine residues in the submucosa during hydrogel formation [30]. However, differing from the expectations derived from the postoperative adhesion characteristics observed on POD0, no hydrogel remained on POD7. Theoretically, hydrogels could exhibit long-term stability both in vitro and in vivo because they lack bonds that would break easily at low pH levels and undergo enzymatic degradation [16]. Hence, at this stage, we speculated that the physical stress caused by the postoperative diet led to the removal of the hydrogel. However, additional endoscopic observations over short periods are required to clarify the underlying mechanisms involved. The specimens in the second control group underwent partial resection, which made it impossible to measure the area accurately. Overall, the hydrogels could have affected the ulcer healing process, but the small number of experiments made this evaluation difficult. Further studies are required to explore the relationships among hydrogel and ulcer healing properties. The developed catheter was confirmed to be suitable for use with an actual endoscope without causing problems. In addition, we confirmed that it was possible to cover post-ESD ulcers in a porcine stomach under the hydrogel conditions that were investigated in a preliminary experiment.
Histologic evaluation
We assessed the extent of angiogenesis, inflammatory cells, and fibrosis by staining tissue sections with HE and picrosirius red (Fig. 5). When comparing the number of new blood vessels, 40 new blood vessels per high-power field (HPF) were detected in the hydrogel group, whereas only 11 new blood vessels were detected in the control group. In terms of inflammatory cells, the control group had 51 inflammatory cells per 100 μm² compared to 47 inflammatory cells in the hydrogel group. Angiogenesis was considered critical tissue regeneration and repair, particularly when applying biomaterials [31]. In this study, further angiogenesis was observed in the hydrogel group. Future experiments on porcine models that repeatedly show this trend would suggest that hydrogels could enhance tissue regeneration and repair properties. Additionally, no significant differences were observed in the number of inflammatory cells between the two groups. Because inflammatory cells tended to increase in the surrounding area when implanted biomaterials caused foreign body reactions (FBRs) [32], we inferred that the present hydrogel did not induce excessive or chronic inflammation in the body.
Stained sections of the ulcers. HE-stained images (left; a and d), magnified images (center; b and e), and picrosirius red-stained images (right; c and f) of the control groups (top) and hydrogel groups (bottom). The scale bars for (a, d) are 1000 μm, those for (b, e) are 100 μm, and those for (c, f) are 200 μm
Additionally, we assessed the degrees of tissue damage, inflammatory cell infiltration, angiogenesis, granulation tissue formation, epithelialization, and fibrosis (Table 2). There were no significant differences observed, except for the degree of neovascularization; these results were similar to those obtained by direct counting. In this single large animal experiment, the hydrogel group showed no undesirable results in terms of ulcer healing compared to the control group. However, it was too early to draw conclusions from this pilot study alone. Further experiments would be necessary to fully investigate the effects of hydrogel coverage on wound healing.
Conclusion
In the animal experiments performed in this study, it was demonstrated that by optimizing the parameters of the application device and hydrogel, the hydrogel could cover the base of a post-ESD ulcer. Histological evaluations suggested that the hydrogel did not induce chronic inflammation, and they indicated the potential for angiogenesis at the ulcer sites. However, further experiments are needed to verify the clinical significance of these findings. The future design of hydrogels with diverse physical properties and extended observations will contribute to the enhancement in the understanding of the relationships among material and ulcer healing properties.
References
Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–9.
Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72:1217–25.
Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and emr for early gastric cancer. Gastrointest Endosc. 2012;76:763–70.
Tsuji Y, Fujishiro M, Kodashima S, Ono S, Niimi K, Mochizuki S, et al. Polyglycolic acid sheets and fibrin glue decrease the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms (with video). Gastrointest Endosc. 2015;81:906–12.
Subramaniam S, Kandiah K, Chedgy F, Fogg C, Thayalasekaran S, Alkandari A, et al. A novel self-assembling peptide for hemostasis during endoscopic submucosal dissection: A randomized controlled trial. Endoscopy. 2021;53:27–35.
Xu X, Xia X, Zhang K, Rai A, Li Z, Zhao P, et al. Bioadhesive hydrogels demonstrating ph-independent and ultrafast gelation promote gastric ulcer healing in pigs. Sci Transl Med. 2020;12:eaba8014.
Zhou T, Mao X, Xu L, Jin H, Cen L, Dong C, et al. A new protective gel to facilitate ulcer healing in artificial ulcers following oesophageal endoscopic submucosal dissection: A multicentre, randomized trial. Sci Rep. 2023;13:6849.
Bang B, Lee E, Maeng J, Kim K, Hwang JH, Hyon SH, et al. Efficacy of a novel endoscopically deliverable muco-adhesive hemostatic powder in an acute gastric bleeding porcine model. PLoS One. 2019;14:e0216829.
Zhao LM, Gong M, Wang R, Yuan QJ, Zhang Y, Pi JK, et al. Accelerating esd-induced gastric ulcer healing using a ph-responsive polyurethane/small intestinal submucosa hydrogel delivered by endoscopic catheter. Regen Biomater. 2021;8:rbaa056.
Sakai T, Matsunaga T, Yamamoto Y, Ito C, Yoshida R, Suzuki S, et al. Design and fabrication of a high-strength hydrogel with ideally homogeneous network structure from tetrahedron-like macromonomers. Macromolecules. 2008;41:5379–84.
Shibayama M, Li X, Sakai T. Gels: From soft matter to biomatter. Ind Eng Chem Res. 2018;57:1121–8.
Okata S, Hoshina K, Hanada K, Kamata H, Fujisawa A, Yoshikawa Y, et al. Hemostatic capability of a novel tetra-polyethylene glycol hydrogel. Ann Vasc Surg. 2022;84:398–404.
Hayashi K, Okamoto F, Hoshi S, Katashima T, Zujur DC, Li X, et al. Fast-forming hydrogel with ultralow polymeric content as an artificial vitreous body. Nat Biomed Eng. 2017;1:1–7.
Ishikawa S, Kamata H, Chung UI, Sakai T. Tissue-adhesive hydrogel spray system for live cell immobilization on biological surfaces. ACS Appl Bio Mater. 2023;6:4613–9.
Miura Y, Tsuji Y, Cho R, Fujisawa A, Fujisawa M, Kamata H, et al. The feasibility of a novel injectable hydrogel for protecting artificial gastrointestinal ulcers after endoscopic resection: An animal pilot study. Sci Rep. 2021;11:18508.
Jansen LE, Negrón-Piñeiro LJ, Galarza S, Peyton SR. Control of thiol-maleimide reaction kinetics in peg hydrogel networks. Acta Biomater. 2018;70:120–8.
Dunn CJ, Goa KL. Fibrin sealant: A review of its use in surgery and endoscopy. Drugs. 1999;58:863–86.
Kurakazu M, Katashima T, Chijiishi M, Nishi K, Akagi Y, Matsunaga T, et al. Evaluation of gelation kinetics of tetra-peg gel. Macromolecules. 2010;43:3935–40.
Fujishiro M. Endoscopic submucosal dissection for stomach neoplasms. World J Gastroenterol. 2006;12:5108–12.
Wei Y, Tang J, Li J, Hou X, Li L, Zhang D, et al. A novel tetra-peg based hydrogel for prevention of esophageal stricture after esd in a porcine model. Colloids Surf B Biointerfaces. 2023;226:113321.
Kamata H, Akagi Y, Kayasuga-Kariya Y, Chung U, Sakai T. Nonswellable” hydrogel without mechanical hysteresis. Science. 2014;343:873–5.
Kamata H, Li X, Chung UI, Sakai T. Design of hydrogels for biomedical applications. Adv Healthc Mater. 2015;4:2360–74.
Akagi Y, Gong JP, Chung U, Sakai T. Transition between phantom and affine network model observed in polymer gels with controlled network structure. Macromolecules. 2013;46:1035–40.
Fujiyabu T, Yoshikawa Y, Chung UI, Sakai T. Structure-property relationship of a model network containing solvent. Sci Technol Adv Mater. 2019;20:608–21.
Song C, Alijani A, Frank T, Hanna GB, Cuschieri A. Mechanical properties of the human abdominal wall measured in vivo during insufflation for laparoscopic surgery. Surg Endosc. 2006;20:987–90.
Weisbach JL. Lehrbuch der ingenieur- und maschinen-mechanik : Ohne unwendung des höhern calcus für den unterricht an technischen lebranstalten sowie zum gebrauche für technifer. Braunschweig: Druck und Verlag von Friedrich Vieweg und Sohn; 1845.
Poiseuille JLoM. Recherches expérimentales sur le mouvement des liquides. Paris; 1844.
Fetters LJ, Lohse DJ, Richter D, Witten TA, Zirkel A. Connection between polymer molecular-weight, density, chain dimensions, and melt viscoelastic properties. Macromolecules. 1994;27:4639–47.
Reynolds O. An experimental investigation of the circumstances which determine whether the motion of water shall be direct or sinuous, and of the law of resistance in parallel channels. Philos Trans R Soc Lond. 1883;174:935–82.
Leichner C, Jelkmann M, Bernkop-Schnurch A. Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature. Adv Drug Deliv Rev. 2019;151:191–221.
Ngo MT, Harley BAC. Angiogenic biomaterials to promote therapeutic regeneration and investigate disease progression. Biomaterials. 2020;255:120207.
Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100.
Acknowledgements
This study was supported by JSPS KAKENHI (grant number 21K12680/23K08327/21H04688), JST CREST JPMJCR1992, the MEXT Program: Data Creation and Utilization-Type Material Research and Development Project JPMXP1122714694, and a research grant from Nipro.
Funding
Open Access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hiroyuki Kamata and Takamasa Sakai are employees of Gellycle Co., Ltd., Japan. The Department of Next-Generation Endoscopic Computer Vision is an endowment department supported by an unrestricted grant from AI Medical Service, Inc.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cho, R., Kamata, H., Tsuji, Y. et al. Optimizing a self-solidifying hydrogel as an endoscopically deliverable hydrogel coating system: a proof-of-concept study on porcine endoscopic submucosal dissection-induced ulcers. Polym J (2024). https://doi.org/10.1038/s41428-024-00921-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41428-024-00921-w
- Springer Nature Limited