Abstract
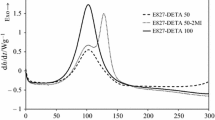
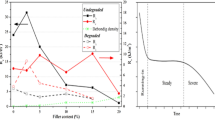
Amine-cured epoxy resins bearing ester moieties were synthesized, and their properties, hydrolytic degradation behavior, and biomineralization were investigated. Neopentyl glycol diglycidate (NPG) was used as the epoxide and was cured with diethylenetriamine (DETA) and isophoronediamine (IPD) at different ratios. The glass transition temperatures were controlled using the composition of DETA and IPD. The cured materials containing IPD units were tolerant to neutral water but were degraded under acidic and basic conditions. Degradation in the presence of lipase also proceeded in phosphate buffer, while degradation proceeded gradually in the absence of lipase. To demonstrate their potential application as degradable biomedical materials for bone and dental repair, composites containing hydroxyapatite (HA) were prepared by curing NPG and the amines in the presence of HA. Biological bone-like apatite was grown on an NPG-IPD-HA composite by immersion in synthetic biofluid, and the amount of bone-like apatite was greater than that on the glycidyl ether analog.








Similar content being viewed by others
References
Ochi M. Structure and properties of functional epoxy resins. J Adhes Soc Jpn. 2003;39:432–7.
Vidil T, Tournilhac F, Musso S, Robisson A, Leibler L. Control of reactions and network structures of epoxy thermosets. Prog Polym Sci. 2016;62:126–79.
Rahman MM, Islam MA. Application of epoxy resins in building materials: progress and prospects. Polym Bull. 2022;79:1949–75.
Mi XQ, Liang N, Xu HF, We J, Jiang Y, Nie B, Zhang DH. Toughness and its mechanisms in epoxy resins. Prog Mater Sci. 2022;130:100977.
Shundo A, Yamamoto S, Tanaka K. Network formation and physical properties of epoxy resins for future practical applications. JACS Au. 2022;2:1522–42.
Mintenig SM, Löder MGJ, Primpke S, Gerdts G. Low numbers of microplastics detected in drinking water from ground water sources. Sci Total Environ. 2019;648:631–5.
Liu K, Wang XH, Fang T, Xu P, Zhu LX, Li DJ. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci Total Environ. 2019;675:462–71.
Amorim MJB, Scott-Fordsmand JJ. Plastic pollution — A case study with Enchytraeus crypticus — From micro-to nanoplastics. Environ Pollut 2021;271:116363.
Gardiner TH, Waechter JM Jr, Wiedow MA, Solomon WT. Glycidyloxy compounds used in epoxy resin systems: A toxicology review. Regul Toxic Pharmac. 1992;15:S1–S77.
González MP, Helguera AM, Ruiz RM, Fárdales JRG. A topological sub-structural approach of the mutagenic activity in dental monomers. 1. Aromatic epoxides. Polymer. 2004;45:2773–9.
Auvergne R, Caillol S, David G, Boutevin B, Pascault J. Biobased thermosetting epoxy: present and future. Chem Rev. 2014;114:1082–1115.
Mashouf Roudsari G, Mohanty AK, Misra M. Green approaches to engineer tough biobased epoxies: a review. ACS Sustain Chem Eng. 2017;5:9528–41.
Wan J, Zhao J, Zhang X, Fan H, Zhang J, Hu D, Jin P, Wang DY. Epoxy thermosets and materials derived from bio-based monomeric phenols: transformations and performances. Prog Polym Sci. 2020;108:101287.
Feng Z, Nikafshar S, Hegg EL, Nejad M. Biobased divanillin as a precursor for formulating biobased epoxy resin. ACS Sustain Chem Eng. 2020;8:9095–103.
Shen MJ, Robertson ML. Degradation behavior of biobased epoxy resins in mild acidic media. ACS Sustain Chem Eng. 2021;9:438–47.
Yokoyama Y, Yasui T, Takeda A, Ogino K, Kanehashi S. Novel bio-based flexible bisphenol epoxy resin derived from cashew nut shell liquid. Polym J. 2023;55:859–67.
Silau H, Garcia AG, Woodley JM, Dam-Johansen K, Daugaard AE. Bio-based epoxy binders from lignin derivatized with epoxidized rapeseed fatty acids in bimodal coating systems. ACS Appl Polym Mater. 2022;4:444–51.
Ma S, Liu Z, Jiang Y, Tang Z, Zhang C, Zhu J. Bio-based epoxy resin from itaconic acid and its thermosets cured with anhydride and comonomers. Green Chem. 2013;15:245–54.
Deng J, Liu X, Li C, Jiang Y, Zhu J. Synthesis and properties of a bio-based epoxy resin from 2,5-furandicarboxylic acid (FDCA). RSC Adv. 2015;5:15930–9.
Miao JT, Yuan L, Guan Q, Liang G, Gu A. Biobased heat resistant epoxy resin with extremely high biomass content from 2,5-furandicarboxylic acid and eugenol. ACS Sustain Chem Eng. 2017;5:7003–11.
Wang J, Li YC, Song JF, He MY, Song JJ, Xia K. Recycling of acrylonitrile-butadiene-styrene (ABS) copolymers from waste electrical and electronic equipment (WEEE), through using an epoxy-based chain extender. Polym Degrad Stabil. 2015;112:167–74.
Turel T, Daglar O, Eisenreich F, Tomovic Z. Epoxy thermosets designed for chemical recycling. Chem Asian J. 2023;18:e202300373.
Ning C, Lin CSK, Hui DCW, McKay G. Waste printed circuit board (PCB) recycling techniques. Top Curr Chem. 2017;375:43.
Hao J, Wang Y, We Y, Guo F. Metal recovery from waste printed circuit boards: a review for current status and perspectives. Res Conserv Recycl. 2020;157:104787.
Ochiai B, Hirano T. Facile Synthesis of glycidates via oxidation of acrylates with aqueous solution of NaOCl in the presence of ammonium salts. Heterocycles. 2014;89:487–93.
Ochiai B, Soegawa K. Glycidate as a high-strength epoxy adhesive curable with amine under ambient conditions. Polymers. 2022;14:957.
Ochiai B, Yashima M, Soegawa K, Matsumura Y. Biodegradable epoxy thermosetting system with high adhesiveness based on glycidate-acid anhydride curing. ACS Macro Lett. 2023;12:54–58.
Ochiai B, Yano S, Soegawa K. Novel polyfunctional glycidic ester compound and production method thereof, and polymer prepared using the same. JP-Patent. 2015, 2015-214497A.
Moriwaki Y, Akaishi R. Production of α,β-epoxycarboxylic acid derivative. JP-Patent. 1993, H5-39277.
Zhang H, Lin X, Chin S, Grinstaff MW. Synthesis and characterization of poly(glyceric acid carbonate): a degradable analogue of poly(acrylic acid). J Am Chem Soc. 2015;137:12660–6.
Beharaj A, Ekladious I, Grinstaff MW. Poly(alkyl glycidate carbonate)s as degradable pressure-sensitive adhesives. Angew Chem Int Ed. 2019;58:1407–11.
Sheikh Z, Najeeb S, Khurshid Z, Verma V, Rashid H, Glogauer M. Biodegradable materials for bone repair and tissue engineering applications. Materials. 2015;8:5744–94.
Gigli M, Fabbri M, Lotti N, Gamberini R, Rimini B, Munari A. Poly(butylene succinate)-based polyesters for biomedical applications: A review. Eur Polym J. 2016;75:431–60.
Ng HM, Bee ST, Sin LT, Ratnam CT, Rahmat AR. Hydroxyapatite for poly(α-hydroxy esters) biocomposites applications. Polym Rev. 2019;59:187–239.
Fu Z, Cui J, Zhao B, Shen SGF, Lin K. An overview of polyester/hydroxyapatite composites for bone tissue repairing. J Orthop Transl. 2021;28:118–30.
Abdulrazzaq B, Al-Bakhsh J, Shafiei F, Hashemian A, Shekofteh K, Bolhari B, Behroozibakhsh M. In-vitro bioactivity evaluation and physical properties of an epoxy-based dental sealer reinforced with synthesized fluorine-substituted hydroxyapatite, hydroxyapatite and bioactive glass nanofillers. Bioact Mater. 2019;4:322–33.
Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–15.
Wegmann A. Chemical resistance of waterborne epoxy/amine coatings. Prog Org Coat. 1997;32:231–9.
Gorovin VA, Il’in AB. Composite protective coatings. Resistance to acid penetration of coatings based on epoxy resins. Int J Corros Scale Inhib. 2020;9:1530–49.
Lynn DM, Langer R. Degradable poly(β-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122:10761–10768.
Zhong Z, Song Y, Engbersen JFJ, Lok MC, Hennink WE, Feijen J. A versatile family of degradable non-viral gene carriers based on hyperbranched poly(ester amine)s. J Controlled Rel. 2005;109:317–29.
Zhang J, Fredin NJ, Janz JF, Sun B, Lynn DM. Structure/property relationships in erodible multilayered films: influence of polycation structure on erosion profiles and the release of anionic polyelectrolytes. Langmuir. 2006;22:239–45.
Pattanayak DK, Yamaguchi S, Matsushita T, Nakamura T, Kokubo T. Apatite-forming ability of titanium in terms of pH of the exposed solution. J R Soc Inter. 2012;9:2145–55.
Acknowledgements
We are thankful for the financial support from JSPS KAKENHI (grant number JP19K22212), the Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-STEP) from Japan Science and Technology Agency (JST) (grant number AS2621327M, and Nagase ChemteX Co., Inc.), and the kind advice from Mr. Tetsuya Hosomi of Nagase ChemteX Co., Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ochiai, B., Nakazawa, Y., Matsumura, Y. et al. Hydrolytic degradation and biomineralization of amine-cured epoxy resin based on glycidate. Polym J 56, 409–417 (2024). https://doi.org/10.1038/s41428-023-00880-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00880-8
- Springer Nature Limited