Abstract
Oxide-based thermoelectric materials that show a high figure of merit are promising because of their good chemical and thermal stabilities and their relative harmlessness compared with chalcogenide-based state-of-the-art thermoelectric materials. Although several high-ZT thermoelectric oxides (ZT > 1) have been reported thus far, their reliability levels are low due to the lack of careful observations of their stabilities at elevated temperatures. Herein, we review the epitaxial film growth and thermoelectric properties of representative p-type layered cobalt oxides: Na3/4CoO2, Ca1/3CoO2, Sr1/3CoO2, Ba1/3CoO2, and Ca3Co4O9. Among these specimens, Ba1/3CoO2 and Ca3Co4O9 are stable in air at elevated temperatures (~600 °C). The ZT of Ba1/3CoO2 reaches ~ 0.55 at 600 °C in air, which is reliable and the highest among thermoelectric oxides. Moreover, this value is comparable to those of p-type PbTe and p-type SiGe.
Similar content being viewed by others
Thermoelectrics
Today, most energy resources are discharged as waste heat into the environment without being applied. Such exhaust heat reaches approximately 2/3 of the primary energy. Hence, thermoelectric energy conversion technology has attracted great attention for converting waste heat into electricity1,2. The principle of thermoelectric energy conversion was first discovered by T.J. Seebeck in 18213. He found that a voltage is generated between two ends of a metal bar by introducing a temperature difference. Thus, when electric loads are connected at both ends of a metal bar, an electric current can be obtained. This phenomenon is called the Seebeck effect. Conversely, in 1834, J.C.A. Peltier discovered that heating or cooling of the junctions can occur during electric current application to a heterogeneous metal circuit. This phenomenon is called the Peltier effect, and it has been commercially applied in electronic refrigerators, among other applications.
Generally, the performance of thermoelectric materials is evaluated in terms of a dimensionless figure of merit, ZT = S2·σ·T·κ−1, where Z is a figure of merit, T is the absolute temperature, S is the thermopower (≡ Seebeck coefficient), σ is the electrical conductivity, and κ is the thermal conductivity. The energy conversion efficiency from transforming the temperature difference into electricity increases as ZT increases. Thus, to realize efficient thermoelectric energy conversion, three physical properties are needed for thermoelectric materials: (1) low κ, which is needed to introduce a large temperature difference into both ends of the material; (2) high σ, which is needed to reduce the internal resistance of the material; and (3) high S, which is needed to obtain a high voltage.
The ZT values of practical thermoelectric materials, such as Bi2Te3 and PbTe, are ~1, which is the lowest value needed for practical applications4,5. Recently, several high-ZT materials (ZT > 1) have been developed sequentially based on heavy metal alloys, including SnSe, PbSe, GeTe, and oxychalcogenides (BiCuSeO)5,6,7,8,9,10,11,12,13,14,15,16,17. Since thermoelectric devices can directly convert a temperature difference into electricity, some automobile companies have developed thermoelectric-assisted hybrid automobiles18,19,20,21 while considering the outside temperature of the exhaust pipe to be ~700 °C. However, these thermoelectric materials are not appealing, particularly when operating at such high temperatures, because decomposition, vaporization, and melting of the constituents can easily occur. Furthermore, the use of these heavy metals should be limited to specific environments, such as space, because they are mostly toxic, low in abundance as natural resources, and not environmentally benign.
To overcome these issues, metal oxides have attracted much attention as thermoelectric power generation materials at high temperatures based on their potential advantages over heavy metal alloys in terms of chemical and thermal robustness22,23,24. From the 1950s to the 1970s, there was a boom in the search for oxide thermoelectric materials. At this time, researchers in the United States used thermoelectric effects to investigate the intrinsic properties of grown oxide single crystals. Since the 1990s, the search for oxide thermoelectric materials, which originated in Japan, has spread all over the world, including in Europe, the United States, Asia, and India. To date, it has been reported that some oxides could exhibit parameters of ZT > 1, exceeding that of PbTe.
History of thermoelectric oxides
There is a long history of thermoelectric oxides. From the 1950s to the 1970s (1st boom), the thermoelectric properties of many simple conducting oxides, including CdO (1949, Hogarth et al.25), NiO (1957, Parravano26), ZnO (1959, Hutson27), In2O3 (1962, Arvin28), SrTiO3 (1964, Frederikse et al.29), rutile-TiO2 (1965, Thurber et al.30), SnO2 (1965, Marley and Dockerty31), Cu2O (1969, Young and Schwartz32), and Fe3O4 (1970, Griffiths et al.33), were studied to obtain the fundamental physical properties of conducting oxides, such as carrier effective mass. After the discovery of cuprous oxide-based high-Tc superconducting oxides in 198634, the thermoelectric properties of superconducting oxides, including La2CuO4 (1987, Cooper et al.35), La-Ba-Cu-O (1987, Chen et al.36), YBa2Cu3O7−δ (1988, Lee et al.37), and Tl-Ca-Ba-Cu-O (1988, Mitra et al.38), were reported sequentially to clarify the superconducting transition (2nd boom).
After the research boom in the field of high-Tc superconductors, two Japanese researchers, Ohtaki and Terasaki, reported that oxides had good thermoelectric performance, including CaMnO3 (1995, Ohtaki et al.39), Al-doped ZnO (1996, Ohtaki et al.40), and NaxCoO2 (1997, Terasaki et al.41). These reports triggered the 3rd boom of thermoelectric oxide research in the 2000s. As a result of energetic exploratory research on good thermoelectric oxides, Ca3Co4O9 (2000, Masset et al.42 and Funahashi et al.43) and electron-doped SrTiO3 (2001, Okuda et al., La-doped44, 2005, Ohta et al. Nb-doped45,46) were discovered. In 2012, Fergus reviewed the thermoelectric properties of promising oxides (mostly bulk ceramics), including Ca3Co4O9, NaxCoO2, SrTiO3, CaMnO3, and ZnO47 (also see the references therein). Recently, several scholars that have reported rather high ZT values for oxide ceramics. Acharya et al.48 reported that SrTi0.85Nb0.15O3 ceramics sintered with graphite flakes exhibit ZT = 1.4 at 1050 K, and Biswas et al.49 reported that Al-doped ZnO ceramics sintered with reduced graphene oxide exhibit ZT = 0.5 at 1100 K. Although these reported ZTs are very attractive as practical thermoelectric materials, there is still no practical application for them, probably due to the low reliability of the ZT values.
To clarify the intrinsic thermoelectric properties of oxides, we focused on high-quality epitaxial films with stepped and terraced surfaces. As a result, we fabricated high-quality epitaxial films of several thermoelectric oxides, including Na3/4CoO250,51, Sr1/3CoO252, Ca1/3CoO253, Ba1/3CoO254, Ca3Co4O953,55, SrTiO3:Nb46, TiO2:Nb56 and SrO(SrTiO3):Nb57. Among these oxides, we found that Ba1/3CoO2 epitaxial films exhibited a ZT value of ~0.55 at 600 °C in air, which is the highest and most reliable value among the reported thermoelectric oxides54. In this context, we reviewed the epitaxial film growth and thermoelectric properties of four representative p-type layered cobalt oxide films based on our efforts: Na3/4CoO250,51, Sr1/3CoO252, Ca1/3CoO253, and Ba1/3CoO254.
Epitaxial film growth of A xCoO2 (A x = Na3/4, Ca1/3, Sr1/3, and Ba1/3)
Na3/4CoO2 50,51
Figure 1 shows the schematic crystal structure of AxCoO2 (Ax = Na3/4, Ca1/3, Sr1/3, and Ba1/3). In the case of AxCoO2, the rigid CoO2 layer and mobile Ax layer are alternately stacked along the c-axis. In 1997, Terasaki et al. discovered that a NaCo2O4 (≡NaxCoO2, x ~ 3/4) single crystal with a two-dimensional layered structure exhibits a very large power factor S2 ∙ σ of 5 mW m−1 K−2 in the in-plane direction at room temperature41. After this discovery, the electronic structure58,59,60, crystal structure61,62, and Na-composition dependence of the thermoelectric properties62 of NaxCoO2 were energetically studied to understand the origin of the unusually large S. In 2001, Fujita and coworkers fabricated NaxCoO2 single crystals and reported that they exhibited ZT values of ~1.2 at 800 K63. This material has attracted much attention because it can be converted into a superconductor (Tc ~ 4.7 K) by introducing H2O molecules into a layer between the two adjacent CoO2− layers64.
To clarify the intrinsic thermoelectric properties of Na3/4CoO2, we fabricated thin epitaxial films of Na3/4CoO2. First, we tried to fabricate Na3/4CoO2 epitaxial films by the conventional pulsed laser deposition (PLD) technique, but we failed. Several reports have been written on the thin film growth of Na3/4CoO2 by PLD. However, the film quality in terms of crystallographic orientation, surface morphology, and lateral grain size is insufficient. In the case of Na3/4CoO2 film growth by PLD, it is very difficult to control the Na concentrations in the films at high temperatures in a vacuum environment due to the high vapor pressure of the Na species.
To overcome this difficulty, we modified the reactive solid-phase epitaxy (R-SPE)50 method that was developed to fabricate single-crystal films of InGaO3(ZnO)m (m = integer). Figure 2 schematically illustrates the R-SPE procedure. Step 1: A highly (111)-oriented CoO epitaxial film was deposited on a (0001)-α-Al2O3 substrate at 700 °C by the PLD technique using a Co3O4 sintered disk as a target. Step 2: The surface of the PLD-deposited CoO film was fully capped by an yttria-stabilized zirconia (YSZ) single-crystalline plate to keep the surface clean. Step 3: NaHCO3 powder was put on the YSZ plate. Step 4: The sandwich specimen was annealed at 700 °C for 1 h in air. Notably, several researchers have used this R-SPE method for fabricating NaxCoO2 epitaxial films65,66,67 since the resultant film quality, especially in surface morphology, is better than that of PLD-grown films68,69.
The out-of-plane (Fig. 3a) and in-plane (Fig. 3b) X-ray diffraction patterns of the resultant NaxCoO2 film clearly indicate that epitaxial growth occurred, showing the effectiveness of the R-SPE method. The chemical composition of the obtained film was evaluated to be x = 0.83 by X-ray fluorescence (XRF) measurements. The Na content in the present film was slightly higher than the reported values in the as-grown bulk sample x = 0.770, likely because the amorphous layer at the interface contained some Na ions51. Figure 3c shows an atomic force microscopy (AFM) image of the NaxCoO2 film. A step-like structure composed of several flake-like domains could be observed. The step increment was approximately 3 nm, which was three times longer than the c-axis length of NaxCoO2, suggesting that step bunching occurred during annealing at 700 °C.
As described in the next section, the R-SPE-grown Na0.7CoO2 epitaxial film was very useful for fabricating epitaxial films of LiCoO271, Sr0.5CoO252, Ca0.33CoO255,72, and Ca3Co4O955. Furthermore, the Na0.7CoO2 epitaxial film could be converted into a superconducting sodium cobalt oxyhydrate, Na0.3CoO2·1.3H2O (Tc ~ 4 K), by dipping in HNO3 for oxidation treatment followed by dipping in NaCl aqueous solution for hydration treatment73. Furthermore, peeling-off of the Na0.7CoO2 epitaxial film from the α-Al2O3 substrate was possible, and the peeled film could be pasted on the other substrate51.
Ca1/3CoO2
Powder syntheses of CaxCoO2 (x = 0.3, 0.35 and 0.5) were reported in 1996 by Cushing et al.74,75 The scholars used specimens of the sodium cobalt oxide NaxCoO2 (0.6 ≤ x ≤ 1.0) as precursors and performed multivalent ion-exchange reactions.
Stoichiometric amounts of the AxCoO2 precursors were combined with anhydrous Ca(NO3)2 in evacuated sealed glass tubes and heated at 350 °C for 48 h.
There is an interesting feature in the CaxCoO2 system: there are two superstructures of cation ordering. In 2006, Yang et al. discovered that there are two common well-defined cation ordered states corresponding to the \(2a\,\times \,\sqrt{3}a\) orthorhombic superstructure at approximately x = 1/2 and the \(\sqrt{3}a\,\times \,\sqrt{3}a\) hexagonal superstructure at approximately x = 1/376. In 2006, Sugiura et al. fabricated high-quality Ca0.48CoO2 epitaxial films by ion-exchange reactions. Na3/4CoO2 epitaxial films were heated together with Ca(NO3)2 powder at 300 °C for 0.5 h in air55. In 2008, Huang et al. directly observed two instances of Ca ordering in Ca1/3CoO2 epitaxial films using high-angle annular dark-field (HAADF) scanning transmission electron microscopy (STEM)77,78.
In 2009, Sugiura et al. found that the structural transformation of Ca1/3CoO2 occurred at approximately 300 °C72. The \(\sqrt{3}a\,\times \,\sqrt{3}a\) hexagonal phase transformed into the \(2a\,\times \,\sqrt{3}a\) orthorhombic phase when heated in air. The scholars found that the orthorhombic phase showed insulating electron transport, whereas the hexagonal phase showed metallic transport. Interestingly, the temperature dependence of the thermopower of Ca1/3CoO2 epitaxial films was similar, independent of the crystallographic phases.
Sr1/3CoO2
The most serious drawback of Na3/4CoO2 is its low chemical stability against water. Na3/4CoO2 is easily decomposed into insulating Co(OH)2 under high-humidity conditions (temperature, 80 °C; humidity, ~80%) because Na+ ions can easily dissolve in water. To address this issue, Sugiura and coworkers hypothesized that modification of the chemical composition improves the chemical stability without degrading the thermoelectric performance.
In 2002, Ishikawa et al. reported that the thermoelectric properties of the Sr1/3CoO2 ceramic synthesized by sintering at 400 °C were quite low relative to those of Na3/4CoO2. Although a high-density and single-crystal Sr1/3CoO2 ceramics are preferable for clarifying the intrinsic properties, this process is extremely difficult because the phase transition of Sr1/3CoO2 occurs at a relatively low temperature (~400 °C). To examine the intrinsic thermoelectric properties of Sr1/3CoO2, Sugiura and coworkers fabricated high-quality epitaxial films of Sr1/3CoO2 because epitaxial films generally exhibit intrinsic carrier transport properties, similar to those of bulk single crystals. The Sr1/3CoO2 epitaxial films exhibits better chemical stability than Na3/4CoO2 while retaining good thermoelectric properties.
Ba1/3CoO2
Recently, we found a reliable high-ZT thermoelectric oxide, Ba1/3CoO2. The crystal structure and electrical properties of the Ba1/3CoO2 epitaxial films were maintained to 600 °C. The power factor gradually increased to ~1.2 mW m−1 K−2, and the thermal conductivity gradually decreased to ~1.9 W m−1 K−1 with increasing temperature to 600 °C. Consequently, the ZT reached ~0.55 at 600 °C in air, which was the highest value among oxides and comparable to those of p-type PbTe and p-type SiGe.
Notably, initial investigations on BaxCoO2-based thermoelectric materials have been conducted by Liu et al.79,80 The scholars fabricated BaxCoO2 (x = 0.19, 0.28, 0.30, 0.33) ceramics by using BaxCoO2 powders synthesized through the ion-exchange technique from Na0.7CoO2. The researchers measured the thermoelectric properties to 800 K and found that ZT values ranging from 0.14 to 0.21 were dependent on Ba content. In their research, thermal conductivity did not experience effective suppression from polycrystalline grain boundaries, while electron transport properties deteriorated substantially. Therefore, high-quality epitaxial films are ideal for elucidating the intrinsic properties of BaxCoO2-based thermoelectric materials, which is essential for developing high-performance thermoelectric oxides.
Thermoelectric properties of A xCoO2 (A x = Na3/4, Ca1/3, Sr1/3, and Ba1/3) epitaxial films
Systematic investigations on the thermoelectric properties of AxCoO2 (Ax = Na3/4, Ca1/3, Sr1/3, and Ba1/3) started from our findings that heavy ion substitution at the A-site of AxCoO2 effectively reduces the in-plane thermal conductivity81. By fabricating AxCoO2 (Ax = Li1, Na0.75, Ca0.33, Sr0.33, La0.3) epitaxial films on (0001) α-Al2O3 substrates by conducting R-SPE and an ion exchange process, we clarify the A-site ion mass-dependent thermal conductivity of AxCoO250,51. As shown in Fig. 4, the in-plane thermal conductivity (κ||) obviously decreases with the A-site ion mass due to the mismatch of the impedance between the cation layer and CoO2 layers. This impedance hinders coupling of the vibrational modes, while the cross-plane thermal conductivity (κ⊥) mainly depends on the interfacial scattering.
As the mass of the A-site ions increases, the in-plane thermal conductivity (κ||) and cross-plane thermal conductivity (κ⊥) show decreasing tendencies. The inset shows a schematic illustration of the phonon propagation of AxCoO2. Heavy ions display a stronger thermal conductivity suppression effect than light ions. This figure was reproduced with permission81.
By conducting heavy ion substitution to reduce thermal conductivity, we further fabricate AxCoO2 (Ax = Na3/4, Ca1/3, Sr1/3, and Ba1/3) epitaxial films on (0001) α-Al2O3 and (111) YSZ substrates and compare their room temperature thermoelectric properties82. Fig. 5 presents a summary of the room-temperature electrical conductivity (σip), thermopower (Sip), power factor (PFip), thermal conductivity (κip) and figure of merit (ZTip) values along the in-plane direction of AxCoO2 epitaxial films. The electrical conductivity and thermopower values of all the films show stable changing patterns, resulting in a consistent power factor (Fig. 5a–c). This consistent value suggests perfect electron–phonon decoupling between the A-site ion layer and CoO2 layer, where ion substitution has almost no effect on the electrical conductivity of the CoO2 layers. Moreover, the thermal conductivity along the layered direction decreases with the atomic mass of Ax, thereby enhancing the ZT value (Fig. 5d, e). The highest ZT value of ~0.11 can be obtained in the Ba1/3CoO2 epitaxial film, reaching a peak value among layered cobalt oxides. In this research, the in-plane thermal conductivity has been deduced based on the experimental results of cross-plane thermal conductivities for differently oriented epitaxial films by varying the substrate orientations. In our latest report, we have directly confirmed the in-plane thermal conductivity through AC calorimetric measurements by using a freestanding Ba1/3CoO2 single-crystalline film, yielding a consistent result83.
a In-plane electrical conductivity (σip), b in-plane thermopower (Sip), c in-plane power factor (PFip), d in-plane and out-of-plane thermal conductivity (κip, κop), and e in-plane figure of merit (ZTip) as a function of the atomic mass Ax in AxCoO2. The κip shows a clear decreasing tendency, whereas the PF shows a slight increasing tendency. These trends result in an increasing tendency of ZT. ZT reached 0.11 when Ax = Ba1/3. This figure was reproduced with permission82.
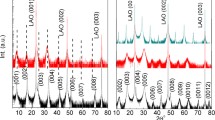
As an emerging candidate for high-performance oxide-based thermoelectric materials, Ba1/3CoO2 has promising prospects in applications at elevated temperatures. To elucidate the high-temperature thermoelectric performance, we further conducted high-temperature characterizations of AxCoO2 epitaxial films54. First, the thermal stabilities of the Na3/4CoO2, Ca1/3CoO2, Sr1/3CoO2, and Ba1/3CoO2 epitaxial films were tested by annealing at an elevated temperature for 0.5 h in air. Figure 6 shows the room temperature XRD patterns after heat treatment. The 0002 Na3/4CoO2 diffraction peak shrinks above 450 °C, whereas the 111 Co3O4 peak appears due to the evaporation of Na. In contrast, the 0002 Ca1/3CoO2, 0002 Sr1/3CoO2, and 0002 Ba1/3CoO2 peaks appear below 650 °C (Fig. 6a). However, the in-plane XRD patterns (Fig. 6b) demonstrate that a phase transition from hexagonal to orthorhombic occurs in the Ca1/3CoO2 film when the annealing temperature is above 200 °C72. The Sr1/3CoO2 film shows a hexagonal–orthorhombic hybridized phase below 450 °C and a single orthorhombic phase above ~450 °C. Only the Ba1/3CoO2 film can maintain a stable phase composition to 600 °C, which suggests a strong thermal robustness and a high potential for high-temperature Ba1/3CoO2 applications. We have confirmed a similar temperature-dependent behavior from the resistivity variation after heat treatment.
a Out-of-plane XRD patterns measured at room temperature after each annealing step. The 0002 Na3/4CoO2 diffraction peak intensity decreases above 450 °C due to decomposition into Co3O4, whereas those for Ca1/3CoO2, Sr1/3CoO2, and Ba1/3CoO2 remain stable to 650 °C. b In-plane XRD patterns measured at room temperature after each annealing step. The 1/3 and 2/3 diffraction peaks of the Ca1/3CoO2 film disappear at approximately 200 °C, and the 1/2 diffraction peak appears above 200 °C. The 1/3 and 2/3 diffraction peaks of the Sr1/3CoO2 film disappear at approximately 450 °C, and the 1/2 diffraction peak appears above 450 °C. The 1/3 and 2/3 diffraction peaks of the Ba1/3CoO2 film are stable to 600 °C. This figure was reproduced with permission54.
Finally, we calculated the temperature-dependent ZT of AxCoO2 epitaxial films. The ZT values increase with temperature for all films (Fig. 7a). Due to the strongest thermal robustness, the Ba1/3CoO2 epitaxial film displays the highest ZT of ~0.55 at 600 °C, which is higher than those of the Ca1/3CoO2 and Sr1/3CoO2 films. This high ZT value of Ba1/3CoO2 is reproducible and reliable. This value is comparable to those of p-type PbTe and p-type SiGe, indicating that Ba1/3CoO2 is a suitable candidate for high-temperature thermoelectric applications (Fig. 7b).
a Comparison among the four AxCoO2 (A = Na3/4, Ca1/3, Sr1/3, and Ba1/3) films. In all cases, the ZT values increase as the temperature increases. The Ba1/3CoO2 epitaxial film exhibits the highest ZT among the four AxCoO2 epitaxial films and reaches a ZT value of ~0.55 at 600 °C. b Comparison against commercially available p-type thermoelectric materials. ZT of the Ba1/3CoO2 epitaxial film at 600 °C is comparable to the values of p-type PbTe and p-type SiGe. This figure was reproduced with permission54.
Summary and prospects
We have reviewed the thermoelectric properties of representative layered cobalt oxides: AxCoO2 (A = Na, Ca, Sr, and Ba) and Ca3Co4O9. Although several high-ZT thermoelectric oxides (ZT > 1) have been reported thus far, their reliability is low due to a lack of careful observation of their stabilities at elevated temperatures. We have explained that Ba1/3CoO2 is stable in air even at 600 °C and exhibits a high ZT value of 0.55, which is comparable to p-type PbTe. Bulk crystals (single crystal and sintered) are essential for incorporating Ba1/3CoO2 into thermoelectric conversion elements. To date, we are researching the growth of large single crystals and are proceeding with the production of sintered bodies. Moreover, better thermoelectric performance may be realized by optimizing the compositions and nanostructures of these crystals.
References
Rowe, D. M. CRC Handbook of TheThe authors declare no competing interestsrmoelectrics. (CRC Press, 1995).
Goldsmid, H. J. Introduction to Thermoelectricity. (Springer, 2010).
Seebeck, T. J. Abh. K. Akad. Wiss. 265 (1823).
Tritt, T. M. & Subramanian, M. A. Thermoelectric materials, phenomena, and applications: A bird’s eye view. MRS Bull. 31, 188–194 (2006).
Snyder, G. J. & Toberer, E. S. Complex thermoelectric materials. Nat. Mater. 7, 105–114 (2008).
Venkatasubramanian, R., Siivola, E., Colpitts, T. & O’Quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 413, 597–602 (2001).
Zhao, L. D. et al. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 508, 373 (2014).
Rhyee, J. S. et al. Peierls distortion as a route to high thermoelectric performance in In4Se3–δ crystals. Nature 459, 965–968 (2009).
Snyder, G. J., Christensen, M., Nishibori, E., Caillat, T. & Iversen, B. B. Disordered zinc in Zn4Sb3 with phonon-glass and electron-crystal thermoelectric properties. Nat. Mater. 3, 458–463 (2004).
Zhao, L. D. et al. Ultrahigh power factor and thermoelectric performance in hole-doped single-crystal SnSe. Science 351, 141–144 (2016).
Hsu, K. F. et al. Cubic AgPbmSbTe2+m: Bulk thermoelectric materials with high figure of merit. Science 303, 818–821 (2004).
Chung, D. Y. et al. CsBi4Te6: A high-performance thermoelectric material for low-temperature applications. Science 287, 1024–1027 (2000).
Kim, S. I. et al. Dense dislocation arrays embedded in grain boundaries for high-performance bulk thermoelectrics. Science 348, 109–114 (2015).
Jiang, B. et al. High-entropy-stabilized chalcogenides with high thermoelectric performance. Science 371, 830–834 (2021).
Jiang, B. et al. High figure-of-merit and power generation in high-entropy GeTe-based thermoelectrics. Science 377, 208–213 (2022).
Zhao, L.-D. et al. BiCuSeO oxyselenides: new promising thermoelectric materials. Energy Environ. Sci. 7, 2900–2924 (2014).
Liu, Y. et al. Remarkable Enhancement in Thermoelectric Performance of BiCuSeO by Cu Deficiencies. J. Am. Chem. Soc. 133, 20112–20115 (2011).
Yang, J. H. & Stabler, F. R. Automotive Applications of Thermoelectric Materials. J. Electron. Mater. 38, 1245–1251 (2009).
Korzhuev, M. A. & Katin, I. V. On the Placement of Thermoelectric Generators in Automobiles. J. Electron. Mater. 39, 1390–1394 (2010).
Kim, S. K. et al. Thermoelectric Power Generation System for Future Hybrid Vehicles Using Hot Exhaust Gas. J. Electron. Mater. 40, 778–783 (2011).
Vining, C. B. An inconvenient truth about thermoelectrics. Nat. Mater. 8, 83–85 (2009).
Koumoto, K. et al. Thermoelectric Ceramics for Energy Harvesting. J. Am. Ceram. Soc. 96, 1–23 (2013).
Koumoto, K., Terasaki, I. & Funahashi, R. Complex oxide materials for potential thermoelectric applications. MRS Bull. 31, 206–210 (2006).
Koumoto, K., Wang, Y. F., Zhang, R. Z., Kosuga, A. & Funahashi, R. Oxide ‘Thermoelectric Materials: A Nanostructuring Approach. Annu. Rev. Mater. Res. 40, 363–394 (2010).
Hogarth, C. A. & Andrews, J. P. Variation with Oxygen Pressure of the Thermoelectric Power of Cadmium Oxide. Philos. Mag. 40, 273–282 (1949).
Parravano, G. Thermoelectric Behavior of Nickel Oxide. J. Chem. Phys. 23, 5–10 (1955).
Hutson, A. R. Electronic Properties of ZnO. J. Phys. Chem. Solids 8, 467–472 (1959).
Arvin, M. J. Electrical Conductivity and Thermoelectric Power of Indium Oxide. J. Phys. Chem. Solids 23, 1681 (1962).
Frederikse, H. P. R., Thurber, W. R. & Hosler, W. R. Electronic transport in strontium titanate. Phys. Rev. 134, A442 (1964).
Thurber, W. R. & Mante, A. J. H. Thermal Conductivity and Thermoelectric Power of Rutile (TiO2). Phys. Rev. 139, 1655 (1965).
Marley, J. A. & Dockerty, R. C. Electrical Properties of Stannic Oxide Single Crystals. Phys. Rev. 140, A304 (1965).
Young, A. P. & Schwartz, C. M. Electrical Conductivity and Thermoelectric Power of Cu2O. J. Phys. Chem. Solids 30, 249-& (1969).
Griffiths, B. A., Elwell, D. & Parker, R. Thermoelectric Power of System NiFe2O4–Fe3O4. Philos. Mag. 22, 163 (1970).
Bednorz, J. G. & Muller, K. A. Possible High-Tc Superconductivity in the Ba–La–Cu–O System. Z. Phys. B Con. Mat. 64, 189–193 (1986).
Cooper, J. R., Alavi, B., Zhou, L. W., Beyermann, W. P. & Gruner, G. Thermoelectric-Power of Some High-Tc Oxides. Phys. Rev. B 35, 8794–8796 (1987).
Chen, J. T., Mcewan, C. J., Wenger, L. E. & Logothetis, E. M. Determination of Charge-Carriers in Superconducting La-Ba-Cu-O by Thermoelectric Measurements. Phys. Rev. B 35, 7124–7125 (1987).
Lee, S. C. et al. Thermoelectric-Power and Superconducting Properties of YBa2Cu3O7-δ and RBa2Cu3O7-δ. Phys. Rev. B 37, 2285–2288 (1988).
Mitra, N. et al. Thermoelectric-Power of the Tl-Ca-Ba-Cu-O Superconductor. Phys. Rev. B 38, 7064–7066 (1988).
Ohtaki, M., Koga, H., Tokunaga, T., Eguchi, K. & Arai, H. Electrical-Transport Properties and High-Temperature Thermoelectric Performance of (Ca0.9M0.1)MnO3 (M = Y, La, Ce, Sm, In, Sn, Sb, Pb, Bi). J. Solid State Chem. 120, 105–111 (1995).
Ohtaki, M., Tsubota, T., Eguchi, K. & Arai, H. High-temperature thermoelectric properties of (Zn1–xAlx)O. J. Appl. Phys 79, 1816–1818 (1996).
Terasaki, I., Sasago, Y. & Uchinokura, K. Large thermoelectric power in NaCo2O4 single crystals. Phys. Rev. B 56, 12685–12687 (1997).
Masset, A. C. et al. Misfit-layered cobaltite with an anisotropic giant magnetoresistance: Ca3Co4O9. Phys. Rev. B 62, 166–175 (2000).
Funahashi, R. et al. An oxide single crystal with high thermoelectric performance in air. Jpn. J. Appl. Phys. 39, L1127–L1129 (2000).
Okuda, T., Nakanishi, K., Miyasaka, S. & Tokura, Y. Large thermoelectric response of metallic perovskites: Sr1–xLaxTiO3 (0 ≤ x ≤ 0.1). Phys. Rev. B 63, 113104 (2001).
Ohta, S., Nomura, T., Ohta, H. & Koumoto, K. High-temperature carrier transport and thermoelectric properties of heavily La- or Nb-doped SrTiO3 single crystals. J. Appl. Phys. 97, 034106 (2005).
Ohta, S. et al. Large thermoelectric performance of heavily Nb-doped SrTiO3 epitaxial film at high temperature. Appl. Phys. Lett. 87, 092108 (2005).
Fergus, J. W. Oxide materials for high temperature thermoelectric energy conversion. J. Eur. Ceram. Soc. 32, 525–540 (2012).
Acharya, M., Jana, S. S., Ranjan, M. & Maiti, T. High performance (ZT > 1) n-type oxide thermoelectric composites from earth abundant materials. Nano Energy 84, 105905 (2021).
Biswas, S. et al. Selective Enhancement in Phonon Scattering Leads to a High Thermoelectric Figure-of-Merit in Graphene Oxide-Encapsulated ZnO Nanocomposites. ACS Appl. Mater. Interfaces 13, 23771–23786 (2021).
Ohta, H. et al. Reactive solid-phase epitaxial growth of NaxCoO2 (x ~0.83) via lateral diffusion of Na into a cobalt oxide epitaxial layer. Cryst. Growth Des. 5, 25–28 (2005).
Ohta, H. et al. Surface modification of glass substrates for oxide heteroepitaxy: Pasteable three-dimensionally oriented layered oxide thin films. Adv. Mater. 18, 1649 (2006).
Sugiura, K. et al. Fabrication and thermoelectric properties of layered cobaltite, γ-Sr0.32Na0.21CoO2 epitaxial films. Appl. Phys. Lett. 88, 082109 (2006).
Sugiura, K. et al. Thermoelectric properties of the layered cobaltite Ca3Co4O9 epitaxial films fabricated by topotactic ion-exchange method. Mater. Trans. 48, 2104–2107 (2007).
Zhang, X. et al. Ba1/3CoO2: A Thermoelectric Oxide Showing a Reliable ZT of ~0.55 at 600 °C in Air. ACS Appl. Mater. Interfaces 14, 33355–33360 (2022).
Sugiura, K. et al. High electrical conductivity of layered cobalt oxide Ca3Co4O9 epitaxial films grown by topotactic ion-exchange method. Appl. Phys. Lett. 89, 032111 (2006).
Kurita, D., Ohta, S., Sugiura, K., Ohta, H. & Koumoto, K. Carrier generation and transport properties of heavily Nb-doped anatase TiO2 epitaxial films at high temperatures. J. Appl. Phys. 100, 096105 (2006).
Lee, K. H., Ishizaki, A., Kim, S. W., Ohta, H. & Koumoto, K. Preparation andthermoelectric properties of heavily Nb-doped SrO(SrTiO3)1 epitaxial films. J. Appl. Phys. 102, 033702 (2007).
Singh, D. J. Electronic structure of NaCo2O4. Phys. Rev. B 61, 13397–13402 (2000).
Koshibae, W. & Maekawa, S. Electronic state of a CoO layer with hexagonal structure: A Kagome lattice structure in a triangular lattice. Phys. Rev. Lett. 91, 257003 (2003).
Koshibae, W. & Maekawa, S. Effects of spin and orbital degeneracy on the thermopower of strongly correlated systems. Phys. Rev. Lett. 87, 236603 (2001).
Wang, Y. Y., Rogado, N. S., Cava, R. J. & Ong, N. P. Spin entropy as the likely source of enhanced thermopower in NaxCo2O4. Nature 423, 425–428 (2003).
Lee, M. et al. Large enhancement of the thermopower in NaxCoO2 at high Na doping. Nat. Mater. 5, 537–540 (2006).
Fujita, K., Mochida, T. & Nakamura, K. High-temperature thermoelectric properties of NaxCoO2–δ single crystals. Jpn. J. Appl. Phys. 40, 4644–4647 (2001).
Takada, K. et al. Superconductivity in two-dimensional CoO2 layers. Nature 422, 53–55 (2003).
Venimadhav, A. et al. Structural and transport properties of epitaxial NaxCoO2 thin films. Appl. Phys. Lett. 87, 172104 (2005).
Chang, W. J. et al. Fabrication and low temperature thermoelectric properties of NaxCoO2 (x = 0.68 and 0.75) epitaxial films by the reactive solid-phase epitaxy. Appl. Phys. Lett. 90, 061917 (2007).
Sugiura, K. et al. Anisotropic carrier transport properties in layered cobaltate epitaxial films grown by reactive solid-phase epitaxy. Appl. Phys. Lett. 94, 152105 (2009).
Krockenberger, Y. et al. Epitaxial growth of NaxCoO2 thin films by pulsed-laser deposition. Appl. Phys. Lett. 86, 191913 (2005).
Brinks, P. et al. Enhanced Thermoelectric Power Factor of NaxCoO2 Thin Films by Structural Engineering. Adv. Energy Mater. 4, 201301927 (2014).
Lee, M. et al. Enhancement of the thermopower in NaxCoO2 in the large-x regime (x ≥ 0.75). Phys. B 403, 1564–1568 (2008).
Mizutani, A., Sugiura, K., Ohta, H. & Koumoto, K. Epitaxial film growth of LixCoO2 (0.6 ≤ x ≤ 0.9) via topotactic ion exchange of Na0.8CoO2. Cryst. Growth Des. 8, 755 (2008).
Sugiura, K. et al. Structural Transformation of Ca-Arrangements and Carrier Transport Properties in Ca0.33CoO2 Epitaxial Films. Appl. Phys. Express 2, 035503 (2009).
Sugiura, K. et al. Epitaxial film growth and superconducting behavior of sodium-cobalt oxyhydrate, NaxCoO2-yH2O (x ~0.3, y ~1.3). Inorg. Chem. 45, 1894–1896 (2006).
Cushing, B. L., Falster, A. U., Simmons, W. B. & Wiley, J. B. A multivalent ion exchange route to lamellar calcium cobalt oxides, CaxCoO2 (x ≤ 0.5). Chem Commun 23, 2635–2636 (1996).
Cushing, B. L. & Wiley, J. B. Topotactic routes to layered calcium cobalt oxides. J. Solid State Chem. 141, 385–391 (1998).
Yang, H. X. et al. Structural properties and cation ordering in layered hexagonal CaxCoO2. Phys. Rev. B 73 (2006).
Huang, R. et al. Direct observations of Ca ordering in Ca0.33CoO2 thin films with different superstructures. Appl. Phys. Lett. 93, 014109 (2008).
Huang, R. et al. Microstructure evolution of Ca0.33CoO2 thin films investigated by high-angle annular dark-field scanning transmission electron microscopy. J. Mater. Res. 24, 279–287 (2009).
Liu, J., Huang, X., Yang, D., Xu, G. & Chen, L. Synthesis and physical properties of layered BaxCoO2. Dalton Trans. 43, 15414 (2014).
Liu, J., Huang, X., Yang, D., Wan, S. & Xu, G. High-temperature thermoelectric properties of layered BaxCoO2. Scr. Mater. 100, 63–65 (2015).
Cho, H. J., Takashima, Y., Nezu, Y., Onozato, T. & Ohta, H. Anisotropic Heat Conduction in Ion-Substituted Layered Cobalt Oxides. Adv. Mater. Interfaces 7, 1901816 (2020).
Takashima, Y. et al. Layered Cobalt Oxide Epitaxial Films Exhibiting Thermoelectric ZT = 0.11 at Room Temperature. J. Mater. Chem. A 9, 274–280 (2021).
Kang, K. et al. Fabrication and Thermoelectric Properties of Freestanding Ba1/3CoO2 Single-Crystalline Films. ACS Appl. Electron. Mater. 5, 5749–5754 (2023).
Acknowledgements
The authors thank Drs. Xi Zhang, Hai Jun Cho, Akihiro Tsuruta, Masashi Mikami, and Profs. Yuichi Ikuhara and Bin Feng, and many students at Nagoya University and Hokkaido University for their support. Y.Z. acknowledges the National Natural Science Foundation of China (Grant No. 52202242), the Ministry of Science and Technology of PRC (Grant No. G2022014136L), the National Science Foundation of the Jiangsu Higher Education Institutions of China (Grant No. 22KJB430002), and the Start-Up Fund of Jiangsu University (Grant No. 5501310015). H.O. was supported by Grants-in-Aid for Innovative Areas (19H05791) and Scientific Research A (22H00253) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Contributions
H.O. conceived the theme and directed the project. All the authors contributed to writing, reviewing, and editing the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Ohta, H. Recent progress in thermoelectric layered cobalt oxide thin films. NPG Asia Mater 15, 67 (2023). https://doi.org/10.1038/s41427-023-00520-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41427-023-00520-w
- Springer Japan KK