Abstract
Chimeric antigen receptor engineered T (CAR T) cell therapy has developed rapidly in recent years, leading to profound developments in oncology, especially for hematologic malignancies. However, given the pressure of immunosuppressive tumor microenvironments, antigen escape, and diverse other factors, its application in solid tumors is less developed. Urinary system tumors are relatively common, accounting for approximately 24% of all new cancers in the United States. CAR T cells have great potential for urinary system tumors. This review summarizes the latest developments of CAR T cell therapy in urinary system tumors, including kidney cancer, bladder cancer, and prostate cancer, and also outlines the various CAR T cell generations and their pathways and targets that have been developed thus far. Finally, the current advantages, problems, and side effects of CAR T cell therapy are discussed in depth, and potential future developments are proposed in view of current shortcomings.
Similar content being viewed by others
Facts
-
The latest progress of CAR T cell therapy in urinary system tumors.
-
The advantages, problems, and side effects of CAR T cell therapy in urinary system tumors.
-
Mechanism and clinical application of novel immunotherapies in urinary system tumors.
-
The various studies of CAR T generations and their pathways and targets in urinary system tumors.
Open questions
-
How to ensure the specificity and personalization of CAR T cell therapy in patients with urinary system tumors?
-
What are the possible future directions of CAR T cell therapy in urinary system tumors?
-
How can the safety and efficacy of CAR T cell therapy be improved through more extensive clinical trials and long-term follow-up?
Introduction
According to statistics reported in the 2024 edition of CA, a Cancer Journal for Clinicians, urinary system tumors are among the most common malignancies in the United States, accounting for ~24% of all new cancers [1], with a rising incidence recently. In addition to surgery, some traditional treatment options, such as chemotherapy for urothelial carcinoma (UC) or androgen deprivation therapy (ADT) for prostate cancer (PCa), result in suboptimal clinical outcomes. For example, even after radical treatment of PCa, recurrence remains possible, especially for high-risk locally progressive PCa, which has a biochemical recurrence rate of up to 50% within 3 years after surgery [2]. As a result, immunotherapy approaches have gained increased attention in research.
At present, immunotherapy has been applied to multiple types of urinary tumors. In high-risk, non-muscle invasive bladder cancer (NMIBC), Bacille Calmette-Guérin (BCG) has been used to activate the mucosal immune system locally, stimulating an inflammatory response, which reduces recurrence risk [3]. In renal cell carcinoma (RCC), systemic interleukin-2 (IL-2) treatment activates the immune system, mainly by stimulating natural killer (NK) cells and T cells to kill tumor cells [4]. In addition, immune checkpoint inhibitors (ICIs), such as those impacting programmed death 1 (PD-1) and programmed death-ligand 1 (PD-L1), have been used increasingly to treat urinary system tumors [5].
More recently, chimeric antigen receptor engineered T cell (CAR T) therapy, a novel genetically based immunotherapy method, has been applied increasingly in malignancy [6]. CAR T cell therapy has achieved impressive results in treating a variety of hematologic malignancies, including lymphoma, leukemia, and multiple myeloma [7]. The availability of tumor-associated antigens in urinary system tumors, such as prostate stem cell antigen (PSCA), prostate specific membrane antigen (PSMA), and epidermal growth factor receptor (EGFR), makes CAR T cell therapy possible for urinary system tumors. However, the immunosuppressive tumor microenvironment (TME), antigen escape, tumor heterogeneity, and the diminished proliferation capacity, insufficient migration ability, and short survival time of CAR T cells, limit their impact for solid tumors [8].
In this manuscript, we systematically review the research progress, potential value, and difficulties of utilizing CAR T cells to treat urinary system tumors, to provide novel approaches for urinary system tumor treatment.
CAR T cell therapy in tumor immunotherapy
Immunotherapy recently has developed rapidly for both cancer and regenerative medicine. In oncology, multiple clinical trials of autologous engineered T cells are underway, with the goal of improving anti-tumor immune responses, and this approach may change cancer treatment standards [9].
CAR T cell therapy is one type of adoptive cell therapy, with the major advantages of not being restricted by the major histocompatibility complex, bypassing antigen presentation, priming and activating T cells, which directly attack cancer cells, killing them [10]. CAR T cell therapy has proven efficacious for treating hematological malignancies, especially B-cell malignancies [11]. However, for solid tumors, such as UC, CAR T cell therapy requires further development before it becomes widespread.
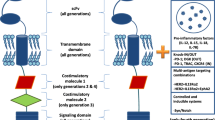
The engineered receptors of CAR T cells consist of three major components: an extracellular domain specific for tumor antigens [single chain fragment variable (scFv) fragment], a transmembrane domain, and an intracellular signaling domain that activates T lymphocyte responses (including costimulatory molecules) [12] (Fig. 1). Initial in vivo testing of the first-generation of CAR T cells, which had basic architecture, revealed poor activation and persistence of the engineered T cells [13]. Further studies revealed that full T cell activation requires the first signal be transmitted upon binding of the extracellular antigen binding domain to the antigen, as well as a second signal transmitted by the binding of costimulatory molecule receptors to their ligands, but these ligands are usually not expressed on the tumor cell surface. Enhanced CAR T cells have come from optimizing T cell proliferation and tumor kill by including intracellular costimulatory molecules in the receptor. The design structure of the first-generation of CAR T cells was relatively simple, with the CD3ζ costimulatory domain as the only intracellular domain included, with none of the costimulatory signals necessary to fully activate the CAR T cells, and their effect in clinical trials was suboptimal. Based on the design of the first generation of CAR T cells, the costimulatory domains CD28 or 4-1BB were introduced in the second generation of CAR T cells, significantly improving their immune activation and durability [14, 15]. CD28 and 4-1BB exhibited different anti-tumor properties. CD28-activated T cells had a strong transient mortality, while 4-1BB-activated T cells had better anti-tumor persistence [16].
The first-generation of CAR T had only CD3ζ chains. The second-generation of CAR T added co-stimulatory domain 1 (CD28 or 4-1BB) on the basis of the first-generation. The third-generation of CAR T added co-stimulatory domain 2 (CD28, 4-1BB, CD27, OX40, and ICOS) on the basis of the second-generation. The fourth-generation of CAR T added co-expressed cytokines on the basis of the second-generation. The next-generation of CAR T was also based on the second-generation, with the addition of co-stimulatory domains for the activation of other signaling pathways, such as IL-2Rβ-JAK/STAT pathway.
Expanding on these results, the third-generation of CAR T cell receptors contained two co-stimulatory domains, usually CD28 and 4-1BB, but also sometimes CD27, OX40, or ICOS [17,18,19,20]. In preclinical models, third-generation anti-CD19 CAR T cells with both CD28 and 4-1BB co-stimulatory domains provided better therapeutic outcomes than did second-generation CAR T cells. These cells showed balanced tumor-killing and increased persistence, with an elevated CD8/CD4 ratio and decreased exhaustion [21]. However, there are significant differences in cytokine production and anti-tumor activity in these third-generation of CAR T cells compared with the second-generation. This difference may be due to impaired mitochondrial function caused by over-stimulation of CAR T cells, leading to CAR T cell exhaustion [22].
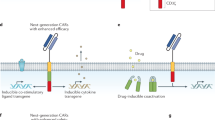
Based on these observations, the fourth-generation of CAR T cells added intracellular co-expressed cytokines (IL-7, IL-12, IL-18, IL-21, CCL19, et al.) to positively regulate CAR T cells [23,24,25,26,27]. These have an additional inducible domain responsible for transgenic cytokine release upon CD3ζ-containing CARs engaging with their specific target. The activation of these receptors leads to secretion of the cytokine to improve CAR T cell persistence and antitumor effects [28, 29]. For example, IL-12 is eventually produced and released in target-initiated CD3ζ-containing CAR T cells. IL-12 promotes T cell activation, modulates the immune and vascular tumor environment, and recruits additional immune cells to attack tumor cells not recognized by the CAR T cells [30, 31]. Therefore, the fourth-generation of CAR T cells are also termed T cells redirected for universal cytokine-mediated killing (TRUCKs).
The fifth-generation of CAR T cells, also based on the second-generation, have added an additional intracellular IL-2 receptor domain that enables antigen-dependent activation of the JAK/STAT pathway [32, 33]. This induces the CAR T cells to produce memory T cells, facilitating a more durable long-term response. The fifth-generation of CAR T cells also have additional intracellular domains with truncated cytokine receptor fragments, such as IL-2R chain fragments, containing transcription factor binding motifs. Therefore, these secreted signals not only drive CAR T cell activity persistence and memory T cell generation, but also reactivate and stimulate the immune system [32] (Fig. 1). Naeem et al. [34] also tried to circumvent other challenges with these cells by using clustered regularly interspaced short palindromic repeats (CRISPR) genome editing technology to inhibit dominant negative receptors (PD-1, TGF-β, and B2M), reduce the risk of cytokine release syndrome (CRS), and regulate CAR T cell function in the TME.
CAR T cell therapy in urologic neoplasms
Given the impressive therapeutic effects of CAR T cells for multiple hematological malignancies [7], investigations related to urinary system tumors recently have been conducted with increasing frequency. CAR T cell therapy is made possible by the expression of tumor-associated antigens in urinary system tumors, permitting a novel approach for the treatment of urinary system tumors, including RCC, PCa, and bladder cancer (BCa) (Table 1).
CAR T cell therapy in RCC
In the United States, RCC is twice as common in men as in women, with ~81,610 new cases and 14,390 deaths expected in 2024 [1]. The incidence of RCC has recently stabilized, rather than improving, despite advances in surgical treatment, such as robot-assisted surgical treatment, suggesting the need for new treatments [35, 36]. Since RCC does not respond to radiotherapy and chemotherapy, IL-2 and interferon-α are the traditional non-specific immunotherapies used [37, 38], but multiple studies have questioned their effectiveness [39]. More recently, for advanced RCC, the current standard first-line immunotherapies include dual ICIs and ICIs combined with vascular endothelial growth factor receptor tyrosine kinase inhibitors [40]. Therefore, new immunotherapy has become an area of active interest.
CAR T cell therapy has been studied in RCC. Suarez et al. [41] developed CAR T cells targeting human carbonic anhydrase IX (CAIX) to secrete human anti-PD-L1 antibodies at the tumor sites (Fig. 2A). In a humanized mouse model of RCC, tumor suppression was evident after treatment with CAIX-CAR-T cells. Panowski et al. generated a panel of anti-CD70 scFv-based CAR T cells [42]. In their study, CD70 CAR T cells showed potent anti-tumor activity against RCC cell lines and in patient-derived mouse xenograft models (Fig. 2B). In addition, Mori et al. [43] found that cellular-mesenchymal epithelial transition factor (c-Met) could be used as a therapeutic target for RCC, creating anti-human c-Met targeted CAR T cells (Fig. 2C) and studying their anti-tumor effects in an orthotopic mouse model. They found that anti-c-Met CAR T cells could infiltrate tumor tissues and inhibit tumor growth.
A The fourth-generation of CAR T (CD28) targeted CAIX to secrete anti-PD-L1 antibody to block T cells exhaustion in RCC. B The second-generation of CAR T (CD27) targeted CD70 in RCC. C The third-generation of CAR T (CD28 and 4-1BB) targeted c-Met in RCC. D The second-generation of CAR T (4-1BB) targeted CAIX in RCC. E The second-generation of CAR T (CD28) targeted PD-1 in BCa. F The second-generation of CAR T (CD28) targeted MUC1 in BCa. G The second-generation of CAR T (CD28) targeted EGFR in BCa. H The second-generation of CAR T (4-1BB) targeted PSMA in PCa. I The second-generation of CAR T (CD28) targeted PSMA in PCa. J The fourth-generation of CAR T (IL-23mAb-PSMA-CARs) was more effective in PCa. K The second-generation of CAR T (4-1BB) targeted PSCA in PCa. (L). The second-generation of CAR T (4-1BB) targeted STEAP1 in PCa. M The second-generation of CAR T (CD28) targeted B7-H3 in PCa.
Multiple studies have found that CAR T cells combined with targeted drugs may have an improved anti-tumor effect compared with CAR T cells alone. Li et al. [44] constructed second-generation CAR T cells that recognize the human RCC-specific antigen, CAIX (Fig. 2D). In their investigation, the combination of CAIX-CAR-T and sunitinib had a synergistic effect on lung metastasis in a mouse model of human RCC. The CAIX-CAR-T cells in the combined treatment group had increased proliferation and invasion compared to that observed in the single treatment group. Mori et al. [43] had similar results. After the construction of c-Met-specific CAR T cells, they studied these in combination with axitinib, finding that axitinib synergistically enhanced anti-tumor CAR T cell responses. These results suggest that the combination of CAR T cells with targeted agents may also be effective for solid tumor therapy.
CAR T cell therapy in BCa
In the United States, BCa, with ~83,190 new cases and 16,840 deaths expected in 2024, is more common in men [1], and BCa treatment is mainly based on risk classification. With the standardization of treatment, BCa prognosis has significantly improved recently. For example, NMIBC has a 5-year survival rate of up to 90%. However, outcomes for metastatic BCa (mBCa) remain particularly poor, with a 5-year survival rate of <15% [45, 46], with a median survival for various chemotherapy regimens of only 13–15 months [47, 48]. While ICIs have been considered an optional first-line treatment for patients who are not candidates for chemotherapy, the results remain unsatisfactory [49], highlighting the need for new immunotherapies. BCa expresses multiple tumor-associated antigens, suggesting favorable conditions for CAR T cell therapy.
Many pan-cancer tumor targets, including human epidermal growth factor receptor 2 (HER2), mucin 1 (MUC1), and EGFR, are also expressed at high levels in UCs and may serve as therapeutic targets [50, 51]. One current ongoing clinical trial targets HER2 (NCT03740256). However, this is a basket trial, and BCa is just one of several malignancies included. We look forward to learning the specific results in BCa. Parriott et al. [52] developed CAR T cells targeting PD-1, finding an anti-tumor effect for CHPD1-expressing T cells in a variety of isogenic mouse solid cancer models, including BCa (Fig. 2E). Yu et al. [53] produced second-generation CAR T cells targeting MUC1 in vitro (Fig. 2F), finding a significant, specific cytotoxic effect of MUC1-CAR-T on MUC1-positive tumors such as BCa, further validating the feasibility of using MUC1-CAR-T cells to treat BCa. In addition, Grunewald et al. [54] used decitabine in combination with EGFR and CD44V6-specific CAR T cells for anti-BCa studies (Fig. 2G), finding that the combination was an attractive therapeutic approach to enhance tumor-specific killing in BCa.
CAR T cell therapy in PCa
With an estimated 300,000 new cases and 35,250 deaths anticipated in the United States in 2024, PCa is the most common malignancy in men and the second leading cause of cancer mortality [1]. The 5-year survival rate of PCa reaches 98%, but in metastatic PCa (mPCa), survival drops to 30% [55]. After years of experience, PCa treatment has become standardized. PCa growth and development depend on androgens, so ADT is the cornerstone of PCa treatment. Standard treatment for localized PCa includes surgery, radiation, and active surveillance [56]. However, the poor prognosis of patients with mPCa, especially castration-resistant PCa (CRPC), remains concerning. Diverse treatment options exist for advanced PCa, including taxane chemotherapy, Sipuleucel-T, androgen receptor (AR) pathway inhibitors, and ICIs [57]. PCa is often described as a “cold” tumor because of its limited sensitivity to ICIs [58]. The non-response to ICIs spurs interest in novel immunotherapy methods, such as CAR T cell therapy.
A variety of tumor specific antigens are expressed in PCa. In addition to the well-known PSA, there are PSMA and PSCA, which may serve as targets for CAR T cell therapy. PSMA is currently the most studied target for CAR T cell therapy in PCa. For example, Shanghai Changzheng Hospital has just registered a single-center, single-arm, open-label, investigator-initiated clinical trial evaluating the safety and efficacy of enhanced autologous PSMA-specific CAR T cells to treat refractory CRPC (NCT06228404). In addition, multiple preclinical and clinical investigations found that PSMA-specific CAR-T cells have an anti-PCa effect. Kloss et al. [59] enhanced the effect of CAR T cells targeting PSMA by co-expression of a dominant-negative TGF-βRII (dnTGF-βRII), which further increased lymphocyte proliferation and cytokine secretion, leading to killing of PCa cells (Fig. 2H). Subsequently, they conducted a phase I trial (NCT03089203) consisting of 18 patients, demonstrating that treatment with TGF-β-resistant CAR T cells is feasible and generally safe [60]. Alzubi et al. [61] developed a novel D7 single-chain antibody fragment-derived anti-PSMA CAR that showed promising activity both in vitro and in vivo (Fig. 2I). In addition, the combination of PSMA-CAR-T cells and non-ablative low-dose chemotherapy, such as low-dose docetaxel, controlled tumor growth. Wang et al. [62] constructed a group of IL-23 mAb-PSMA-CARs and found that IL-23 mAb combined with PSMA-specific CAR T cells were more effective in eradicating PCa than were PSMA CAR T cells alone (Fig. 2J).
In addition to PSMA, other targets have been evaluated. Priceman et al. [63] found the intracellular co-stimulatory signaling domain can determine the sensitivity of CAR T cells to tumor antigen expression. Inclusion of a 4-1BB intracellular co-stimulatory signal domain provided better tumor killing than did a PSCA-CAR containing a CD28 co-stimulatory signal domain (Fig. 2K). A phase Ib clinical trial (NCT05805371) of PSCA-targeting CAR T cells to treat metastatic CRPC is ongoing and will evaluate this question. The six transmembrane epithelial antigen of the prostate 1 (STEAP1) can be used as a target antigen for PCa therapy [64, 65]. Bhatia et al. [66] designed a STEAP1-targeted CAR T cell therapy with high antigen specificity (Fig. 2L), finding that STEAP1 CAR T cells showed reactivity at low antigen density, anti-tumor activity in mPCa models, and safety in human STEAP1 knock-in mouse models. Furthermore, the addition of tumor-localized IL-12 in the form of a collagen binding domain-IL-12 fusion protein enhanced the anti-tumor effect of the STEAP1 CAR T cells. Zhang et al. [67] reported that B7-H3 CAR T cells combined with fractionated irradiation (FIR) was more effective than FIR or CAR T cells alone for radioresistant PCa stem cells (PCSCs) (Fig. 2M).
Advances and challenges
Multiple CAR T cell therapies have proven effective for treating even refractory B-cell malignancies, providing a new approach for cancer treatment and leading to progress for treatment of other malignancies. As mentioned earlier, tumor-associated antigens are crucial for the application of CAR T cell therapy. Tumor-related antigens commonly expressed in urinary system tumors and other solid tumors have shown good anti-tumor activity in preclinical or clinical studies, such as CAR T cells targeting PSMA, EGFR, MUC1, etc. Therefore, the use of CAR T cell therapy in urologic tumors may be more favorable, given the availability of target antigens expressed more frequently than is observed in other solid tumors.
In addition, CAR T cells still require a certain process before administration to patients, which creates challenges for delivering CAR T cell therapy in a timely fashion. However, urologic tumors progress relatively slowly compared to other malignancies, such as those of hematological, respiratory, and digestive system cancers, providing sufficient preparation time for specific and individualized CAR T cells. To shorten the time to CAR T cell infusion and reduce costs, researchers have evaluated using cells from healthy donors, that is, allogeneic T cells, to produce CAR T cells. However, two major problems exist when using allogeneic CAR T cells. First, allogeneic T cells may cause fatal graft-versus-host disease (GVHD). Second, these allogeneic T cells may be rapidly eliminated by the host immune system, limiting their effectiveness [68]. Fortunately, several studies have shown that the GVHD from CAR T cells is not a cause for concern. Studies of donor-derived CAR T cells therapy have exhibited that alloreactive T cells expressing CD28 co-stimulated CD19 CARs, thereby further significantly reducing the incidence of GVHD [69,70,71]. Further study is needed to confirm this observation.
CAR T cell therapy still has many shortcomings and faces serious challenges. Firstly, the current use of CAR T cells for solid tumors, including urological tumors, remains very limited. Secondly, from a safety perspective, the side effects continue to be significant, including CRS, immune effector cell-associated neurotoxicity syndrome (ICANS), and the resulting organ dysfunction [72].
Although CAR T cell therapy offers many advantages for urological tumors, its efficacy has not reached that of CAR T cells for hematological malignancies. In addition, the heterogeneity of the TME poses challenges for CAR T cells. The TME has multiple anti-tumor effects, most notably on T cell activity [73]. A large number of immunosuppressive cells, including regulatory T cells (Tregs), myeloid-derived suppressor cells, and tumor-associated macrophages, are usually present in the solid TME. Co-stimulatory molecules, such as CD28, may facilitate CAR T cell activation and survival at tumor sites [74, 75]. Various common cytokines also affect the efficacy of immunotherapy for urological tumors. For example, transforming growth factor-β (TGF-β), which is abundantly expressed in the TME of BCa and PCa, downregulates CD8+ T cell function and promotes Treg maturation [76]. Future investigations will examine how to overcome the inhibition of TGF-β on CAR T cells [77, 78].
Conversely, cytokines such as IL-2 and IL-12 can enhance the anti-tumor effects of CAR T cells [79]. IL-12, in particular, can alter the TME and prolong T cell survival, improving immunotherapy efficacy [30, 31, 80]. However, neither IL-2 nor IL-12 are normally present in the TME of urological tumors, but intravesical therapy with BCG after BCa surgery induces IL-2 and IL-12 in the TME, implying that CAR T cells expressing these cytokines may be advantageous for BCa [77]. Further studies evaluating the role of the TME on CAR T cells in solid tumors, especially urological tumors, are needed to improve the applicability of CAR T cells for these malignancies.
The most popular immunotherapy currently uses ICIs, which are also widely used in urological tumors and are coming to be accepted as a first-line treatment for advanced RCC and BCa [81, 82]. When ICIs prove ineffective, it is uncertain how impactful CAR T cell therapy, as another immunotherapy, will be. This question may be addressed by clinical trials that are currently underway. For example, the inclusion criteria of the clinical trials NCT05420519, targeting CD70, and NCT04969354, targeting CAIX, both had patients with advanced RCC who have failed first-line and second-line therapies (including ICIs). In addition, several preclinical and clinical trials in other tumors have shown that ICIs combined with CAR-T cell therapy can enhance anti-tumor activity, offering hope for this combination in urological tumors [83,84,85]. Ren et al. [86] also found that CAR T cells that use CRISPR to knock down the PD-1 encoding gene, PDCD1, exhibited potent anti-tumor activity both in vitro and in animal models, suggesting that such CAR T cells lacking this checkpoint receptor might be beneficial.
However, ICIs have limited utility for PCa, predominantly because of the previously-mentioned immunosuppressive TME. Beyond ongoing effort to enhance immune signals and remodel the immunosuppressive “cold” TME, the success of ICIs in combination with CAR T cells, especially PD-1 knockdown CAR T cells, provides novel approaches for improvement. Another possibility involves cancer-associated fibroblasts (CAFs), which are the most abundant component of the PCa TME [87]. If CAR T cells can be engineered to target the specific surface antigens of CAFs, might this inhibit the growth and infiltration of PCa? All of these ideas merit further exploration and validation.
Another challenge is CRS, a physiological inflammatory state triggered by the interaction of inflammatory cytokines and chemokines released by CAR T cells. These cytokines include granulocyte-macrophage colony-stimulating factor, interferon-γ, and tumor necrosis factor-α, and are released after T cells engage with their corresponding target antigens [88, 89]. CRS has been observed in many patients receiving CAR T cell therapy [72, 88, 89] and are the main side effect of CAR T cell therapy. Interestingly, CRS is not the result of CAR T cells themselves, but inflammatory cytokines released by macrophages in response to the activated CAR T cells, such as IL-1, IL-2, IL-6, and especially IL-6. When these inflammatory cytokines are released in large amounts, they cause an inflammatory response in the vascular endothelium, which further releases IL-6, leading to a positive feedback loop for CRS [89,90,91,92]. This effect disrupts vascular integrity in patients with CRS and leads to hemodynamic instability, capillary leakage, and consumptive coagulopathy [93, 94] (Supplementary Fig. S1). To predict CRS occurrence in high-risk patients in advance, Hay et al. [95] performed classification-tree modeling using data from 133 patients with CRS. They found that the monocyte chemoattractant protein 1 may be a useful prediction marker, and additional predictive markers with good specificity and sensitivity are being explored. Further clinical experience and practice are still needed for the management of CRS.
Neurotoxicity, another common acute side effect of CAR T cell therapy, has been observed in 64% of clinical trials [72]. ICANS may occur simultaneously with CRS, after resolution of CRS, or independent of CRS. Clinical manifestations of ICANS can include decreased consciousness, coma, seizures, motor weakness, and cerebral edema [93]. The pathophysiological mechanism of ICANS remains unclear, but hypotheses include the passive diffusion of cytokines during the transport of CAR T cells in the central nervous system, endothelial cell activation and blood-brain barrier destruction, and N-methyl-D-aspartate receptor agonists [93, 94, 96].
More extensive clinical trials and long-term follow-up are needed to improve the safety and efficacy of CAR T cells. Moreover, the high cost of CAR T cells are a barrier, highlighting the need to improve affordability for patients. The fourth and subsequent generations of CAR T cells are being designed to enhance their lethality, extend persistence after infusion, and enhance their ability to invade solid tumor tissues and regulate the TME [97,98,99]. The ongoing development of CAR T cells should also focus on optimizing target selection and construction methods, reducing toxicity, stabilizing the construction system, and improving invasiveness.
Conclusion
In conclusion, numerous studies have demonstrated the potential utility of CAR T cells for urologic tumors. However, adoptive T cell immunotherapy still faces many challenges, including the viability of tumor cells, the complex regulatory mechanisms of the TME, and side effects such as CRS and ICANS. The ongoing optimization of CAR T cell therapy should focus on improving the role of CAR T cells in solid tumors. Cytokines that improve the structure of CAR T cells or certain pathways may achieve this goal. With the development and progress of a multiple areas of investigation, CAR T cells are poised to become increasingly useful for the treatment of solid tumors, including urinary system tumors.
References
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49.
Mano R, Eastham J, Yossepowitch O. The very-high-risk prostate cancer: a contemporary update. Prostate Cancer Prostatic Dis. 2016;19:340–8.
Malmström P-U, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56:247–56.
Krigel RL, Padavic-Shaller KA, Rudolph AR, Konrad M, Bradley EC, Comis RL. Renal cell carcinoma: treatment with recombinant interleukin-2 plus beta-interferon. J Clin Oncol. 1990;8:460–7.
Carosella ED, Ploussard G, LeMaoult J, Desgrandchamps F. A systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur Urol. 2015;68:267–79.
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–5.
Majzner RG, Mackall CL. Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med. 2019;25:1341–55.
Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17:147–67.
Finck AV, Blanchard T, Roselle CP, Golinelli G, June CH. Engineered cellular immunotherapies in cancer and beyond. Nat Med. 2022;28:678–89.
Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–40.
Atrash S, Bano K, Harrison B, Abdallah A-O. CAR-T treatment for hematological malignancies. J Investig Med. 2020;68:956–64.
Gill S, June CH. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev. 2015;263:68–89.
Thistlethwaite FC, Gilham DE, Guest RD, Rothwell DG, Pillai M, Burt DJ, et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol Immunother. 2017;66:1425–36.
Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–6.
Wang X, Popplewell LL, Wagner JR, Naranjo A, Blanchard MS, Mott MR, et al. Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood. 2016;127:2980–90.
Philipson BI, O’Connor RS, May MJ, June CH, Albelda SM, Milone MC. 4-1BB costimulation promotes CAR T cell survival through noncanonical NF-κB signaling. Sci Signal. 2020;13:eaay8248.
Roselli E, Boucher JC, Li G, Kotani H, Spitler K, Reid K, et al. 4-1BB and optimized CD28 co-stimulation enhances function of human mono-specific and bi-specific third-generation CAR T cells. J Immunother Cancer. 2021;9:e003354.
Hombach AA, Heiders J, Foppe M, Chmielewski M, Abken H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology. 2012;1:458–66.
Song D-G, Ye Q, Poussin M, Harms GM, Figini M, Powell DJ. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood. 2012;119:696–706.
Guedan S, Chen X, Madar A, Carpenito C, McGettigan SE, Frigault MJ, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–80.
Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, Gunset G, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28:415–28.
Huang Y, Si X, Shao M, Teng X, Xiao G, Huang H. Rewiring mitochondrial metabolism to counteract exhaustion of CAR-T cells. J Hematol Oncol. 2022;15:38.
Markley JC, Sadelain M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood. 2010;115:3508–19.
Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4:e994446.
Hu B, Ren J, Luo Y, Keith B, Young RM, Scholler J, et al. Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep. 2017;20:3025–33.
Wang Y, Jiang H, Luo H, Sun Y, Shi B, Sun R, et al. An IL-4/21 inverted cytokine receptor improving CAR-T cell potency in immunosuppressive solid-tumor microenvironment. Front Immunol. 2019;10:1691.
Luo H, Su J, Sun R, Sun Y, Wang Y, Dong Y, et al. Coexpression of IL7 and CCL21 increases efficacy of CAR-T cells in solid tumors without requiring preconditioned lymphodepletion. Clin Cancer Res. 2020;26:5494–505.
Zhao L, Cao YJ. Engineered T cell therapy for cancer in the clinic. Front Immunol. 2019;10:2250.
Pinto IS, Cordeiro RA, Faneca H. Polymer- and lipid-based gene delivery technology for CAR T cell therapy. J Control Release. 2023;353:196–215.
Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther. 2011;19:751–9.
Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–706.
Kagoya Y, Tanaka S, Guo T, Anczurowski M, Wang C-H, Saso K, et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med. 2018;24:352–9.
Shah D, Soper B, Shopland L. Cytokine release syndrome and cancer immunotherapies - historical challenges and promising futures. Front Immunol. 2023;14:1190379.
Naeem M, Hazafa A, Bano N, Ali R, Farooq M, Razak SIA, et al. Explorations of CRISPR/Cas9 for improving the long-term efficacy of universal CAR-T cells in tumor immunotherapy. Life Sci. 2023;316:121409.
Carbonara U, Simone G, Capitanio U, Minervini A, Fiori C, Larcher A, et al. Robot-assisted partial nephrectomy: 7-year outcomes. Minerva Urol Nephrol. 2021;73:540–3.
Calpin GG, Ryan FR, McHugh FT, McGuire BB. Comparing the outcomes of open, laparoscopic and robot-assisted partial nephrectomy: a network meta-analysis. BJU Int. 2023;132:353–64.
Messing EM, Manola J, Wilding G, Propert K, Fleischmann J, Crawford ED, et al. Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma: an eastern cooperative oncology group/intergroup trial. J Clin Oncol. 2003;21:1214–22.
Clark JI, Atkins MB, Urba WJ, Creech S, Figlin RA, Dutcher JP, et al. Adjuvant high-dose bolus interleukin-2 for patients with high-risk renal cell carcinoma: a cytokine working group randomized trial. J Clin Oncol. 2003;21:3133–40.
Passalacqua R, Caminiti C, Buti S, Porta C, Camisa R, Braglia L, et al. Adjuvant low-dose interleukin-2 (IL-2) plus interferon-α (IFN-α) in operable renal cell carcinoma (RCC): a phase III, randomized, multicentre trial of the Italian oncology group for clinical research (GOIRC). J Immunother. 2014;37:440–7.
Rathmell WK, Rumble RB, Van Veldhuizen PJ, Al-Ahmadie H, Emamekhoo H, Hauke RJ, et al. Management of metastatic clear cell renal cell carcinoma: ASCO guideline. J Clin Oncol. 2022;40:2957–95.
Suarez ER, Chang D-K, Sun J, Sui J, Freeman GJ, Signoretti S, et al. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7:34341–55.
Panowski SH, Srinivasan S, Tan N, Tacheva-Grigorova SK, Smith B, Mak YSL, et al. Preclinical development and evaluation of allogeneic CAR T cells targeting CD70 for the treatment of renal cell carcinoma. Cancer Res. 2022;82:2610–24.
Mori J-I, Adachi K, Sakoda Y, Sasaki T, Goto S, Matsumoto H, et al. Anti-tumor efficacy of human anti-c-met CAR-T cells against papillary renal cell carcinoma in an orthotopic model. Cancer Sci. 2021;112:1417–28.
Li H, Ding J, Lu M, Liu H, Miao Y, Li L, et al. CAIX-specific CAR-T cells and sunitinib show synergistic effects against metastatic renal cancer models. J Immunother. 2020;43:16–28.
Berdik C. Unlocking bladder cancer. Nature. 2017;551:S34–5.
Cumberbatch MGK, Jubber I, Black PC, Esperto F, Figueroa JD, Kamat AM, et al. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018;74:784–95.
von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, inblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2023;41:3881–90.
Sternberg CN, de Mulder P, Schornagel JH, Theodore C, Fossa SD, van Oosterom AT, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006;42:50–4.
Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79:82–104.
Li Z, Chen S, Feng W, Luo Y, Lai H, Li Q, et al. A pan-cancer analysis of HER2 index revealed transcriptional pattern for precise selection of HER2-targeted therapy. EBioMedicine. 2020;62:103074.
Hussein S, Fathi A, Abouhashem NS, Amer S, Hemeda M, Mosaad H. SATB-1 and Her2 as predictive molecular and immunohistochemical markers for urothelial cell carcinoma of the bladder. Cancer Biomark. 2021;30:249–59.
Parriott G, Deal K, Crean S, Richardson E, Nylen E, Barber A. T-cells expressing a chimeric-PD1-Dap10-CD3zeta receptor reduce tumour burden in multiple murine syngeneic models of solid cancer. Immunology. 2020;160:280–94.
Yu L, Li Z, Mei H, Li W, Chen D, Liu L, et al. Patient-derived organoids of bladder cancer recapitulate antigen expression profiles and serve as a personal evaluation model for CAR-T cells in vitro. Clin Transl Immunol. 2021;10:e1248.
Grunewald CM, Haist C, König C, Petzsch P, Bister A, Nößner E, et al. Epigenetic priming of bladder cancer cells with decitabine increases cytotoxicity of human EGFR and CD44v6 CAR engineered T-cells. Front Immunol. 2021;12:782448.
Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77:38–52.
Desai K, McManus JM, Sharifi N. Hormonal therapy for prostate cancer. Endocr Rev. 2021;42:354–73.
Yamada Y, Beltran H. The treatment landscape of metastatic prostate cancer. Cancer Lett. 2021;519:20–9.
Wang C, Zhang Y, Gao W-Q. The evolving role of immune cells in prostate cancer. Cancer Lett. 2022;525:9–21.
Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ, Lacey SF, et al. Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol Ther. 2018;26:1855–66.
Narayan V, Barber-Rotenberg JS, Jung I-Y, Lacey SF, Rech AJ, Davis MM, et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med. 2022;28:724–34.
Alzubi J, Dettmer-Monaco V, Kuehle J, Thorausch N, Seidl M, Taromi S, et al. PSMA-directed CAR T cells combined with low-dose docetaxel treatment induce tumor regression in a prostate cancer xenograft model. Mol Ther Oncolytics. 2020;18:226–35.
Wang D, Shao Y, Zhang X, Lu G, Liu B. IL-23 and PSMA-targeted duo-CAR T cells in prostate cancer eradication in a preclinical model. J Transl Med. 2020;18:23.
Priceman SJ, Gerdts EA, Tilakawardane D, Kennewick KT, Murad JP, Park AK, et al. Co-stimulatory signaling determines tumor antigen sensitivity and persistence of CAR T cells targeting PSCA+ metastatic prostate cancer. Oncoimmunology. 2018;7:e1380764.
Hubert RS, Vivanco I, Chen E, Rastegar S, Leong K, Mitchell SC, et al. STEAP: a prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc Natl Acad Sci USA. 1999;96:14523–8.
Lee JK, Bangayan NJ, Chai T, Smith BA, Pariva TE, Yun S, et al. Systemic surfaceome profiling identifies target antigens for immune-based therapy in subtypes of advanced prostate cancer. Proc Natl Acad Sci USA. 2018;115:E4473–82.
Bhatia V, Kamat NV, Pariva TE, Wu L-T, Tsao A, Sasaki K, et al. Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. Nat Commun. 2023;14:2041.
Zhang Y, He L, Sadagopan A, Ma T, Dotti G, Wang Y, et al. Targeting radiation-resistant prostate cancer stem cells by B7-H3 CAR T Cells. Mol Cancer Ther. 2021;20:577–88.
Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19:185–99.
Jacoby E, Yang Y, Qin H, Chien CD, Kochenderfer JN, Fry TJ. Murine allogeneic CD19 CAR T cells harbor potent antileukemic activity but have the potential to mediate lethal GVHD. Blood. 2016;127:1361–70.
Anwer F, Shaukat A-A, Zahid U, Husnain M, McBride A, Persky D, et al. Donor origin CAR T cells: graft versus malignancy effect without GVHD, a systematic review. Immunotherapy. 2017;9:123–30.
Ghosh A, Smith M, James SE, Davila ML, Velardi E, Argyropoulos KV, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med. 2017;23:242–9.
Schubert M-L, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32:34–48.
Ma S, Li X, Wang X, Cheng L, Li Z, Zhang C, et al. Current progress in CAR-T cell therapy for solid tumors. Int J Biol Sci. 2019;15:2548–60.
Koehler H, Kofler D, Hombach A, Abken H. CD28 costimulation overcomes transforming growth factor-beta-mediated repression of proliferation of redirected human CD4+ and CD8+ T cells in an antitumor cell attack. Cancer Res. 2007;67:2265–73.
Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G, Brenner MK. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–28.
Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21:104.
Hatogai K, Sweis RF. The tumor microenvironment of bladder cancer. Adv Exp Med Biol. 2020;1296:275–90.
Kang J, La Manna F, Bonollo F, Sampson N, Alberts IL, Mingels C, et al. Tumor microenvironment mechanisms and bone metastatic disease progression of prostate cancer. Cancer Lett. 2022;530:156–69.
Wallace A, Kapoor V, Sun J, Mrass P, Weninger W, Heitjan DF, et al. Transforming growth factor-beta receptor blockade augments the effectiveness of adoptive T-cell therapy of established solid cancers. Clin Cancer Res. 2008;14:3966–74.
Xia A-L, Wang X-C, Lu Y-J, Lu X-J, Sun B. Chimeric-antigen receptor T (CAR-T) cell therapy for solid tumors: challenges and opportunities. Oncotarget. 2017;8:90521–31.
Powles T, Albiges L, Bex A, Grünwald V, Porta C, Procopio G, et al. ESMO clinical practice guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann Oncol. 2021;32:1511–9.
Lopez-Beltran A, Cimadamore A, Blanca A, Massari F, Vau N, Scarpelli M, et al. Immune checkpoint inhibitors for the treatment of bladder cancer. Cancers. 2021;13:131.
Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–44.
Gargett T, Yu W, Dotti G, Yvon ES, Christo SN, Hayball JD, et al. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol Ther. 2016;24:1135–49.
Li AM, Hucks GE, Dinofia AM, Seif AE, Teachey DT, Baniewicz D, et al. Checkpoint inhibitors arugment CD19-directed chimeric antigen receptor (CAR) T cell therapy in relapsed B-cell acute lymphoblastic leukemia. Blood. 2018;132:556.
Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res. 2017;23:2255–66.
Chiarugi P, Paoli P, Cirri P. Tumor microenvironment and metabolism in prostate cancer. Semin Oncol. 2014;41:267–80.
Sachdeva M, Duchateau P, Depil S, Poirot L, Valton J. Granulocyte-macrophage colony-stimulating factor inactivation in CAR T-cells prevents monocyte-dependent release of key cytokine release syndrome mediators. J Biol Chem. 2019;294:5430–7.
Titov A, Petukhov A, Staliarova A, Motorin D, Bulatov E, Shuvalov O, et al. The biological basis and clinical symptoms of CAR-T therapy-associated toxicites. Cell Death Dis. 2018;9:897.
Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–8.
Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–48.
Obstfeld AE, Frey NV, Mansfield K, Lacey SF, June CH, Porter DL, et al. Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: clinicopathological insights. Blood. 2017;130:2569–72.
Gust J, Hay KA, Hanafi L-A, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017;7:1404–19.
Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8:958–71.
Hay KA, Hanafi L-A, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–306.
Gust J, Finney OC, Li D, Brakke HM, Hicks RM, Futrell RB, et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann Neurol. 2019;86:42–54.
Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15:1145–54.
Labanieh L, Mackall CL. CAR immune cells: design principles, resistance and the next generation. Nature. 2023;614:635–48.
Hong M, Clubb JD, Chen YY. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell. 2020;38:473–88.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 82072835) to KW, 345 Talent Project of Shengjing Hospital of China Medical University (Grant No. M0366) to KW, Outstanding Scientific Fund of Shengjing Hospital (Grant No. 202205) to KW.
Author information
Authors and Affiliations
Contributions
KW, CZ and YW conceived the review; XW and CZ reviewed the information. YW and FD arranged the format of the figures. GZ, SL, and YW wrote the manuscript. KW, CZ, and XW critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Hans-Uwe Simon
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, G., Wang, Y., Lu, S. et al. Molecular understanding and clinical outcomes of CAR T cell therapy in the treatment of urological tumors. Cell Death Dis 15, 359 (2024). https://doi.org/10.1038/s41419-024-06734-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-024-06734-2
- Springer Nature Limited