Abstract
Type-17 immune response, mediated mainly by IL-17, plays a critical role in ulcerative colitis. Previously, we showed that madecassic acid (MA), the main active ingredient of Centella asiatica herbs for anti-colitis effect, ameliorated dextran sulfate sodium (DSS)-induced mouse colitis through reducing the level of IL-17. Here, we explore the effect of MA on the activation of γδT17 cells, an alternative source of IL-17 in colitis. In DSS-induced colitis mice, oral administration of MA decreased the number of γδT17 cells and attenuated the inflammation in the colon, and the anti-colitis effect of MA was significantly counteracted by redundant γδT17 cells, suggesting that the decrease in γδT17 cells is important for the anti-colitis effect of MA. In vitro, MA could inhibit the activation but not the proliferation of γδT17 cells at concentrations without evident cytotoxicity. Antibody microarray profiling showed that the inhibition of MA on the activation of γδT17 cells involved PPARγ–PTEN/Akt/GSK3β/NFAT signals. In γδT17 cells, MA could reduce the nuclear localization of NFATc1 through inhibiting Akt phosphorylation to promote GSK3β activation. Moreover, it was confirmed that MA inhibited the Akt/GSK3β/NFATc1 pathway and the activation of γδT17 cells through activating PPARγ to increase PTEN expression and phosphorylation. The correlation between activation of PPARγ, decrease in γδT17 cell number, and amelioration of colitis by MA was validated in mice with DSS-induced colitis. In summary, these findings reveal that MA inhibits the activation of γδT17 cells through PPARγ–PTEN/Akt/GSK3β/NFAT pathway, which contributes to the amelioration of colitis.
Similar content being viewed by others
Introduction
Ulcerative colitis (UC) is a chronic relapsing–remitting inflammatory disease with a high risk of developing colorectal cancer in long-term patients1. The greatest increase in the incidence of UC is observed in newly industrialized countries, and the highest incidence and prevalence is recorded in Western countries, particularly in Europe and America2. Although the etiology of UC remains unclear, studies in recent years emphasize the importance of the dysregulation of immune responses, especially type-17 immune response, in the initiation, augmentation, and perpetuation of UC3,4. The type 17 immune response is mediated mainly by the activation of IL-17-producing cells, including αβT cells, γδT cells, and innate lymphoid cells (ILCs)5,6,7. The signature cytokines of type-17 immune response include IL-17, IL-21, IL-22, IL-23, IL-6, TNF-α, GM-CSF, and so on8,9, among which IL-17 is closely associated with the development and progression of UC10. Therefore, reducing the level of IL-17, produced by type-17 immune cells, is a practical therapeutic strategy for treating UC.
Madecassic acid (MA), a triterpenoid constituent in Centella asiatica herbs, has anti-inflammatory11, antidiabetic12, anti-oxidant13, neuroprotective14, and anticancer effects15. In our previous studies16, MA, orally administered, was shown to obviously attenuate DSS-induced colitis in mice. MA could reduce the expression of RORγt and IL-17 in the colon tissues of mice, suggesting that the anti-colitis mechanism is probably associated with the downregulation of the IL-17 level in colon tissue. However, under in vitro Th17-polarizing condition, MA only weakly decreased the expression of RORγt and IL-17, implying that other IL-17-producing cells are involved in the anti-colitis effect of MA. Accumulative evidence indicates that γδT cells are the alternative contributors of IL-17 in addition to CD4+αβT cells in type-17 immune response of colitis4,17,18,19. The IL-17-producing γδT (γδT17) cells might be the target cells of MA for the anti-colitis effect.
This study aims to identify the effect of MA on the activation of γδT17 cells and the relation to the anti-colitis effect. The underlying mechanism of MA is also explored.
Results
γδT17 cells are the main target cells of MA for reducing the expression of IL-17 in the colon of colitis mice
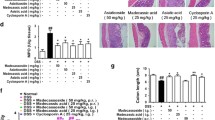
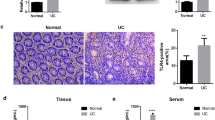
Colitis was induced in female C57BL/6 mice by drinking with 2.5% DSS for 7 days, and followed by normal drinking water for 3 days. MA (25 mg/kg/day) and cyclosporin A (CsA, 25 mg/kg/day), as positive control, were orally gavaged for 10 days. The mice treated with DSS exhibited weight loss, diarrhea, and blood, and an elevated cumulative disease activity index (DAI). On the 10th day, all mice were sacrificed. The DSS-treated mice showed shorter colons, higher activity of myeloperoxidase (MPO), and promoted expression of Il17a and IL-17A in the colon tissues. Consistent with our previous report16, orally administered MA and CsA showed significant ameliorative effect on colitis, and reduced the expression of IL-17 in colon tissues (Fig. 1a–e). The anti-colitis effects of MA and CsA showed no significant difference.
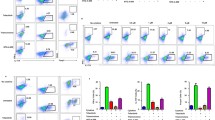
Colitis was induced in female C57BL/6 mice by drinking with 2.5% DSS for 7 days, and followed by normal drinking water for 3 days. Madecassic acid (MA, 25 mg/kg) and cyclosporin A (CsA, 25 mg/kg) were orally gavaged for 10 days consecutively. a Disease activity index (DAI). b Colon length. c Myeloperoxidase (MPO) activity. d The expression of Il17a mRNA as assessed by real-time PCR. e The levels of IL-17A as assessed by enzyme-linked immunosorbent assay. f, g The representative flow cytometry and percentage of CD3+IL-17+ cells in the mononuclear cells of lamina propria from mouse colon tissues. h The representative flow cytometry of IL-17 expression in CD8+ T cells (Tc17), CD4+ T cells (Th17), and γδTCR+ T cells (γδT17) in CD3+ cells. i The percentages of IL-17A+ subpopulations present in the CD8+, CD4+, and γδTCR+ T cells. j The absolute number of Tc17, Th17, and γδT17 cells in CD3 + cells (104). The data are expressed as means ± SEM of six mice per group. ##P < 0.01 versus the normal group; *P < 0.05, **P < 0.01 versus the DSS group, $$P < 0.01 versus the Tc17 cell group.
To identify the target cells of MA for reducing the expression of IL-17 in the colon of colitis mice, single-cell suspensions were prepared from the colon tissues and analyzed by flow cytometry. The data showed that CD3+ cells were the main IL-17-producing cells, and the percentage of CD3+IL-17+ cells was significantly decreased by MA treatment (Fig. 1f, g). In the colon tissues, CD3+IL-17+ cells mainly include Th17, Tc17, and γδT17 cells. The numbers of Th17 and γδT17 cells significantly increased in the lamina propria of the colons of DSS-treated mice, and the absolute numbers of γδT17 and Th17 cells were far more than Tc17 cells. MA treatment markedly decreased the absolute numbers of γδT17 and Th17 cells, but not Tc17 cells (Fig. 1h, j). Moreover, the percentage of γδT17 cells was more greatly decreased than Th17 cells with the treatment of MA (Fig. 1h, i). In combination with our previous findings that MA hardly affects the generation of Th17 cells16, we speculate that γδT17 cells are the main target cells of MA for reducing IL-17 expression in colon tissues of colitis mice.
Decrease in γδT17 cell number plays a critical role in MA ameliorating DSS-induced mouse colitis
To recognize if the decrease in γδT17 cell number is critical for the anti-colitis effect of MA, an adoptive transfer experiment was performed. The γδT cells, purified from the spleens of normal mice, were activated by stimulation with IL-1β and IL-23, and were then adoptively transferred to mice with colitis. As expected, the transfer of activated γδT17 cells led to an increased number of γδT17 cells in the lamina propria of mouse colons (Fig. 2a), and an elevated expression of Il17a and IL-17A (Fig. 2b, c). Accordingly, colitis was aggravated by the transfer of γδT17 cells, evidenced by higher DAI, shorter colon length, higher MPO activity, and worse pathological lesion in the colon tissues (Fig. 2d–g). The anti-colitis effects of MA and CsA (a selective inhibitor for the activation of T cells) were markedly diminished in the mice transferred with activated γδT17 cells (Fig. 2d–g), suggesting that the reduction of the number or activation of γδT17 cells plays a critical role in MA ameliorating DSS-induced colitis in mice.
The γδT17 cell rescue experiment was performed. The γδT cells were stimulated with IL-1β (10 ng/mL) and IL-23 (10 ng/mL) for 72 h; then, they were adoptively transferred into the recipient mice (105 cells/mouse in 200 μL of PBS) on the 3rd and 7th days. The same volume of PBS was injected as the control. a The representative flow cytometry and percentages of IL-17A+ subpopulations present in the γδTCR+ T cells from mouse colon tissues. b The expression of Il17a mRNA as assessed by real-time PCR. c The levels of IL-17A as assessed by enzyme-linked immunosorbent assay. d Disease activity index (DAI). e Colon length. f Myeloperoxidase (MPO) activity. g Hematoxylin and eosin (H&E) staining (scale bar, 100 μm). The data are expressed as means ± SEM of six mice per group. *P < 0.05, **P < 0.01 versus the indicated group.
Madecassic acid inhibits the activation of γδT17 cells in vitro
Unlike conventional αβT cells that differentiate into effector cells when encountered with stimulus signals in peripheral tissues, γδT effector subsets were functionally preprogrammed during ontogeny in the thymus before their distribution to the periphery20,21,22. Therefore, the detectable γδT17 cell number represents the activation extent of γδT17 cells in colon tissues.
To evaluate whether MA can regulate the activation of γδT17 cells, the γδT cells were purified from the spleen cells of mice by magnetic-activated cell sorting (MACS) (Supplementary Fig. S1). In vitro, the γδT cells were stimulated with IL-1β and IL-23, and then exposed to different concentrations (1, 3, and 10 μM) of MA for 72 h. The results showed that MA (3 and 10 μM) decreased the percentage of TCRγ/δ+IL-17+ T cells (Fig. 3a). Consistently, MA (3 and 10 μM) downregulated the expression of cytokines, including Il17a, Il17f, Il21, and Il22, which were produced from γδT17 cells under the stimulation of IL-1β and IL-23 (Fig. 3b). The expression of γδT17 cell-specific transcription factors, such as Rorc, Sox4, Sox13, and Plzf, was also reduced by MA treatment (Fig. 3c). In addition, MA lowered the expression of Il1r1 and Il23r, and enriched the expression of Ccr6 without significant impact on the expression of Ccr2 (Fig. 3d), resulting in the lower sensitivity of γδT17 cells to cytokine stimulation. In order to eliminate the interference of cytotoxicity and proliferation, MTT and proliferation assays were performed. The results showed that MA had no obvious effect on the viability of γδT cells at concentrations lower than 30 μM for 72 h, and did not influence the expansion of γδT cells at concentrations lower than 30 μM for 2 weeks (Supplementary Fig. S2). Consequently, we argue that the activation rather than proliferation of γδT17 cells was inhibited by MA at concentrations without cytotoxicity in vitro.
The purified γδT cells were cultured with IL-1β (10 ng/mL) and IL-23 (10 ng/mL) for 72 h in the presence of madecassic acid (MA, 1, 3, or 10 μM). a The representative flow cytometry and percentages of IL-17A+ subpopulations present in the γδTCR+ T cells. b The expression levels of the cytokines in γδT17 cells, including Il17a, Il17f, Il21, and Il22, as assessed by real-time PCR. c The expression levels of the transcription factors of γδT17 cells, including Rorc, Sox4, Sox13, and Plzf, as assessed by real-time PCR. d The expression levels of the surface receptors of γδT17 cells, including Il1r1, Il23r, Ccr2, and Ccr6, as assessed by real-time PCR. The data are expressed as means ± SEM from three independent experiments. *P < 0.05, **P < 0.01 versus the untreated group; #P < 0.05, ##P < 0.01 versus IL-1β and the IL-23-stimulated group.
Screening of the signaling pathway accounting for madecassic acid inhibiting the activation of γδT17 cells
At present, the activation mechanism of γδT17 cells remains obscure. To explore the potential pathways by which MA inhibits the activation of γδT17 cells, an antibody microarray analysis was performed to identify the changes in protein expression and phosphorylation following MA treatment (Supplementary Fig. S3). In the activated γδT17 cells, MA (10 μM) significantly inhibited the phosphorylation of PPARγ (Fig. 4a, b), and reduced the expression and phosphorylation of PTEN (Fig. 4a, c). MA hardly affected the phosphorylation of PI3K (Fig. 4a, d), but suppressed the phosphorylations of Akt (Fig. 4a, e) and mTOR (Fig. 4a, g), and promoted the phosphorylation of GSK3β (Fig. 4a, f). Notably, the inactivation of mTOR by rapamycin was unable to inhibit the activation of γδT17 cells, and an overactivation of mTOR caused by the treatment of L-leucine did not affect the inhibitory effect of MA on the activation of γδT17 cells (Supplementary Fig. S4), suggesting that mTOR might be unlikely to be essential for the inhibition of MA on γδT17 cell activation. In addition, the promotion of the phosphorylation of GSK3β, an enhancer of nuclear export of NFAT (nuclear factor of activated T cells), suggests a possibility that MA can reduce the nuclear localization of NFATc1. Further immunofluorescence assay demonstrated that MA was indeed able to impede the nuclear localization of NFATc1 in the activated γδT17 cells (Fig. 4h). Moreover, FK506 (a NFAT inhibitor) could inhibit the activation of γδT17 cells and the expression of Il17a and Rorc (Supplementary Fig. S5). These findings suggest that inhibition of γδT17 cell activation by MA might involve the PPARγ–PTEN/Akt/GSK3β/NFAT pathway.
The γδT cells were stimulated with IL-1β (10 ng/mL) and IL-23 (10 ng/mL) for 72 h in the presence or absence of madecassic acid (MA, 10 μM). a The fluorescent images of the relative protein phosphorylation and expression as detected by the phospho explorer antibody array. Data are presented as fold change after normalization to untreated cells. b, g The expression and phosphorylation levels of PPARγ, PTEN, PI3K, AKT, GSK3β, and mTOR, as detected by western blot. h The localization of NFATc1 as visualized by immunofluorescence analysis (scale bar, 20 μm). i Schematic diagram of activated or deactivated proteins of the signaling pathway upon MA treatment (according to www.phosphosite.org). The data are expressed as means ± SEM from six technical replicates in microarray experiments or from three independent experiments. **P < 0.01 versus the untreated group; ##P < 0.01 versus IL-1β and IL-23-stimulated group.
Madecassic acid reduces the nuclear localization of NFATc1 in γδT17 cells through inhibition of Akt phosphorylation
In αβT cells, αβTCR signal-induced phosphorylation of Akt requires CD28 costimulation23. In contrast, in γδT cells, the γδTCR signal can stimulate Akt activation in the absence of costimulatory signals24. In order to recognize the role that Akt plays in the inhibition of MA on γδT17 cell activation, we used MK-2206 (a highly selective inhibitor of Akt) to inhibit the phosphorylation of Akt, and SC-79 (an activator of Akt phosphorylation) to restore Akt activation in γδT17 cells. It was shown that, similar to MA, MK-2206 could inhibit the activation of γδT17 cells and the expression of Il17a and Rorc. When MA was used in combination with SC-79, the inhibitory effect of MA on the activation of γδT17 cells nearly disappeared (Fig. 5a). Likewise, SC-79 almost abolished the inhibitory effect of MA on the expression of Il17a and Rorc (Fig. 5b, c). These results indicate that the inhibition of Akt phosphorylation by MA substantially contributes to the prevention of γδT17 cell activation.
The γδT cells were stimulated with IL-1β (10 ng/mL) and IL-23 (10 ng/mL) for 72 h in the presence or absence of madecassic acid (MA, 10 μM) or in combination with MK-2206 (5 μM) or SC-79 (10 μM). a The representative flow cytometry and percentages of IL-17A+ subpopulations present in the γδTCR+ T cells. b, c The expression levels of Il17a and Rorc as assessed by real-time PCR. d–g The expression and phosphorylation levels of PPARγ, PTEN, PI3K, AKT, GSK3β, and mTOR as detected by western blot. f The expression level of Pten as assessed by real-time PCR. h The localization of NFATc1 as visualized by immunofluorescence analysis (scale bar, 20 μm). The data are expressed as means ± SEM from three independent experiments. *P < 0.05, **P < 0.01 versus the indicated group.
In addition to mTOR that was excluded to participate in the inhibition of MA on γδT17 cell activation (Supplementary Fig. S4), GSK3β is another important downstream molecule of Akt, and phosphorylated GSK3β is capable of promoting NFAT translocation from the nucleus into the cytoplasm25. Our further studies showed that MK-2206 increased the phosphorylation of GSK3β, and reduced the nuclear localization of NFATc1 in γδT17 cells (Fig. 5d, g, h), but MK-2206 had no effect on the phosphorylation of PPARγ and PTEN (Fig. 5d–f). When used in combination with SC-79, MA failed to promote the phosphorylation of GSK3β (Fig. 5d, g) and prevent the nuclear localization of NFATc1 in γδT17 cells (Fig. 5h), but SC-79 did not alter the effect of MA on the phosphorylation of PPARγ and PTEN (Fig. 5d–f). These results suggest that MA promotes GSK3β activation and further reduces the nuclear localization of NFATc1 in γδT17 cells through inhibition of Akt phosphorylation.
Madecassic acid inhibits the PTEN/PI3K–Akt/GSK3β/NFAT signal pathway in γδT17 cells by activating PPARγ
Our previous studies demonstrated that MA is a PPARγ agonist16. The activation of PPARγ could increase PTEN expression, and inhibit Akt phosphorylation26. A recent report indicated that the activation of γδT17 cells could be promoted by SF1670 (an inhibitor of PTEN)27. To further verify the key role of PPARγ in the inhibitory effect of MA on γδT17 cell activation, we used rosiglitazone (RGZ, a selective PPARγ agonist) to activate PPARγ and GW9662 (a selective and irreversible PPARγ antagonist) to inhibit the activation of PPARγ in γδT17 cells. It was shown that RGZ inhibited the activation of γδT17 cells and the expression of Il17a and Rorc (Fig. 6a–c). In addition, in combination with GW9662, the inhibitory effect of MA on the activation of γδT17 cells nearly disappeared (Fig. 6a). Likewise, GW9662 almost abolished the inhibition of MA on the expression of Il17a and Rorc (Fig. 6b, c). Therefore, we argued that MA-mediated PPARγ activation contributed to the inhibition of γδT17 cell activation. As expected, RGZ increased the phosphorylation of PPARγ in γδT17 cells (Fig. 6d, e). Further experiments showed that RGZ increased the expression and phosphorylation of PTEN (Fig. 6d–f), and promoted the phosphorylation of Akt (Fig. 6d, g). Correspondingly, the phosphorylation of GSK3β was promoted (Fig. 6d, g), and the nuclear localization of NFATc1 was reduced by RGZ (Fig. 6h). In addition, the promotion effect of MA on the expression and phosphorylation of PTEN (Fig. 6d–f) and the inhibitory effect of MA on phosphorylation of Akt were restored (Fig. 6d, g) when γδT17 cells were treated with GW9662 and MA. In contrast, the promotion effect of MA on the phosphorylation of GSK3β nearly disappeared (Fig. 6d, g) and the nuclear localization of NFATc1 in γδT17 cells was restored (Fig. 6h) when γδT17 cells were treated with GW9662 and MA. These results suggest that MA inhibits the PTEN/Akt/GSK3β/NFAT signal pathway in γδT17 cells by activating PPARγ.
The γδT cells were stimulated with IL-1β (10 ng/mL) and IL-23 (10 ng/mL) for 72 h in the presence or absence of madecassic acid (MA, 10 μM) or in combination with GW9662 (1 μM) or rosiglitazone (RGZ, 2 μM). a The representative flow cytometry and percentages of IL-17A+ subpopulations present in the γδTCR+ T cells. b, c The expression levels of Il17a and Rorc as assessed by real-time PCR. d–g The expression and phosphorylation levels of PPARγ, PTEN, PI3K, AKT, GSK3β, and mTOR, as detected by western blot. f The expression level of Pten as assessed by real-time PCR. h The localization of NFATc1, as visualized by immunofluorescence analysis (scale bar, 20 μm). The data are expressed as means ± SEM from three independent experiments. *P < 0.05, **P < 0.01 versus the indicated group.
Madecassic acid alleviates colitis through inhibiting the activation of γδT17 cells dependent on PPARγ
To further ascertain the causal link between PPARγ activation, inhibition of γδT17 activation, and anti-colitis effects of MA, MA was orally administered in combination with GW9662 to colitis mice. The results showed that GW9662 counteracted the inhibitory effect of MA on the activation of γδT17 cells in the colons of colitis mice, but it did not affect the inhibitory effect of CsA (Fig. 7a). A plausible explanation for this phenomenon is that CsA prevents nuclear import of NFAT by blocking calcineurin activity28; however, MA reduces the nuclear localization through the PPARγ–PTEN/Akt/GSK3β pathway. As expected, MA inhibited the expression of Il17a and IL-17A in the colons, and these effects were counteracted by GW9662, but the reduction of IL-17A expression caused by CsA was not markedly weakened by GW9662 (Fig. 7b, c). These results indicate that MA, different from CsA, inhibits the activation of γδT17 cells by activating PPARγ. In addition, GW9662 markedly weakened the anti-colitis effect of MA as evidenced by higher DAI (Fig. 7d), shorter colon length (Fig. 7e), higher MPO activity (Fig. 7f), and more serious pathological lesion (Fig. 7g) in the colon tissues. These findings reveal that MA alleviates colitis through inhibiting the activation of γδT17 cells dependent on PPARγ.
Colitis was induced in female C57BL/6 mice by drinking with 2.5% dextran sulfate sodium (DSS) for 7 days, and followed by normal drinking water for 3 days. Madecassic acid (MA, 25 mg/kg), cyclosporin A (CsA, 25 mg/kg), or GW9662 (1 mg/kg) were orally administered for 10 days consecutively. a The representative flow cytometry and percentages of IL-17A+ subpopulations present in the γδTCR+ T cells from the colon tissues of mice. b The levels of IL-17A as assessed by enzyme-linked immunosorbent assay. c The expression level of Il17a as assessed by real-time PCR. d Disease activity index (DAI). e Colon length. f Myeloperoxidase (MPO) activity. g Hematoxylin and eosin (H&E) staining (scale bar, 100 μm). The data are expressed as means ± SEM of six mice per group. *P < 0.05, **P < 0.01 versus the indicated group.
Discussion
IL-17, an acknowledged pro-inflammatory cytokine, plays an important role in UC. Clinical studies showed that IL-17 was highly expressed in intestinal mucosal tissues of patients with UC29,30. In animal studies, DSS-induced colitis was IL-17-dependent31,32. These findings indicated that IL-17 is associated with the development and progression of UC, and suggested that IL-17 is an attractive therapeutic target. For many years, researchers have been exploring the new IL-17-based treatment schemes33,34,35, although the results of clinical trials with anti-IL-17 antibodies in inflammatory bowel disease (IBD) were discouraging36. Our previous study demonstrated that orally administered MA could obviously attenuate colitis by downregulating the expression of IL-17 in colon tissue of colitis mice16, but the IL-17-producing effector cells remain undefined.
It is generally accepted that IL-17 is produced mainly by Th17-type lymphocytes in colon tissue of colitis37,38. Some studies have also pointed out that IL-17 is also largely secreted by γδT cells39,40 or CD8+ T cells41,42. This study found that MA could reduce the level of IL-17 and the number of γδT17 cells in colon tissues of colitis mice, and attenuate colitis in mice. In the absence of suitable genetic tools for the ablation of IL-17 specifically in γδT-cell lineage, some conclusions, based on associations of phenotypes of mice lacking γδT cells or IL-17, are unconvincing43. The contribution of γδT17 cells to diseases can be verified by adoptive transfer of γδT17 cells43. This strategy has been employed in colitis mouse models44. In this study, the anti-colitis effect of MA was counteracted by adoptive transfer of γδT17 cells, which suggested that γδT17 was the main target cell of MA ameliorating colitis and reducing the expression of IL-17 in colon tissues of colitis mice.
Unlike conventional type-17 αβT effector cells, γδT17 cells acquire their effector potential in the thymus before their distribution to the periphery21,22,23. These preprogrammed effector cells can rapidly switch from rest to activated state and secrete a large amount of cytokines during the immune response45,46,47. Recent researches have shown that γδT17 cells are deeply involved in multiple inflammation and autoimmune diseases, such as colitis17, psoriasis48, asthma49, diabetes50, encephalomyelitis51, and so on. The IL-17 produced from γδT cells can independently promote inflammation development52. On the other hand, γδT17 cells can also amplify Th17 responses in inflammation53,54. In this study, the number of activated γδT17 cells in colon tissues of colitis mice was decreased by orally administering MA. Moreover, the activation of γδT17 cells was inhibited by MA at concentrations without cytotoxicity in vitro.
It is reported that TCR signaling is a major determinant of the function of effector γδT cells55. However, little is known about the mechanism of γδTCR to drive IL-17 secretion in γδT cells. A study identified that γδ17 cells are absent in mice with defective Zap-70 function56. However, another recent study indicated the Syk signaling pathway was one means to control the production of IL-17 in γδT cells27. In fact, stimulation with IL-1β and IL-23 is sufficient to trigger abundant secretion of IL-17 by γδT cells in vitro, even in the absence of TCR stimulation43. IRF4 cooperates with STAT3 to regulate gene expression, and links IL-1R and IL-23R signaling pathways to IL-17 production3. STAT3 activation is crucial for RORγt expression in Th17 cells and also important for IL-17 production in γδT cells57,58. Furthermore, both classical and noncanonical NF-κB signaling pathways are important for γδT17. RelA or RelB conditional deficiency leads to reduction of γδT17 cells through reducing Il17a and Rorc expression at the transcriptional level, and p52, not p50, was also required for IL-17 production59. NF-κB-inducing kinase, which is required for noncanonical NF-κB signaling, is essential for IL-17 production as NF-κB-inducing kinase depletion led to impairing Rorc and Sox13 expression60. Notch signaling and its downstream target Hes1, one of the basic helix–loop–helix proteins, are indispensable for the IL-17-producing function of mature γδT cells in the periphery61. In this study, we found that MA could inhibit the activation of γδT17 cells through the PPARγ–PTEN/Akt/GSK3β/NFAT pathway.
The nuclear receptor PPARγ, a ligand-activated transcription factor that plays an important role in the control of gene expression linked to a variety of physiological processes, is highly expressed in the colon62,63. Many preclinical researchers have pointed out that the activation of PPARγ plays a protective role in mice with colitis64,65,66. Many studies have shown that various compounds, acting as the agonists of PPARγ (such as rosiglitazone, troglitazone, and thiazolidinediones), have immunoprotective roles in experimental colitis67,68,69. In our previous study, MA has shown to induce Th17 toward Treg cells in a PPARγ-dependent manner and ameliorate experimental colitis by activating PPARγ16. In this study, MA can ameliorate experimental colitis through inhibiting the activation of γδT17 cells in a PPARγ-dependent manner.
In conclusion, γδT17 cells play an important role in the anti-colitis effect of MA. MA is able to inhibit the activation of γδT17 cells through PPARγ–PTEN/Akt/GSK3β/NFAT pathway, reduce the level of IL-17 in colon tissues of colitis mice, and ameliorate DSS-induced colitis in mice. MA and other PPARγ agonists are promising for the prevention and treatment of γδT17-related IBD or other diseases.
Materials and methods
Media and reagents
Madecassic acid (CAS no. 18449-41-7) with a purity of 98.5% was purchased from Jiangsu Yongjian Pharmaceutical Technology Co., Ltd (Taizhou, China). DSS (MW: 36–50 kDa) was purchased from MP Biomedicals Inc. (Irvine, CA, USA). Mouse IL-17 ELISA kit was purchased from Lianke Biotech Co., Ltd (Hangzhou, China). MPO activity assay kit was obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). FITC-anti-mouse CD3, PE-anti-mouse TCR γ/δ, PE-anti-mouse CD4, PE-anti-mouse CD8, and APC-anti-mouse IL-17A were purchased from eBioscience, Inc. (San Diego, CA, USA). Murine IL-1β and IL-23 were purchased from Sino Biological Inc. (Beijing, China). Mouse TCRγ/δ T Cell Isolation Kit was purchased from Miltenyi Biotec Inc. (Bergisch Gladbach, Germany). TRIzol reagent was purchased from SunShine Biotechnology Co., Ltd (Nanjing, China). PPARγ, phosphorylated (p)-PPARγ, PTEN, p-PTEN, PI3K, p-PI3K, Akt, p-Akt, GSK3β, p-GSK3β, mTOR, p-mTOR, NFATc1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mAb were purchased from HuaBio Inc. (Hangzhou, China). Cyclosporin A, GW9662, MK-2206, and rapamycin were obtained from CSNpharm (Chicago, USA). Rosiglitazone, FK506, LY294002, and SC-97 were purchased from Selleck (Houston, USA).
Animals
Female C57BL/6 mice (20 ± 2 g) were obtained from the Comparative Medicine Center of Yangzhou University (Yangzhou, China). Mice were maintained in the animal center of China Pharmaceutical University, a specific pathogen-free environment, with light:dark (12 h:12 h) condition and fed with commercial diet and water ad libitum. All animal experiments were approved by the animal ethics committee of the China Pharmaceutical University, and were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
DSS-induced colitis in mice
All mice were assigned to different groups according to a randomized block experimental design. Experimental colitis was induced in mice by freely drinking of 2.5% DSS for 7 days followed by normal drinking water for 3 days. Madecassic acid (25 mg/kg) and cyclosporin A (25 mg/kg) were orally gavaged daily for 10 days, and carboxyl methyl cellulose sodium (CMC-Na, 0.5%) was used as the vehicle control. Body weight, diarrhea, and rectal bleeding were measured every day. The DAI was accounted by the mean value of the following three elements. Briefly, (a) body weight loss (0 = none, 1 = 1–5%, 2 = 5–10%, 3 = 10–15%, and 4 = over 15%), (b) stool consistency (0 = normal, 2 = loose stools, and 4 = diarrhea), and (c) gross bleeding (0 = normal, 2 = hemoccult, and 4 = gross bleeding).
On day 10, mice were sacrificed, the colon length was measured, and then the colon was fixed in 4% PBS-buffered formaldehyde as a roll and embedded in paraffin. The 5-mm tissue sections were stained with hematoxylin and eosin (H&E). Histological scores were graded as follows: (a) severity of inflammation: 0 = none, 1 = slight, 2 = moderate, and 3 = severe, (b) sites of inflammation: 0 = none, 1 = mucosa, 2 = mucosa and submucosa, and 3 = transmural, and (c) lesions of crypt: 0 = none, 1 = basal 1/3 damaged, 2 = basal 2/3 damaged, 3 = only surface epithelium intact, and 4 = entire crypt and epithelium lost. The histological score was assessed by the average of the three evaluations with a maximal score of 10.
Real-time quantitative PCR assay
The Trizol reagent was employed to extract total mRNA from colonic homogenates or cultured cells. The mRNA was reverse-transcribed using 5×All-In-One RT MasterMix kit according to the manufacturer’s instructions. RT-qPCR analysis was performed with EvaGreen 2×qPCR MasterMix kit on a Bio-Rad iQ Real Time PCR system (Hercules, CA, USA). The RT-qPCR conditions were activation at 95 °C for 10 min, followed by 35 cycles of amplification with denaturation at 95 °C for 15 s, and then annealing and extension at 60 °C for 60 s. The primer sequences used are listed in Table 1. GAPDH was used as an internal control. The expression of each gene was normalized to the corresponding GAPDH threshold cycle (Ct) values by the 2−ΔΔCt method.
Enzyme-linked immunosorbent assay
Colonic tissues were homogenated, and the supernatants were collected for the detection of IL-17A level by enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions.
Extracellular staining and flow cytometry
For extracellular staining of immune markers, we prepared lamina propria mononuclear cell suspensions by mechanic dispersion and enzymatic digestion of colon tissues. Lamina propria mononuclear cells and γδT cells were resuspended in RPMI-1640 supplemented with 10% fetal bovine serum labeled with FITC-anti-mouse CD3, and PE-anti-mouse TCR γ/δ or PE-anti-mouse CD4 or PE-anti-mouse CD8 at 4 °C for 30 min. After fixation and permeabilization, the cells were labeled with APC-anti-mouse IL-17A at 4 °C for 30 min. All flow cytometric measurements were conducted on FACS Calibur (BD Biosciences, USA). The results were analyzed by Flowjo 10.0.1 (Flowjo LLC, USA).
Western blot analysis
Cell and tissue lysate homogenates were prepared by NP-40 buffer. Following the mixture with SDS-PAGE protein sample buffer, proteins were divided by SDS-PAGE (10%). Subsequently, the protein was transferred to nitrocellulose filter membranes. After that, the nitrocellulose filter membranes were transiently blocked by 5% defatted milk for about 2 h at room temperature, and then incubated with primary antibodies overnight at 4 °C. After washing with TBST, the membranes were incubated with the appropriate secondary antibody for 2 h at room temperature. After washing, the hybridized bands were obtained with the help of Odyssey Infrared Imaging System.
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde for 15 min and permeabilized by 0.1% Triton X-100 for 15 min. After blocking in 4% bovine serum albumin for 2 h, the cells were incubated with primary antibodies at 4 °C overnight, and next with fluorophore rhodamine- or FITC-conjugated IgG for 2 h, and nuclei were counterstained with DAPI for 10 min in the dark, followed by three washes with PBST. Anti-fluorescence quenching agent was added and photographed using a fluorescence microscope.
Microarray analysis
The phospho explorer antibody microarray, which was designed and manufactured by Full Moon Biosystems, Inc. (Sunnyvale, CA), contains 304 antibodies. Each of the antibodies has six replicates that are printed on coated glass microscope slides, along with multiple positive and negative controls. The antibody array experiment was performed by Huaying Biotechnology (Shanghai, China), according to their established protocol. Briefly, the cell lysates, which were obtained from γδT cells treated with IL-1β and IL-23 or madecassic acid, were biotinylated with an Antibody Array Assay Kit. The antibody microarray slides were first blocked in a blocking solution. Then the biotin-labeled cell lysate was placed on preblocked microarray slides. After washing, bound biotinylated proteins were detected using Cy3-conjugated streptavidin. The slides were scanned on a GenePix 4000 scanner, and the images were analyzed with GenePix Pro 6.0 (Molecular Devices, Sunnyvale, CA). Where indicated, protein phosphorylation data were confirmed by western blotting.
Sorting and purification of γδT cells
Spleens were harvested and cut into small fragments mechanically. Cut fragments were crushed between nylon mesh (300 meshes). Then red blood cells were depleted by red blood cell lysis buffer, and the pellet was washed in phosphate-buffered saline (PBS).
The γδT cells were isolated from single-spleen cell suspensions using a mouse γδTCR+ T Cell Isolation Kit (MACS) according to the manufacturer’s instruction. Briefly, the isolation of mouse γδT cells is performed in a two-step procedure. To pre-enrich the target cells, non-T cells are magnetically labeled with a cocktail of CD45R and CD11b antibodies conjugated to MicroBeads. Concomitantly, γ/δTCR+ T cells are labeled with Anti-γ/δTCR-Biotin included in the cocktail. The magnetically labeled, unwanted cells are subsequently depleted by separation over a MACS Column. In the second step, γ/δTCR+ T cells are indirectly magnetically labeled with Anti-Biotin MicroBeads and isolated by positive selection from the pre-enriched T-cell fraction. The magnetically labeled γ/δTCR+ T cells are retained on the column and eluted after removal of the column from the magnetic field. Then, the absolute cell numbers were calculated.
The purified γδT cells were incubated in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin–streptomycin, 5 μM zoledronate, and 100 IU/mL IL-2 at 37 °C, 5% CO2 for 2 weeks, which was refreshed twice weekly. γδT-cell purity was determined by flow cytometry with purity over 90%, which can be used for further experiments.
Adoptive transfer of γδT cells
γδT cells were cultured with IL-1β (10 ng/mL) and IL-23 (10 ng/mL) for 72 h. Then, the cells were adoptively transferred into the recipient mice by intravenous injection at the 3rd and 7th day (105 cells/mouse in 200 μL of PBS). The same volume of PBS was used as the control.
Assessment of colonic MPO activity
The activity of MPO is a mark of neutrophil infiltration, which reflects the number and distribution of neutrophils in the tissues. The colon tissues were precisely weighed and homogenized with PBS (1:9, w/v). The supernatants were collected. The MPO activity was measured according to the manufacturer’s instructions from JianCheng Bioengineering Institute (Nanjing, China).
Histological evaluation of the colon
To assess the inflammatory level, murine colonic tissue of the colons was collected and fixed in 10% neutral buffered formalin at room temperature, embedded in paraffin, and sliced. For histological evaluation, 5-μm-thick tissue sections were stained with H&E and examined under the light microscope. The investigators were blinded to the group allocation when assessing the outcome.
Statistical analysis
The data are expressed as the mean ± SEM. Statistically significant differences between the groups were determined by one-way analysis of variance (ANOVA) followed by Tukey’s multiple- comparison test. At least three independent replicates of each experiment were conducted. Differences were considered statistically significant at *P < 0.05 or **P < 0.01.
References
Mishra, S. K. Ulcerative colitis and colorectal cancer: challenges and opportunities. Cell 103, 311–320 (2017).
Ungaro, R., Mehandru, S., Allen, P. B., Peyrin-Biroulet, L. & Colombel, J. F. Ulcerative colitis. Lancet 389, 1756–1770 (2017).
Chen, H. H. & Simmons, A. Becalming type 17 inflammation in ulcerative colitis. Immunity 50, 1029–1031 (2019).
Rosen, M. J. et al. Mucosal expression of type 2 and type 17 immune response genes distinguishes ulcerative colitis from colon-only Crohn’s disease in treatment-naive pediatric patients. Gastroenterology 152, 1345–1357 (2017).
Sparber, F. et al. The skin commensal yeast Malassezia triggers a type 17 response that coordinates anti-fungal immunity and exacerbates skin inflammation. Cell Host Microbe 25, 389–403 (2019).
Chung, L. et al. Interleukin-17 and senescence regulate the foreign body response to synthetic material implants in mice and humans. Sci Transl Med. 12, eaax3799 (2020).
Cohen, J. A. et al. Cutaneous TRPV1+ neurons trigger protective innate type 17 anticipatory immunity. Cell 178, 919–932 (2019).
Abboud, A. et al. Computational analysis supports an early, type 17 cell-associated divergence of blunt trauma survival and mortality. Crit. Care Med. 44, e1074 (2016).
Bain, K. A. et al. Alopecia areata is characterized by dysregulation in systemic type 17 and type 2 cytokines, which may contribute to disease-associated psychological morbidity. Br. J. Dermatol. 182, 130–137 (2020).
McGeachy, M. J., Cua, D. J. & Gaffen, S. L. The IL-17 family of cytokines in health and disease. Immunity 50, 892–906 (2019).
Won, J.-H. et al. Anti-inflammatory effects of madecassic acid via the suppression of NF-κB pathway in LPS-induced RAW 264.7 macrophage cells. Planta Med. 76, 251–257 (2010).
Hsu, Y.-M., Hung, Y.-c, Hu, L., Lee, Y.-j & Yin, M.-c Anti-diabetic effects of madecassic acid and rotundic acid. Nutrients 7, 10065–10075 (2015).
Yang, B. et al. Madecassic Acid protects against hypoxia-induced oxidative stress in retinal microvascular endothelial cells via ROS-mediated endoplasmic reticulum stress. Biomed. Pharmacother. 84, 845–852 (2016).
Wang, P., Long, T.-F., Wang, L.-P. & Song, J. Synergistic effect of asiatic acid and madecassic acid against antioxidant deficit in rat peripheral nervous system. Pharmacology 15, 837–843 (2019).
Valdeira, A. S. et al. Madecassic acid derivatives as potential anticancer agents: synthesis and cytotoxic evaluation. J. Nat. Prod. 82, 2094–2105 (2019).
Xu, X. T. et al. Madecassic acid, the contributor to the anti-colitis effect of madecassoside, enhances the shift of Th17 toward Treg cells via the PPAR gamma/AMPK/ACC1 pathway. Cell Death Dis. 8, e2723 (2017).
Castro-Dopico, T. et al. Anti-commensal IgG drives intestinal inflammation and type 17 immunity in ulcerative colitis. Immunity 50, 1099–1114 (2019).
Sun, X. et al. Innate γδT17 cells play a protective role in DSS-induced colitis via recruitment of Gr-1+ CD11b+ myeloid suppressor cells. Oncoimmunology 6, e1313369 (2017).
McKenzie, D. R., Comerford, I., Silva-Santos, B. & McColl, S. R. The emerging complexity of γδT17 cells. Front. Immunol. 9, 796 (2018).
Zuberbuehler, M. K. et al. The transcription factor c-Maf is essential for the commitment of IL-17-producing γδ T cells. Nat. Immunol. 20, 73–85 (2019).
Paget, C. et al. CD3bright signals on γδ T cells identify IL-17A-producing Vγ6Vδ1+ T cells. Immunol. Cell Biol. 93, 198–212 (2015).
Narayan, K. et al. Intrathymic programming of effector fates in three molecularly distinct γδ T cell subtypes. Nat. Immunol. 13, 511–518 (2012).
Frauwirth, K. A. et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002).
Blanco, R., Borroto, A., Schamel, W., Pereira, P. & Alarcon, B. Conformational changes in the T cell receptor differentially determine T cell subset development in mice. Sci. Signal. 7, ra115–ra115 (2014).
Wu, M. et al. Gα13 negatively controls osteoclastogenesis through inhibition of the Akt-GSK3β-NFATc1 signalling pathway. Nat. Commun. 8, 1–13 (2017).
Kabelitz, D. & Dechanet-Merville, J. Recent advances in gamma/delta T cell biology: new ligands, new functions, and new translational perspectives. Front. Immunol. 6, 371 (2015).
Muro, R. et al. γδTCR recruits the Syk/PI3K axis to drive proinflammatory differentiation program. J. Clin. Investig. 128, 415–426 (2018).
Crabtree, G. R. & Olson, E. N. NFAT signaling: choreographing the social lives of cells. Cell 109, S67–S79 (2002).
Fonseca-Camarillo, G., Mendivil-Rangel, E., Furuzawa-Carballeda, J. & Yamamoto-Furusho, J. K. Interleukin 17 gene and protein expression are increased in patients with ulcerative colitis. Inflamm. Bowel Dis. 17, E135–E136 (2011).
Zhang, H. et al. Expression and clinical significance of IL-17 and IL-17 receptor in ulcerative colitis. J. Huazhong Univ. Sci. Technol. Med. Sci. 36, 37–40 (2016).
Aguiar, S. L. F. et al. High-salt diet induces IL-17-dependent gut inflammation and exacerbates colitis in mice. Front. Immunol. 8, 1969 (2018).
Ito, R. et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem. Biophys. Res. Commun. 377, 12–16 (2008).
Igaki, K. et al. Pharmacological effects of TAK-828F: an orally available RORγt inverse agonist, in mouse colitis model and human blood cells of inflammatory bowel disease. Inflamm. Res. 68, 493–509 (2019).
Abdelmegid, A. M., Abdo, F. K., Ahmed, F. E. & Kattaia, A. A. Therapeutic effect of gold nanoparticles on DSS-induced ulcerative colitis in mice with reference to interleukin-17 expression. Sci. Rep. 9, 1–16 (2019).
Zhang, Z. et al. TAOK1 negatively regulates IL-17-mediated signaling and inflammation. Cell. Mol. Immunol. 15, 794–802 (2018).
Morales, J. M. G. R. et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: an updated review of the evidence focusing in controversies. Autoimmun. Rev. 19, 102429 (2020).
Saeed, M. A. et al. Protease-activated receptor 1 plays a proinflammatory role in colitis by promoting Th17-related immunity. Inflamm. Bowel Dis. 23, 593–602 (2017).
Saleh, M. M. et al. Colitis-induced Th17 cells increase the risk for severe subsequent Clostridium difficile infection. Cell Host Microbe 25, 756–765 (2019).
Lo Presti, E. et al. Analysis of colon-infiltrating γδ T cells in chronic inflammatory bowel disease and in colitis-associated cancer. J. Leukoc. Biol. 108, 749–760 (2020).
Li, M. et al. Upregulation of intestinal barrier function in mice with DSS-induced colitis by a defined bacterial consortium is associated with expansion of IL-17A producing gamma delta T cells. Front. Immunol. 8, 824 (2017).
Tajima, M. et al. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J. Exp. Med. 205, 1019–1027 (2008).
Ji, T. et al. Aryl hydrocarbon receptor activation down-regulates IL-7 and reduces inflammation in a mouse model of DSS-induced colitis. Dig. Dis. Sci. 60, 1958–1966 (2015).
Papotto, P. H., Ribot, J. C. & Silva-Santos, B. IL-17+ γδ T cells as kick-starters of inflammation. Nat. Immunol. 18, 604–611 (2017).
Park, S.-G. et al. T regulatory cells maintain intestinal homeostasis by suppressing γδ T cells. Immunity 33, 791–803 (2010).
Spidale, N. A., Frascoli, M. & Kang, J. γδTCR-independent origin of neonatal γδ T cells prewired for IL-17 production. Curr. Opin. Immunol. 58, 60–67 (2019).
McKenzie, D. R. et al. IL-17-producing γδ T cells switch migratory patterns between resting and activated states. Nat. Commun. 8, 1–13 (2017).
Hayes, S. M. & Laird, R. M. Genetic requirements for the development and differentiation of interleukin-17-producing γδ T cells. Crit. Rev. Immunol. 32, 81–95 (2012).
Ramírez-Valle, F., Gray, E. E. & Cyster, J. G. Inflammation induces dermal Vγ4+ γδT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc. Natl Acad. Sci. USA 112, 8046–8051 (2015).
Zhang, J.-h, Yang, X., Chen, Y.-p, Zhang, J.-f & Li, C.-q Nrf2 activator RTA-408 protects against ozone-induced acute asthma exacerbation by suppressing ROS and γδT17 cells. Inflammation 42, 1843–1856 (2019).
Han, G. et al. Interleukin-17-producing γδ+ T cells protect NOD mice from type 1 diabetes through a mechanism involving transforming growth factor-β. Immunology 129, 197–206 (2010).
Malik, S., Want, M. Y. & Awasthi, A. The emerging roles of gamma-delta T cells in tissue inflammation in experimental autoimmune encephalomyelitis. Front. Immunol. 7, 14 (2016).
Sutton, C. E. et al. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 (2009).
Singh, K. et al. Deletion of cationic amino acid transporter 2 exacerbates dextran sulfate sodium colitis and leads to an IL-17-predominant T cell response. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G225–G240 (2013).
Do, J.-s, Visperas, A., Dong, C., Baldwin, W. M. & Min, B. Cutting edge: generation of colitogenic Th17 CD4 T cells is enhanced by IL-17+ γδ T cells. J. Immunol. 186, 4546–4550 (2011).
Chen, X., Morrissey, S., Chen, F. & Yan, J. Novel insight into the molecular and metabolic mechanisms orchestrating IL-17 production in γδ T cells. Front. Immunol. 10, 2828 (2019).
Wencker, M. et al. Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nat. Immunol. 15, 80–87 (2014).
Cai, Y. et al. Differential roles of the mTOR-STAT3 signaling in dermal γδ T cell effector function in skin inflammation. Cell Rep. 27, 3034–3048 (2019).
Agerholm, R., Rizk, J., Viñals, M. T. & Bekiaris, V. STAT3 but not STAT4 is critical for γδT17 cell responses and skin inflammation. EMBO Rep. 20, e48647 (2019).
Powolny-Budnicka, I. et al. RelA and RelB transcription factors in distinct thymocyte populations control lymphotoxin-dependent interleukin-17 production in γδ T cells. Immunity 34, 364–374 (2011).
Mair, F. et al. The NFκB-inducing kinase is essential for the developmental programming of skin-resident and IL-17-producing γδ T cells. eLife 4, e10087 (2015).
Shibata, K. et al. Notch-Hes1 pathway is required for the development of IL-17–producing γδ T cells. Blood 118, 586–593 (2011).
Tontonoz, P. & Spiegelman, B. M. Fat and beyond: the diverse biology of PPARγ. Annu. Rev. Biochem. 77, 289–312 (2008).
Glass, C. K. & Saijo, K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat. Rev. Immunol. 10, 365–376 (2010).
Pedersen, G. & Brynskov, J. Topical rosiglitazone treatment improves ulcerative colitis by restoring peroxisome proliferator-activated receptor-γ activity. Am. J. Gastroenterol. 105, 1595–1603 (2010).
Hontecillas, R. & Bassaganya-Riera, J. Peroxisome proliferator-activated receptor γ is required for regulatory CD4+ T cell-mediated protection against colitis. J. Immunol. 178, 2940–2949 (2007).
Hontecillas, R. et al. Immunoregulatory mechanisms of macrophage PPAR-γ in mice with experimental inflammatory bowel disease. Mucosal Immunol. 4, 304–313 (2011).
Adachi, M. et al. Peroxisome proliferator activated receptor γ in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut 55, 1104–1113 (2006).
Hontecillas, R. et al. Dietary abscisic acid ameliorates influenza-virus-associated disease and pulmonary immunopathology through a PPARγ-dependent mechanism. J. Nutr. Biochem. 24, 1019–1027 (2013).
Sanchez-Hidalgo, M., Martin, A., Villegas, I. & De La Lastra, C. A. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, reduces chronic colonic inflammation in rats. Biochem. Pharmacol. 69, 1733–1744 (2005).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 81874361 and 81374038), the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX18_0828), and the “Double First-Class” University project (CPU2018GY10).
Author information
Authors and Affiliations
Contributions
Y.X., Y.D., and X.Y. designed the study. X.Y. performed experiments. Y.F. and C.L. assisted in partial experiments. X.Y. and Y.F. analyzed the data. X.Y., Y.D., and Y.X. wrote the paper. Y.T. and S.Q. helped revise the paper. Y.T. and S.Q. provided suggestions. X.Y., Y.D., and Y.X. revised the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by T. Brunner
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yun, X., Fang, Y., Lv, C. et al. Inhibition of the activation of γδT17 cells through PPARγ–PTEN/Akt/GSK3β/NFAT pathway contributes to the anti-colitis effect of madecassic acid. Cell Death Dis 11, 752 (2020). https://doi.org/10.1038/s41419-020-02969-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-020-02969-x
- Springer Nature Limited