Abstract
Background
The adhesion G-protein-coupled receptors (GPCRs) play crucial roles in tumour pathogenesis, however, their clinical significance in pancreatic ductal adenocarcinoma (PDAC) remains unclear.
Methods
We analysed 796 PDAC patients, including 331 from public data sets (TCGA, ICGC and GSE57495) and 465 from independent cohorts (training: n = 321, validation: n = 144). Using in-vitro studies, we confirmed the biological function of the candidate GPCRs.
Results
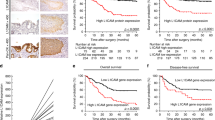
Analysis of all 33 adhesion GPCRs, led to identify GPR115, as the only significant prognostic factor in all public data sets. The patients with high GPR115 expression exhibited significantly poorer prognosis for OS and RFS, in training (P < 0.01, P < 0.01) and validation cohort (P < 0.01, P = 0.04). Multivariate analysis indicated that GPR115 high expression was an independent prognostic factor in both cohorts (HR = 1.43; P = 0.01, HR = 2.55; P < 0.01). A risk-prediction model using Cox regression by incorporating GPR115 and clinicopathological factors accurately predicted 5-year survival following surgery. In addition, GPR115 silencing inhibited cell proliferation and migration in PDAC cells.
Conclusion
We demonstrated that GPR115 has important prognostic significance and functional role in tumour progression; providing a rationale that this may be a potential therapeutic target in patients with PDAC.



Similar content being viewed by others
Data availability
All data are available within the article.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Puleo F, Nicolle R, Blum Y, Cros J, Marisa L, Demetter P, et al. Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology. 2018;155:1999–2013.e1993.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Okada KI, Shimokawa T, Hirono S, Kawai M, Sho M, Satoi S, et al. Effect of neoadjuvant nab-paclitaxel plus gemcitabine therapy on overall survival in patients with borderline resectable pancreatic cancer: a prospective multicenter phase II trial (NAC-GA Trial). Oncology. 2017;93:343–6.
Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–24.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13.
Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248–57.
Ohkawa S, Okusaka T, Isayama H, Fukutomi A, Yamaguchi K, Ikeda M, et al. Randomised phase II trial of S-1 plus oxaliplatin vs S-1 in patients with gemcitabine-refractory pancreatic cancer. Br J Cancer. 2015;112:1428–34.
Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640–8.
Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plusnab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–54.
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl J Med. 2011;364:1817–25.
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6.
Puleo F, Nicolle R, Blum Y, Cros J, Marisa L, Demetter P, et al. Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology. https://doi.org/10.1053/j.gastro.2018.08.033 (2018).
Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10.
O’Reilly EM, Oh DY, Dhani N, Renouf DJ, Lee MA, Sun W, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1431–8.
Katagiri R, Goto A, Nakagawa T, Nishiumi S, Kobayashi T, Hidaka A, et al. Increased levels of branched-chain amino acid associated with increased risk of pancreatic cancer in a prospective case–control study of a large cohort. Gastroenterology. https://doi.org/10.1053/j.gastro.2018.07.033 (2018).
Yoshida K, Toden S, Ravindranathan P, Han H, Goel A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis. 2017;38:1036–46.
Ferrone CR, Kattan MW, Tomlinson JS, Thayer SP, Brennan MF, Warshaw AL. Validation of a postresection pancreatic adenocarcinoma nomogram for disease-specific survival. J Clin Oncol. 2005;23:7529–35.
Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–26.
Groot VP, Gemenetzis G, Blair AB, Rivero-Soto RJ, Yu J, Javed AA, et al. Defining and predicting early recurrence in 957 patients with resected pancreatic ductal adenocarcinoma. Ann Surg. https://doi.org/10.1097/SLA.0000000000002734 (2018).
Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018;267:936–45.
Parikh AA, Maiga A, Bentrem D, Squires MH 3rd, Kooby DA, Maithel SK, et al. Adjuvant therapy in pancreas cancer: does it influence patterns of recurrence? J Am Coll Surg. 2016;222:448–56.
Takahashi H, Ohigashi H, Ishikawa O, Gotoh K, Yamada T, Nagata S, et al. Perineural invasion and lymph node involvement as indicators of surgical outcome and pattern of recurrence in the setting of preoperative gemcitabine-based chemoradiation therapy for resectable pancreatic cancer. Ann Surg. 2012;255:95–102.
Takahashi H, Akita H, Tomokuni A, Kobayashi S, Ohigashi H, Fijiwara Y, et al. Preoperative gemcitabine-based chemoradiation therapy for borderline resectable pancreatic cancer: impact of venous and arterial involvement status on surgical outcome and pattern of recurrence. Ann Surg. 2016;264:1091–7.
Conroy T, Bachet J-B, Ayav A, Huguet F, Lambert A, Caramella C, et al. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur J Cancer. 2016;57:10–22.
Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–406.
Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–81.
Liao W-C, Chien K-L, Lin Y-L, Wu M-S, Lin J-T, Wang H-P, et al. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14:1095–103.
Civelli O, Reinscheid RK, Zhang Y, Wang Z, Fredriksson R, Schioth HB. G protein-coupled receptor deorphanizations. Annu Rev Pharm Toxicol. 2013;53:127–46.
Hamann J, Aust G, Arac D, Engel FB, Formstone C, Fredriksson R, et al. International union of basic and clinical pharmacology. XCIV. Adhesion G protein-coupled receptors. Pharm Rev. 2015;67:338–67.
Hao F, Xu Q, Zhao Y, Stevens JV, Young SH, Sinnett-Smith J, et al. Insulin receptor and GPCR crosstalk stimulates YAP via PI3K and PKD in pancreatic cancer cells. Mol Cancer Res. 2017;15:929–41.
Thakur G, Kumar R, Kim SB, Lee SY, Lee SL, Rho GJ. Therapeutic status and available strategies in pancreatic ductal adenocarcinoma. Biomedicines. 2021;9:178.
Insel PA, Sriram K, Wiley SZ, Wilderman A, Katakia T, McCann T, et al. GPCRomics: GPCR expression in cancer cells and tumors identifies new, potential biomarkers and therapeutic targets. Front Pharm. 2018;9:431.
Wiley SZ, Sriram K, Liang W, Chang SE, French R, McCann T, et al. GPR68, a proton-sensing GPCR, mediates interaction of cancer-associated fibroblasts and cancer cells. FASEB J. 2018;32:1170–83.
He Z, Wu H, Jiao Y, Zheng J. Expression and prognostic value of CD97 and its ligand CD55 in pancreatic cancer. Oncol Lett. 2015;9:793–7.
Hilbig D, Dietrich N, Wandel E, Gonsior S, Sittig D, Hamann J, et al. The Interaction of CD97/ADGRE5 With beta-catenin in adherens junctions is lost during colorectal carcinogenesis. Front Oncol. 2018;8:182.
Ji B, Feng Y, Sun Y, Ji D, Qian W, Zhang Z, et al. GPR56 promotes proliferation of colorectal cancer cells and enhances metastasis via epithelialmesenchymal transition through PI3K/AKT signaling activation. Oncol Rep. 2018;40:1885–96.
Cherry AE, Stella N. G protein-coupled receptors as oncogenic signals in glioma: emerging therapeutic avenues. Neuroscience. 2014;278:222–36.
Gerber PA, Hevezi P, Buhren BA, Martinez C, Schrumpf H, Gasis M, et al. Systematic identification and characterization of novel human skin-associated genes encoding membrane and secreted proteins. PLoS ONE. 2013;8:e63949.
Ozer B, Sezerman U. Analysis of the interplay between methylation and expression reveals its potential role in cancer aetiology. Funct Integr Genomics. 2017;17:53–68.
Wu J, Lei L, Wang S, Gu D, Zhang J. Immunohistochemical expression and prognostic value of CD97 and its ligand CD55 in primary gallbladder carcinoma. J Biomed Biotechnol. 2012;2012:587672.
Ward Y, Lake R, Faraji F, Sperger J, Martin P, Gilliard C, et al. Platelets promote metastasis via binding tumor CD97 leading to bidirectional signaling that coordinates transendothelial migration. Cell Rep. 2018;23:808–22.
Nishiwada S, Sho M, Banwait JK, Yamamura K, Akahori T, Nakamura K, et al. A microRNA signature identifies pancreatic ductal adenocarcinoma patients at risk for lymph node metastases. Gastroenterology. 2020;159:562–74.
Satoi S, Murakami Y, Motoi F, Uemura K, Kawai M, Kurata M, et al. Reappraisal of peritoneal washing cytology in 984 patients with pancreatic ductal adenocarcinoma who underwent margin-negative resection. J Gastrointest Surg. 2015;19:6–14.
Sho M, Murakami Y, Motoi F, Satoi S, Matsumoto I, Kawai M, et al. Postoperative prognosis of pancreatic cancer with para-aortic lymph node metastasis: a multicenter study on 822 patients. J Gastroenterol. 2015;50:694–702.
Nishiwada S, Sho M, Cui Y, Yamamura K, Akahori T, Nakagawa K, et al. A gene expression signature for predicting response to neoadjuvant chemoradiotherapy in pancreatic ductal adenocarcinoma. Int J Cancer. 2021;148:769–79.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Nishiwada S, Cui Y, Sho M, Jun E, Akahori T, Nakamura K, et al. Transcriptomic profiling identifies an exosomal microrna signature for predicting recurrence following surgery in patients with pancreatic ductal adenocarcinoma. Ann Surg. https://doi.org/10.1097/SLA.0000000000004993 (2021).
Ozawa T, Kandimalla R, Gao F, Nozawa H, Hata K, Nagata H, et al. A microRNA signature associated with metastasis of T1 colorectal cancers to lymph nodes. Gastroenterology. 2018;154:844–8.e847.
Takahashi M, Sung B, Shen Y, Hur K, Link A, Boland CR, et al. Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR-200 microRNA family. Carcinogenesis. 2012;33:2441–9.
Okada Y, Takahashi N, Takayama T, Goel A. LAMC2 promotes cancer progression and gemcitabine resistance through modulation of EMT and ATP-binding cassette transporters in pancreatic ductal adenocarcinoma. Carcinogenesis. 2021;42:546–56.
Shimura T, Kandimalla R, Okugawa Y, Ohi M, Toiyama Y, He C, et al. Novel evidence for m(6)A methylation regulators as prognostic biomarkers and FTO as a potential therapeutic target in gastric cancer. Br J Cancer. https://doi.org/10.1038/s41416-021-01581-w (2021).
Ozawa T, Matsuyama T, Toiyama Y, Takahashi N, Ishikawa T, Uetake H, et al. CCAT1 and CCAT2 long noncoding RNAs, located within the 8q.24.21 ‘gene desert’, serve as important prognostic biomarkers in colorectal cancer. Ann Oncol. 2017;28:1882–8.
Haitina T, Olsson F, Stephansson O, Alsio J, Roman E, Ebendal T, et al. Expression profile of the entire family of adhesion G protein-coupled receptors in mouse and rat. BMC Neurosci. 2008;9:43.
Zhang W, Cui Q, Qu W, Ding X, Jiang D, Liu H. TRIM58/cg26157385 methylation is associated with eight prognostic genes in lung squamous cell carcinoma. Oncol Rep. 2018;40:206–16.
Wang Y, Shi M, Yang N, Zhou X, Xu L. GPR115 contributes to lung adenocarcinoma metastasis associated with LAMC2 and predicts a poor prognosis. Front Oncol. 2020;10:577530.
Yoon JH, Cho SG. ADGRF4 regulates non-small cell lung cancer cell invasiveness. Anticancer Res. 2020;40:6835–44.
Acknowledgements
We thank Tatsuhiko Kakisaka, Takeo Toshima, Raju Kandimalla, Goretti Hernandez, Priyanka Sharma, Raju Adduri, and Souvik Ghatak for discussing the how best to set up the experiments and their analyses. We thank Jasjit Banwait for assisting with the analysis. We thank Naoya Ikeda, Takahiro Akahori, and Tadataka Takagi for collecting clinical samples and patient information.
Funding
The present work was supported by the CA72851, CA187956, CA202797 and CA214254 grants from the National Cancer Institute, National Institute of Health; In addition, this work was also supported by a pilot research award from the City of Hope Ludwig Cancer Research-Hilton Foundation Partnership award.
Author information
Authors and Affiliations
Contributions
Study concept and design: SN, MS, AG; Specimen providers: SN, KN, MN, TT, SY, TF, YK, MS; Acquisition of clinical data: SN, KN, MN, TT, SY, TF, YK, MS; Analysis and interpretation of data and statistical analysis: SN, TS, KY, AG; Drafting of the manuscript: SN, MS and AG.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All study-related procedures were performed as per the Declarations of Helsinki, wherein a written informed consent was obtained from each patient, and the institutional review boards of all participating institutions involved approved the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nishiwada, S., Shimura, T., Yamamura, K. et al. Clinical significance and functional role of adhesion G-protein-coupled receptors in human pancreatic ductal adenocarcinoma. Br J Cancer 128, 321–330 (2023). https://doi.org/10.1038/s41416-022-02057-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-02057-1
- Springer Nature Limited