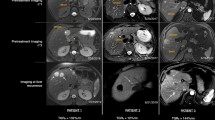
Abstract
Background
The RECIST-based response variably matches the clinical benefit of systemic therapies for liver metastatic uveal melanoma (LMUM). The aims were to determine whether the tumour growth rate (TGR) can help predict the survival in patients with LMUM and to provide information for the management of first-line systemic treatment.
Methods
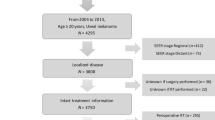
This retrospective study included 147 (training: n = 110, validation: n = 37) patients with LMUM treated with first-line systemic treatment between 2010 and 2021. Two TGR-derived parameters were calculated, TGR0 and TGR3m. Multivariate Cox analyses identified independent predictors of progression-free survival (PFS) and overall survival (OS).
Results
TGR3m was a strong independent prognostic factor of PFS and OS (p < 0.001). The RECIST-based response was no longer significant in the OS analyses. Only immunotherapy regimens correlated with higher OS (HR = 0.2; 95% CI, 0.1–0.5; p < 0.001) in the low-TGR3m (≤50%/m) subgroup. These findings were confirmed in the validation cohort. TGR0, disease-free interval (DFI), and the sum of target lesions at baseline were predictive factors of low TGR3m.
Discussion
The use of TGR3m would improve tumour assessment by identifying patients who would benefit from first-line immunotherapy regimens despite PD. TGR0, DFI and the sum of target lesions were correlated with TGR3m, which can support first-line treatment decision-making for immunotherapy.




Similar content being viewed by others
Data availability
The data generated in this study are available upon request from the corresponding author.
References
Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–5.
Rantala ES, Hernberg M, Kivelä TT. Overall survival after treatment for metastatic uveal melanoma: a systematic review and meta-analysis. Melanoma Res. 2019;29:561–8.
Mariani P, Piperno-Neumann S, Servois V, Berry MG, Dorval T, Plancher C, et al. Surgical management of liver metastases from uveal melanoma: 16 years’ experience at the Institut Curie. Eur J Surg Oncol. 2009;35:1192–7.
Carvajal RD, Schwartz GK, Tezel T, Marr B, Francis JH, Nathan PD. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol. 2017;101:38–44.
Pelster MS, Gruschkus SK, Bassett R, Gombos D, Shephard M, Posada L, et al. Nivolumab and ipilimumab in metastatic uveal melanoma: results from a single-arm phase II study. J Clin Oncol. 2021;39:599–607.
Piulats JM, Espinosa E, de la Cruz Merino L, Varela M, Carrión L, Martín-Algarra S, et al. Nivolumab plus ipilimumab for treatment-naïve metastatic uveal melanoma: an open-label, multicenter, phase II trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). J Clin Oncol. 2021;39:586–98.
Nathan P, Hassel JC, Rutkowski P, Baurain J-F, Butler MO, Schlaak M, et al. Overall Survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385:1196–206.
Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1—Update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7.
Ferté C, Fernandez M, Hollebecque A, Koscielny S, Levy A, Massard C, et al. Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin Cancer Res. 2014;20:246–52.
Carter BW, Murthy R, Balmes G, Wachter EA, Patel S. Response assessment of metastatic uveal melanoma treated with rose bengal disodium. Ann Oncol. 2019;30:xi46.
Shoushtari AN, Collins L, Espinosa E, Sethi H, Stanhope S, Abdullah S, et al. 1757O Early reduction in ctDNA, regardless of best RECIST response, is associated with overall survival (OS) on tebentafusp in previously treated metastatic uveal melanoma (mUM) patients. Ann Oncol. 2021;32:S1210.
Gomez-Roca C, Koscielny S, Ribrag V, Dromain C, Marzouk I, Bidault F, et al. Tumour growth rates and RECIST criteria in early drug development. Eur J Cancer. 2011;47:2512–6.
Lamarca A, Ronot M, Moalla S, Crona J, Opalinska M, Lopez Lopez C, et al. Tumor growth rate as a validated early radiological biomarker able to reflect treatment-induced changes in neuroendocrine tumors: The GREPONET-2 Study. Clin Cancer Res. 2019;25:6692–9.
Ramtohul T, Ait Rais K, Gardrat S, Barnhill R, Román-Román S, Cassoux N, et al. Prognostic implications of mri melanin quantification and cytogenetic abnormalities in liver metastases of uveal melanoma. Cancers. 2021;13:2728.
McShane LM, Altman DG, Sauerbrei W, Taube S, Gion M, Clark G, et al. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Rev Clin Oncol. 2005;2:416–22.
on behalf of the CLARINET Study Group, Dromain C, Pavel ME, Ruszniewski P, Langley A, Massien C, et al. Tumor growth rate as a metric of progression, response, and prognosis in pancreatic and intestinal neuroendocrine tumors. BMC Cancer. 2019;19:66.
Algazi AP, Tsai KK, Shoushtari AN, Munhoz RR, Eroglu Z, Piulats JM, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies: PD-1 Blockade in Uveal Melanoma. Cancer. 2016;122:3344–53.
Najjar YG, Navrazhina K, Ding F, Bhatia R, Tsai K, Abbate K, et al. Ipilimumab plus nivolumab for patients with metastatic uveal melanoma: a multicenter, retrospective study. J Immunother Cancer. 2020;8:e000331.
Heppt MV, Amaral T, Kähler KC, Heinzerling L, Hassel JC, Meissner M, et al. Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multi-center study. J Immunother Cancer. 2019;7:299.
Eskelin S, Pyrhonen S, Summanen P, Hahka-Kemppinen M, Kivela T. Tumor doubling times in metastatic malignant melanoma of the uvea. Ophthalmology. 2000;107:1443–9.
Ferté C, Koscielny S, Albiges L, Rocher L, Soria J-C, Iacovelli R, et al. Tumor growth rate provides useful information to evaluate sorafenib and everolimus treatment in metastatic renal cell carcinoma patients: an integrated analysis of the TARGET and RECORD Phase 3 Trial Data. Eur Urol. 2014;65:713–20.
ten Berge DMHJ, Hurkmans DP, den Besten I, Kloover JS, Mathijssen RHJ, Debets RJEMA, et al. Tumour growth rate as a tool for response evaluation during PD-1 treatment for non-small cell lung cancer: a retrospective analysis. ERJ Open Res. 2019;5:00179–2019.
Khoja L, Atenafu EG, Suciu S, Leyvraz S, Sato T, Marshall E, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol. 2019;30:1370–80.
Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant Nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–35.
Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–801.
Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668–74.
Beaver JA, Hazarika M, Mulkey F, Mushti S, Chen H, He K, et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2018;19:229–39.
Kim JH. Comparison of the RECIST 1.0 and RECIST 1.1 in patients treated with targeted agents: a pooled analysis and review. Oncotarget. 2016;7:13680–7.
Mehrara E, Forssell-Aronsson E, Ahlman H, Bernhardt P. Specific growth rate versus doubling time for quantitative characterization of tumor growth rate. Cancer Res. 2007;67:3970–5.
Wagner NB, Lenders MM, Kühl K, Reinhardt L, André F, Dudda M, et al. Pretreatment metastatic growth rate determines clinical outcome of advanced melanoma patients treated with anti-PD-1 antibodies: a multicenter cohort study. J Immunother Cancer. 2021;9:e002350.
Maitland ML, Wilkerson J, Karovic S, Zhao B, Flynn J, Zhou M, et al. Enhanced detection of treatment effects on metastatic colorectal cancer with volumetric CT measurements for tumor burden growth rate evaluation. Clin Cancer Res. 2020;26:6464–74.
Gerlee P. The model muddle: in search of tumor growth laws. Cancer Res. 2013;73:2407–11.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualisation: TR and VS; methodology: TR, AC and VS; software: TR; validation: all authors; formal analysis: TR and VS; investigation: all authors; resources: SP, NC, LL, DM, SG, GP, PM and VS; data curation: TR, AC and VS; writing—original draft preparation: TR; writing—review and editing: TR, AC, MR, LC, PM and VS; visualisation: all authors; supervision: VS; project administration: VS; funding acquisition: VS. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Institutional Review Board approval was obtained from the Institut Curie prior to the initiation of this study.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ramtohul, T., Cohen, A., Rodrigues, M. et al. Tumour growth rate improves tumour assessment and first-line systemic treatment decision-making for immunotherapy in patients with liver metastatic uveal melanoma. Br J Cancer 127, 258–267 (2022). https://doi.org/10.1038/s41416-022-01793-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01793-8
- Springer Nature Limited