Abstract
Background
This is the first-in-human study of novel anti-angiopoietin-2 (Ang-2) monoclonal antibody LY3127804 as monotherapy and in combination with ramucirumab in advanced solid tumours.
Methods
Patients received intravenous LY3127804 monotherapy (4, 8, 12, 16, 20 and 27 mg/kg) in part A; LY3127804 (8, 12, 16, 20 and 27 mg/kg) with 8 mg/kg ramucirumab in part B; and LY3127804 (20 mg/kg) with 12 mg/kg ramucirumab in part C. Treatments were administered every 2 weeks (Q2W) during 28-day cycles. Dose-escalation was based on cycle 1 dose-limiting toxicities (DLTs).
Results
Sixty-two patients were treated in part A (n = 20), part B (n = 35) and part C (n = 7). Constipation, diarrhoea and fatigue were the most common treatment-emergent adverse events (TEAEs) in part A; hypertension and peripheral oedema were the most frequent TEAE in parts B and C. No DLT was observed and maximum tolerated dose for LY3127804 was not reached. Four patients achieved partial response with combination therapy (clear cell endometrial carcinoma, cervix squamous cell carcinoma, carcinoma of unknown primary and gastroesophageal junction carcinoma), 29 achieved stable disease, and 24 had progressive disease.
Conclusions
LY3127804 monotherapy and its combination with ramucirumab are well tolerated. LY3127804 20 mg/kg was the recommended Phase 2 dose.
Similar content being viewed by others
Background
Angiogenesis is a vital process for growth, proliferation and metastasis of tumours. Multiple vascular factors play an important role in angiogenesis, with vascular endothelial growth factors and receptors (VEGF-VEGFRs) among the most potent angiogenic systems1 in promoting the growth of solid organ malignancies.2 Therefore, anti-VEGF-VEGFR therapies have become the standards of care (SoC) for several solid malignant conditions including colorectal cancer, renal cancer, hepatocellular cancer and ovarian cancer.3
The angiopoietin-Tie receptor axis is another important proangiogenic system that promotes tumour progression. The system comprises the Tie-1 and Tie-2 tyrosine kinase receptors and angiopoietin 1–4 (Ang-1 to Ang4) as ligands to Tie-2.4 Ang-1 and Ang-2 are the most extensively studied Ang ligands. While Ang-1 is responsible for vessel maturation, and mediation of endothelial cell migration, adhesion and survival, Ang-2 promotes apoptosis and vascular regression, and causes disruption of the connections between the endothelium and perivascular cells.4 Several Ang-2 inhibitors have been evaluated for their safety and efficacy in solid tumours. Phase 1 studies of AMG 386 (trebananib; an Ang-1 and Ang-2 inhibitor) as monotherapy5 and in combination with anti-VEGF therapy,6,7 showed good tolerance and antitumour activity in patients with advanced solid tumours. Similar activity results were observed with the selective anti-Ang-2 monoclonal antibody (MAb) MEDI3617 combined with bevacizumab, although further development of the drug was discontinued due to toxicity.8 Monotherapy with nesvacumab (REGN910), another Ang-2 inhibitor, also showed acceptable safety and antitumour activity in patients with treatment-refractory advanced solid tumours.9 In addition, combination of Ang-2 inhibitors and chemotherapy also showed no new safety signals in the recently concluded TRINOVA studies.10,11,12 Simultaneous inhibition of Ang-2/Tie-2 and VEGFA/VEGFR2 pathways may play a role in vessel destabilisation and have an effect on the proliferation and migration of endothelial cells leading to a greater anti-angiogenic effect in the treatment of malignancies. Therefore, a combination of Ang-2 inhibitors and anti-VEGF therapies may be considered promising in patients with multiple tumour types.13
LY3127804 (Eli Lilly and Company; Indianapolis, IN, USA) (https://adisinsight.springer.com/drugs/800043698) is a novel anti-Ang-2, humanised immunoglobulin G4 (IgG4) MAb, which is under clinical development for the treatment of advanced/metastatic solid tumours. LY3127804 and aflibercept combination has shown efficacy in mouse models.14 Furthermore, LY3127804 in combination with DC101, a murine anti-VEGFR2 antibody, had greater efficacy than LY3127804 monotherapy in multiple patient-derived xenograft models including models for non-small cell lung cancer (NSCLC) and ovarian cancer.14 We report the findings of the first-in-human, Phase 1, dose-escalation study JQBA, which assessed the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of LY3127804 in patients with advanced solid tumours. In this trial, LY3127804 was administered as monotherapy or in combination with ramucirumab, a MAb targeting VEGFR2.
Methods
Study design and patient population
The JQBA study was the first-in-human, non-randomised, open-label, dose-escalation, Phase 1 study of LY3127804 in patients with advanced/metastatic solid tumours who had disease progression with SoC or for whom there was no SoC treatment available. Patients from four centres in Belgium, France, Spain and the United States were enrolled between November 19, 2015 and November 23, 2017.
Institutional review boards of each centre approved the study protocol before initiation of patient enrolment. The study adhered to the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, Council for International Organizations of Medical Sciences and applicable local regulations. All patients provided informed consent before enrolment. The study was registered in ClinicalTrials.gov (NCT02597036).15
The study included adult patients with histologically or cytologically confirmed advanced/metastatic solid tumours who were appropriate candidates for experimental therapy in the absence of a standard therapy or for whom available standard therapies had failed to provide clinical benefit for their disease, who refused standard therapies, or who were not a candidate for those therapies, and had progressive disease prior to study entry. Study patients were required to have measurable or evaluable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1;16 life expectancy that would allow completion of at least two treatment cycles; adequate hematologic, hepatic, renal, and coagulation functions; Eastern Cooperative Oncology Group performance status of ≤1; to have discontinued receiving any anticancer therapy for the last 28 days or five half-lives of the previous treatment before enrolment, whichever was shorter, and recovered from the acute effects of therapy.
Patients were excluded if they had any contraindication to LY3127804 or other MAbs, were previously treated with Ang-2 inhibitors, had any second primary active malignancy, or had any bleeding disorder or thromboembolic event. Patients with active/known fungal/bacterial/viral infection, history of major surgery within 28 days prior to randomisation, or placement of a central venous access device within 7 days before receiving treatment, or any planned major surgery during the trial, and patients (with non-small cell lung cancer only) with gross haemoptysis or major blood vessel encasement were also excluded.
Treatment plan
The study was conducted in three parts (Supplementary Fig. S1). Maximum duration of treatment was not predetermined. In part A, dose-escalation followed a 3 + 3 study design and the patients with any solid malignancy were treated in six cohorts (Cohort A1–A6). Cohorts A1–A6 received LY3127804 monotherapy doses of 4, 8, 12, 16, 20 and 27 mg/kg, respectively, intravenously (IV) once every 2 weeks (Q2W). In part B, patients were treated in five cohorts (Cohort B2-B6) with LY3127804 doses of 8, 12, 16, 20 and 27 mg/kg, respectively, IV Q2W in combination with ramucirumab 8 mg/kg Q2W. Patients were treated in part B once the corresponding dose level in part A was declared safe. Part C was conducted after the recommended Phase 2 dose (RP2D) for LY3127804 was determined. In part C, LY3127804 20 mg/kg IV Q2W was administered with ramucirumab 12 mg/kg Q2W.
All doses of LY3127804 and ramucirumab were administered on days 1 and 15 of the 28-day treatment cycle. Ramucirumab was administered at least 60 min after LY3127804 dose. Total doses and planned duration of infusion for LY3127804 and ramucirumab are presented in Supplementary Table S1. Intrapatient dose-escalation was not allowed. Patients discontinued treatment due to unacceptable toxicity, progression of disease, investigator/sponsor decision or withdrawal of informed consent by the patient. Dose-limiting toxicity (DLT) period was defined as the first 28 days after first dosing (cycle 1). Dose-escalation decisions were based primarily on the safety data from the DLT period. PK data were also reviewed before dose-escalation decisions. To accommodate possible schedule conflicts, treatment was administered up to 3 days after the scheduled dosing day. No shortening of the treatment cycle was allowed.
Objectives and assessments
The primary objective was to determine the RP2D and schedule of LY3127804 as monotherapy and combination with ramucirumab. The secondary objectives included characterisation of safety and maximum tolerated dose (MTD), PK-PD assessment of LY3127804, evaluation of limited PK (peak and trough concentrations) of ramucirumab in combination with LY3127804 and antibody formation against LY3127804. All assessments were made for LY3127804 with or without ramucirumab. Data were collected until the MTD was reached or the RP2D was determined, whichever occurred first.
Safety was assessed throughout the study using the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE, v4.0).17 A DLT was defined as any of the following adverse events (AEs) (≥grade 3 nonhematological toxicity despite maximal medical management; grade 4 haematological toxicity for > 5 days; febrile neutropenia; grade 4 thrombocytopenia or Grade 3 thrombocytopenia complicated with ≥ grade 2 bleeding; newly developed oedema characterised by a body weight increase of >10% as compared to the start of therapy; any toxicity deemed significant by investigator), possibly related to LY3127804 and occurring during the DLT period in parts A, B and C. MTD was defined as the highest tested dose in a single-agent setting that has <33% probability of causing a DLT.
PK evaluation included maximum observed drug concentration (Cmax), area under the concentration-time curve (AUC: from time zero to time t, for which t was the last time point with a measurable concentration [AUC0-tlast], from time zero to infinity [AUC0-∞], during a dosing interval at steady state [AUCτ-ss), volume of distribution (Vd) and clearance (CL) for LY3127804 monotherapy and in combination with ramucirumab. We used a standard noncompartmental analysis method for PK parameter estimates for LY3127804. Pre-dose samples of LY3127804 were collected on day 1 and day 15, with post-LY3127804 dose samples collected on day 2 and day 16 of the cycle 1 and 2. For patients receiving LY3127804 + ramucirumab, sampling was done immediately after the end of ramucirumab infusion. In order to determine plasma concentration of LY3127804, 3 mL samples were collected. We used validated antigen capture enzyme-linked immunosorbent assays to determine plasma concentrations of LY3127804 and serum samples of ramucirumab. Sampling for PD/biomarker assessment followed the same schedule as for PK and samples were tested for relationship between LY3127804 concentration and biomarkers such as Ang-2/Ang-1 ratio, placenta growth factor (PLGF), VEGF (VEGFA, VEGFC and VEGFD) and soluble receptors (VEGFR2, sTie-2 and sTie-1).
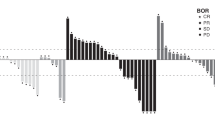
The study was not designed to make an efficacy assessment; we summarised the observed tumour response data in all patients. Until week 8, radiological tumour assessment was performed at the end of each cycle (preferably between day 22 and day 29) and response to treatment was determined using RECIST (v1.1).16 After cycle 8, tumour assessment was done after every 2–4 cycles, as clinically indicated. Best overall response (BOR) was defined as the best response recorded from the start of the study treatment until the earliest of objective progression or start of new anticancer therapy. Disease control rate (DCR) was defined as the proportion of patients who exhibited stable disease (SD) or confirmed complete response or partial response (PR) relative to baseline.
Statistical considerations
The estimated sample size for this study was 45–60 patients. In part A, the planned enrolment was 15–30 patients, which was estimated using a 3 + 3 dose-escalation method and incidence of DLT.18 In parts B and C, estimated patient enrolment was 30 patients (six patients per dose level). Demographic characteristics, patient disposition and all endpoints, except any exploratory analysis, were summarised using descriptive analysis. The analysis included all patients who had received ≥1 dose of LY3127804. All continuous variables were summarised using mean [standard deviation], median (minimum − maximum), whereas categorical variables were summarised using n and percentages.
Results
Patient disposition and baseline characteristics
Between November 2015 and November 2017, 62 patients (mean age 57.3 ± 12.1 years, 58.1% males) with advanced/metastatic solid tumours were enrolled (Table 1), into the following cohorts: part A, n = 20; part B, n = 35; part C, n = 7. At the cut-off date (November 23, 2017), six (9.7%) patients remained on-treatment. Reasons for discontinuation of the study treatment included progression of disease (71%), withdrawal by the patient (4.8%), AEs (3.2%), and death due to study disease (1.6%). Six patients (9.7%) were lost to follow-up.
For LY3127804, the median number of cycles per patient was 2 (1–9), 3 (1–19) and 4 (2–5) in parts A, B and C, respectively. The median duration of treatment in parts A, B and C was 8.6 (4–37) weeks, 11.1 (2–80) weeks and 16.7 (6–20) weeks, respectively. Supplementary Table S2 presents the drug exposure by cohorts in part A and part B.
Safety, toxicity and RP2D
No DLT was reported in any of the cohorts. Therefore, the MTD of LY3127804 was not reached. One patient discontinued the study due to grade 3 hyperbilirubinemia (unrelated to treatment) in cohort B3 and one patient discontinued the study drug due to treatment-related grade 3 hypertension in part C.
Serious AEs (SAEs), regardless of the causality, occurred in 3, 11 and 3 patients in parts A, B and C, respectively (Table 2). Of the four patients with treatment-related SAEs, three were in part B and one was in part C. Grade ≥ 3 events of hypertension (n = 3, 2 in cohorts B5 and B6 and 1 in part C), abdominal pain (n = 1 in cohort B5) and infusion-related reaction (n = 1 in cohort B3) were reported as treatment-related SAEs. Grade ≥ 3 hypertension cases were managed with medical treatment and occurred outside the DLT period. The 20 mg/kg Q2W dose was determined as the RP2D for LY3127804, both as monotherapy and in combination with the two doses of ramucirumab.
Treatment-emergent adverse event (TEAE) occurred in all patients. Treatment-related AEs occurred in 41 patients (66.1%). Grade ≥ 3 TEAEs were reported in 34 patients (54.8%) (Table 2), of which 12 patients (19.30%) had treatment-related grade ≥ 3 AEs. In part A, the most frequently occurring TEAEs included constipation, diarrhoea, fatigue and peripheral oedema, occurring in 20% of patients each (Supplementary Table S3). Fatigue (10%) was the most common treatment-related AEs in part A (Table 3).
In part B, hypertension and peripheral oedema were the most common TEAEs (42.9% each) followed by fatigue (28.6%), headache (25.7%) and vomiting (22.9%; Supplementary Table S4). The most frequent treatment-related AEs in part B were hypertension (34.3%), fatigue (22.9%) and peripheral oedema (20.0%) (Table 3). Hypertension (57.1%) and constipation (42.9%) were the most common TEAEs in part C, with 42.9% of patients having study treatment-related hypertension. Dose-modifications were made in 13 patients overall (21%); four in part A and nine in part B.
Twelve deaths (19.4%) were reported during the study, four in part A (one each in A3 and A5, and two in A6) and eight in part B (one in B3, two each in B4 and B5 and three in B6). Progressive disease caused nine deaths, six during treatment and three after 30 days of discontinuing study treatment. One death due to a TEAE of pharyngeal haemorrhage occurred during treatment in cohort B5 (LY3127804 20 mg/kg + ramucirumab 8 mg/kg). The patient had received high dose radiotherapy to the bleeding area. The event was not considered unequivocally related to study treatment. Two patients died due to an unknown cause 30 days after discontinuing study treatment.
PK-PD evaluation
Mean plasma concentration of LY3127804 after single or multiple doses increased with higher doses (Fig. 1). LY3127804 CL was similar following administration as single agent and in combination with ramucirumab. The combined PK data from all parts showed a constant CL and terminal half-life (t1/2) for LY3127804, irrespective of the dose. Consequently, AUC(0-336) increased in a dose-proportional manner for each dose and each day of dosing (Table 4). Mean CL, Vd and t1/2 for LY3127804 across the study were 16.3 mL/h, 5.2 L and 222 h, respectively. The CL, Vd and t1/2 of LY3127804 at day 1, day 15 and day 29 are presented in Fig. 2.
Ang-2 concentrations increased substantially with LY3127804 therapy, possibly due to the binding of LY3127804 to Ang-2 leading to decrease of Ang-2 clearance or turnover rate. Similarly, an increase in Ang-1 levels was observed under LY3127804 treatment. PLGF concentration increased substantially in the combination arm (part B and C) as has been shown previously with ramucirumab treatment alone, while VEGFC increased moderately with LY3127804 and ramucirumab combination treatments (Supplementary Figs. S2 and S3). The postbaseline, on-treatment VEGFD data are very limited and suggest a moderate increase under treatment, but that trend is within the substantial interpatient CV.
Immunogenicity
We collected 371 samples for immunogenicity analysis, of which 332 (90%) were analysed. Of the 59 patients with evaluable data, treatment-emergent antidrug antibodies (TE ADA) were detected in six patients (10.2%). There was no correlation between the LY3127804 dose and TE ADA detection. Titre range for TE ADA was 1:10–1:80.
Efficacy
Confirmed PR as BOR was achieved by four patients: two patients from cohort B2 (one each with clear cell endometrial carcinoma and cervix squamous cell carcinoma), one patient from cohort B6 (carcinoma of unknown primary) and one patient from part C (gastroesophageal junction adenocarcinoma). Eleven of 20 patients (55.0%) in part A, 13 of 35 patients (37.1%) in part B and five of seven patients (71.4%) in part C achieved SD; whereas 8 (40.0%), 15 (42.9%), and 1 (14.3%) had progressive disease in parts A, B and C, respectively. Three patients were not evaluable for efficacy. Among the tumour types, seven patients with colon carcinoma achieved SD (one each in cohorts A1, A2, A3, A4, B2, B3 and part C), with duration of SD presented in Supplementary Fig. S4. Other tumour types with BOR and the mean duration of treatment in patients in parts A, B and C are presented in Supplementary Fig. S4. BOR was not evaluable in one patient each from part A and part B. Overall response rate (ORR) was zero in part A, 8.6% in part B and 14.3% in part C. DCR was 55.0%, 45.7% and 85.7% in parts A, B and C, respectively.
Discussion
This is the first study to assess the novel anti-Ang-2 MAb LY3127804 as monotherapy and in combination with ramucirumab in patients with advanced solid tumours. The study showed a favourable safety profile for both LY3127804 alone and in combination with ramucirumab. As no DLT occurred and the MTD was not reached, LY3127804 20 mg/kg was defined as the RP2D. The LY3127804 PK, being linear, did not support determination of a target-mediated drug disposition PK model. Consequently, LY3127804 RP2D was determined based on the observed safety/tolerability profile, the on-target effects of hypertension and oedema indicated that the relevant biological dose had been reached. In addition, objective responses were observed at doses below 27 mg/kg. Consequently 20 mg/kg was considered a reasonable RP2D, both for monotherapy and combination.
In our study, fatigue and constipation were the most common treatment-related AEs with LY3127804 monotherapy. Hypertension, constipation, peripheral oedema, fatigue and abdominal pain were the main treatment-related AEs with the LY3127804 and ramucirumab combination. In addition, more than half of the patients treated with LY3127804 monotherapy achieved SD, whereas four patients treated with the LY3127804 and ramucirumab combination showed PR. The findings for safety and tolerability in our study were consistent with the findings for other Ang-2 inhibitors. Phase 1 studies of trebananib (AMG 386; first Ang-1 and Ang-2 blocker) also showed acceptable toxicity profile in patients with solid tumours.5,19,20 Fatigue, abdominal pain and peripheral oedema were the main TEAEs,5 as with LY3127804. Trebananib doses up to 15 mg/kg with chemotherapy and up to 30 mg/kg as monotherapy were considered safe in adults.19,20 RP2D of trebananib was 30 mg/kg in paediatric patients with solid tumours.21 The RP2D of nesvacumab was 20 mg/kg, with a TEAE profile similar to that of LY3127804.9 The MTD was not reached with all doses of MEDI3617 monotherapy and its combinations.8 AMG 780 (an Ang-1 and Ang-2 inhibitor),22 and vanucizumab (a bispecific anti-Ang-2/VEGF MAb) were considered safe in patients with advanced solid tumours.23
In this Phase 1 study, LY3127804 monotherapy and its combination with ramucirumab demonstrated dose-dependent-proportional increase in LY3127804 concentration and AUC. This finding characterised LY3127804 pharmacokinetics as being linear in the dose range studied with a constant CL and t1/2. LY3127804 PK properties are consistent with reported PK properties for large MAb molecules. These observations were consistent with findings from prior PK studies evaluating the Cmax, AUC, CL for trebananib monotherapy,20 and the combination of trebananib with chemotherapy19 and in combination with sorafenib and sunitinib.6
Response assessments with LY3127804 monotherapy (SD: 55%; PR: 0%) were consistent with those reported with trebananib in patients with advanced solid tumours (SD: 55%%; PR: 3.5%)5 and nesvacumab (SD: 48.9%; PR: 2.3%).9 Results from the small cohorts of patients receiving various doses of the combination of LY3127804 with ramucirumab suggest a similar SD (42.8%) but with a higher proportion of patients with PR (9.5%). Therefore, LY3127804 as monotherapy and in combination with ramucirumab showed signs of clinical activity.
The future clinical development of Ang-2 inhibitors such as LY3127804 is likely to be in combination with other anti-angiogenic drugs or with compounds with different mechanisms of action, given their favourable toxicity profile in combinatory regimes. Indeed, combination of anti-angiogenic drugs with immune checkpoint inhibitors (ICIs) has already yielded good results in some advanced solid tumours.24 The ICIs reactivate the exhausted T-cell-mediated immune response, which is suppressed by different mechanisms including angiogenesis.25,26 A number of recent trials have demonstrated the efficacy of ICI and anti-angiogenic combinations in patients with different tumour types. Thus, the Phase 1b study of pembrolizumab and axitinib combination treatment in patients with advanced renal cell carcinoma showed a good safety profile and signs of clinical activity.27 The subsequent Phase 3 trial confirmed superiority of the combinatory treatment over sunitinib monotherapy in ORR, progression-free survival (PFS) and overall survival (OS).28 Similar benefits in PFS were observed with the combination of avelumab plus axitinib29 and atezolizumab plus bevacizumab.30
Other combinations, for instance pembrolizumab plus ramucirumab, have been reported to have manageable safety profile and signs of activity in other tumour types such as biliary tract cancer, NSCLC, gastroesophageal cancer, or urothelial carcinomas.31,32 Inhibition of angiogenesis-mediated immune suppression with the anti-Ang-2 therapy trebananib is under exploration in combination with pembrolizumab.33 Based on the available evidence, the effect of LY3127804 combined with ICIs should be explored in further studies.
In summary, this first-in-human, dose-escalation, Phase 1 study reported a favourable safety profile for LY3127804 with 20 mg/kg Q2W dose determined as the RP2D for LY3127804 in patients with advanced solid tumours. Furthermore, achievement of SD with LY3127804 monotherapy and PR with LY3127804 and ramucirumab combination indicates signs of clinical activity.
References
Shibuya, M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2, 1097–1105 (2011).
Smith, N. R., Baker, D., James, N. H., Ratcliffe, K., Jenkins, M., Ashton, S. E. et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin. Cancer Res. 16, 3548–3561 (2010).
Ramjiawan, R. R., Griffioen, A. W. & Duda, D. G. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Angiogenesis 20, 185–204 (2017).
Fagiani, E. & Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 328, 18–26 (2013).
Herbst, R. S., Hong, D., Chap, L., Kurzrock, R., Jackson, E., Silverman, J. M. et al. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J. Clin. Oncol. 27, 3557–3565 (2009).
Hong, D. S., Gordon, M. S., Samlowski, W. E., Kurzrock, R., Tannir, N., Friedland, D. et al. A phase I, open-label study of trebananib combined with sorafenib or sunitinib in patients with advanced renal cell carcinoma. Clin. Genitourin. Cancer 12, 167–77 e2 (2014).
Hong, D. S., Kurzrock, R., Mulay, M., Rasmussen, E., Wu, B. M., Bass, M. B. et al. A phase 1b, open-label study of trebananib plus bevacizumab or motesanib in patients with solid tumours. Oncotarget 5, 11154–11167 (2014).
Hyman, D. M., Rizvi, N., Natale, R., Armstrong, D. K., Birrer, M., Recht, L. et al. Phase I study of MEDI3617, a selective angiopoietin-2 inhibitor alone and combined with carboplatin/paclitaxel, paclitaxel, or bevacizumab for advanced solid tumors. Clin. Cancer Res. 24, 2749–2757 (2018).
Papadopoulos, K. P., Kelley, R. K., Tolcher, A. W., Razak, A. R., Van Loon, K., Patnaik, A. et al. A phase I first-in-human study of nesvacumab (REGN910), a fully human anti-angiopoietin-2 (Ang2) monoclonal antibody, in patients with advanced solid tumors. Clin. Cancer Res. 22, 1348–1355 (2016).
Marth, C., Vergote, I., Scambia, G., Oberaigner, W., Clamp, A., Berger, R. et al. ENGOT-ov-6/TRINOVA-2: Randomised, double-blind, phase 3 study of pegylated liposomal doxorubicin plus trebananib or placebo in women with recurrent partially platinum-sensitive or resistant ovarian cancer. Eur. J. Cancer 70, 111–121 (2017).
Monk, B. J., Poveda, A., Vergote, I., Raspagliesi, F., Fujiwara, K., Bae, D. S. et al. Final results of a phase 3 study of trebananib plus weekly paclitaxel in recurrent ovarian cancer (TRINOVA-1): Long-term survival, impact of ascites, and progression-free survival-2. Gynecol. Oncol. 143, 27–34 (2016).
Vergote, I., Scambia, G., O’Malley, D. M., Van Calster, B., Park, S. Y., Del Campo, J. M. et al. Trebananib or placebo plus carboplatin and paclitaxel as first-line treatment for advanced ovarian cancer (TRINOVA-3/ENGOT-ov2/GOG-3001): a randomised, double-blind, phase 3 trial. Lancet Oncol. 20, 862–876 (2019).
Gerald, D., Chintharlapalli, S., Augustin, H. G. & Benjamin, L. E. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res. 73, 1649–1657 (2013).
Sudhakar, R., Chintharlapalli, J. E. C., Leung, D., Gerald, D., Lu, J., Iversen, P. W., Lee, L. N., Huber, L., Tetreault, J., Almonte-Baldonado, R., Xu, J., Ramamurthy, B., Pereira, J. A., Chow, C-H., Hanauske, A-R., Wacheck, V., Benjamin, L. & Liu, L. LY3127804, a novel anti-Angiopoietin-2 antibody in combination with an anti-VEGFR2 antibody potently inhibits angiogenesis, tumor growth and metastasis. Cancer Res. 76, (14 Suppl) Abstract nr 3259 (2016).
A study of LY3127804 with ramucirumab in participants with advanced solid tumors. https://clinicaltrials.gov/ct2/show/NCT02597036 (2019).
Eisenhauer, E. A., Therasse, P., Bogaerts, J., Schwartz, L. H., Sargent, D., Ford, R. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
SERVICES USDOHAH. Common terminology criteria for adverse events (CTCAE) version 4.0. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (2009).
Le Tourneau, C., Lee, J. J. & Siu, L. L. Dose escalation methods in phase I cancer clinical trials. J. Natl Cancer Inst. 101, 708–720 (2009).
Vergote, I., Schilder, R. J., Pippitt, C. H. Jr., Wong, S., Gordon, A. N., Scudder, S. et al. A phase 1b study of trebananib in combination with pegylated liposomal doxorubicin or topotecan in women with recurrent platinum-resistant or partially platinum-sensitive ovarian cancer. Gynecol. Oncol. 135, 25–33 (2014).
Doi, T., Ohtsu, A., Fuse, N., Yoshino, T., Tahara, M., Shibayama, K. et al. Phase 1 study of trebananib (AMG 386), an angiogenesis targeting angiopoietin-1/2 antagonist, in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 71, 227–235 (2013).
Leary, S. E. S., Park, J. R., Reid, J. M., Ralya, A. T., Baruchel, S., Wu, B. et al. Pediatric phase I trial and pharmacokinetic study of trebananib in relapsed solid tumors, including primary tumors of the central nervous system ADVL1115: A Children’s Oncology Group Phase I Consortium Report. Clin. Cancer Res. 23, 6062–6069 (2017).
Dowlati, A., Vlahovic, G., Natale, R. B., Rasmussen, E., Singh, I., Hwang, Y. C. et al. A phase I, first-in-human study of AMG 780, an angiopoietin-1 and -2 inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 22, 4574–4584 (2016).
Hidalgo, M., Martinez-Garcia, M., Le Tourneau, C., Massard, C., Garralda, E., Boni, V. et al. First-in-human phase I study of single-agent vanucizumab, a first-in-class bispecific anti-angiopoietin-2/anti-VEGF-A antibody, in adult patients with advanced solid tumors. Clin. Cancer Res. 24, 1536–1545 (2018).
Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G. & Jain, R. K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15, 325–340 (2018).
Amin, A. & Hammers, H. The evolving landscape of immunotherapy-based combinations for frontline treatment of advanced renal cell carcinoma. Front. Immunol. 9, 3120 (2018).
Liang, H., Liu, X. & Wang, M. Immunotherapy combined with epidermal growth factor receptor-tyrosine kinase inhibitors in non-small-cell lung cancer treatment. Onco Targets Ther. 11, 6189–6196 (2018).
Atkins, M. B., Plimack, E. R., Puzanov, I., Fishman, M. N., McDermott, D. F., Cho, D. C. et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 19, 405–415 (2018).
Rini, B. I., Plimack, E. R., Stus, V., Gafanov, R., Hawkins, R., Nosov, D. et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1116–1127 (2019).
Motzer, R. J., Penkov, K., Haanen, J., Rini, B., Albiges, L., Campbell, M. T. et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1103–1115 (2019).
Rini, B. I., Powles, T., Atkins, M. B., Escudier, B., McDermott, D. F., Suarez, C. et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 393, 2404–2415 (2019).
Arkenau, H. T., Martin-Liberal, J., Calvo, E., Penel, N., Krebs, M. G., Herbst, R. S. et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced or metastatic biliary tract cancer: nonrandomized, open-label, phase I trial (JVDF). Oncologist 23, 1407–e136 (2018).
Herbst RS, A. H., Santana-Davilla, R., Calvo, E., Paz-Ares, L., Cassier, P. A. et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol. 20, 1109–1123 (2019).
Rahma, O.E. C. J., Schlechter, B. M., Ng, K., Eno, J., Stroiney, A., Giobbie-Hurder, A., McDermott, D. F., Hodi, F. S. Phase Ib study of pembrolizumab and trebananib (angiopoietin-2 inhibitor [Ang-2]): preliminary analysis of the colorectal cancer (CRC) cohort. J. Clin. Oncol. 37, 15_suppl, e14160–e14160 (2019).
Acknowledgements
We thank all participating patients, their families and the personnel at the investigating sites. We also thank Karan Sharma of Eli Lilly Services India Pvt. Ltd. (Bangalore, India) for manuscript writing support.
Author information
Authors and Affiliations
Contributions
Conception and design: J.M.L., J.D.K. and W.Z. Development of methodology: W.Z. Acquisition of data: J.M.L., A.H., P.A., C.J., P.M.R., J.R. and J.B. Analysis and interpretation of data: J.M.L. and J.R. Writing, review and/or revision of the manuscript: J.M.L., A.H., P.A., C.J., P.M.R., J.R., J.D.K., W.Z. and J.B. Administrative, technical or material support: J.D.K. Study supervision: J.M.L.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional review boards of each centre approved the study protocol before initiation of patient enrolment (Supplementary Table S5). The study adhered to the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, Council for International Organizations of Medical Sciences, and applicable local regulations. All patients provided informed consent before enrolment. The study was registered in ClinicalTrials.gov (NCT02597036).
Data availability
Lilly provides access to all individual participant data collected during the trial, after anonymisation, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data-sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report and blank or annotated case report forms, will be provided in a secure data-sharing environment for up to 2 years per proposal. For details on submitting a request, see the instructions provided at www.clinicalstudydatarequest.com.
Competing interests
J.M.L. received lecture fees from Astellas Pharma, Bristol–Myers Squibb, Merck Sharp & Dohme (MSD), Novartis, Pierre Fabre, Pfizer, and Roche; advisory fees from Bristol–Myers Squibb, MSD, Novartis, Pierre Fabre, and Roche; and travel grants from Bristol–Myers Squibb, MSD, Novartis, Pierre Fabre, Pfizer, Roche and Ipsen. A.H. received honoraria/advisory fees from Gritstone Oncology, Eisai Co., Ltd., Amgen, Servier and Merck Serono. P.A. received honoraria/advisory fees from Novartis, Roche, MacroGenics, G1 Therapeutics, Servier, Synthon, Amcure, Amgen and Boehringer Ingelheim; and travel grants from MSD, Pfizer and Roche. C.J. received travel grants from PharmaMar S.A., Bayer and Ipsen. P Martin-Romano: Principal/sub-Investigator of Clinical Trials for AbbVie, Adaptimmune, Aduro Biotech, Agios Pharmaceuticals, Amgen, Argen-X Bvba, Arno Therapeutics, Astex Pharmaceuticals, AstraZeneca, AstraZeneca Ab, Aveo, Bayer Healthcare Ag, Bbb Technologies Bv, Beigene, Bioalliance Pharma, Biontech Ag, Blueprint Medicines, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol–Myers Squibb, Bristol–Myers Squibb International Corporation, Ca, Celgene Corporation, Cephalon, Chugai Pharmaceutical Co., Clovis Oncology, Cullinan-Apollo, Daiichi Sankyo, Debiopharm S.A., Eisai, Eisai Limited, Eli Lilly, Exelixis, Forma Therapeutics, Gamamabs, Genentech, Gilead Sciences, Glaxosmithkline, Glenmark Pharmaceuticals, H3 Biomedicine, Hoffmann La Roche Ag, Incyte Corporation, Innate Pharma, Institut De Recherche Pierre Fabre, Iris Servier, Janssen Cilag, Janssen Research Foundation, Kura Oncology, Kyowa Kirin Pharm. Dev., Lilly France, Loxo Oncology, Lytix Biopharma As, Medimmune, Menarini Ricerche, Merck Kgaa, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Molecular Partners Ag, Nanobiotix, Nektar Therapeutics, Nerviano Medical Sciences, Novartis Pharma, Octimet Oncology Nv, Oncoethix, Oncomed, Oncopeptides, Onyx Therapeutics, Orion Pharma, Oryzon Genomics, Ose Pharma, Pfizer, Pharma Mar, Philogen S.P.A., Pierre Fabre Medicament, Plexxikon, Rigontec Gmbh, Roche, Sanofi Aventis, Sierra Oncology, Sotio A.S, Syros Pharmaceuticals, Taiho Pharma, Tesaro, Tioma Therapeutics, Wyeth Pharmaceuticals France, Xencor, Y’s Therapeutics, Research Grants from AstraZeneca, BMS, Boehringer Ingelheim, Janssen Cilag, Merck, Novartis, Pfizer, Roche, Sanofi. P Martin-Romano also disclosed non-financial support (drug supplied) from AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Johnson & Johnson, Lilly, Medimmune, Merck, NH TherAGuiX, Pfizer, Roche. J.R.A. received personal fees and other from Novartis, Kelun Pharmaceuticals/Klus Pharma, Spectrum Pharmaceuticals Inc, Pfizer, Bayer. Personal fees from Eli Lilly and Company, Orion Pharmaceuticals, Roche Pharmaceuticals, Ellipses Pharma, Certera, Ionctura SA. Also conflict with European Journal of Cancer, VHIO/Ministero De Empleo Y Seguridad Social, Chinese University of Hong Kong, SOLTI, Elsevier, GlaxoSmithKline, ESMO, Department of Defense, Merck Sharp & Dohme, Louisiana State University, Huntsman Cancer Institute, Cancer Core Europe, Karolinska Cancer Institute, King Abdullah International Medical Research Center, WIN Consortium, Janssen, Tocagen, Symphogen, BioAlta, GenMab, CytomX, Kelun-Biotech, Takea-Millenium, and Ipsen. J.D.K. and W.Z. are employees and stockholders in Eli Lilly and Company. J.B. received grants and other from Gilead, Genentech/Roche, BMS, Five Prime, Eli Lilly and Company, Merck, MedImmune, Celgene, Taiho, Macrogenics, GlaxoSmithkline, Novartis, OncoMed, LEAP, TG Therapeutics, AstraZeneca, BI, Daiichi Sankyo, Bayer, Incyte, Apexigen, Array, Sanofi, Agios, Ipsen, Merrimack, Oncogenex, Evelo, Forma, Innate, Arch Oncology, Prelude Therapeutics, Amgen, Seattle Genetics, Bicycle Therapeutics, and Relay Therapeutics. Received grants from EMD Serono, Koltan, SynDev Rex, Abbvie, Onyx, Takeda, EISAI, Celldex, Xytomx, Nektar, Armo, Boston Biomedical, Tarveda, Tyrogenex, Marshall Edwards, Pieris, Mersana, Calithera, Blueprint, Merus, Effector, Novocare, Arrys, Tracon, Sierra, Jacobio, Unum Therapeutics, Vyriad, Harpoon, ADC, Pfizer, Millennium, Imclome, Acerta Pharma, Rgenix, Bellicum, Gossamer Bio, Arcus Bio, Tempest Tx, Shattuck Labs, Synthorx, Inc, Revolution Medicines, Inc, and Zymework. Conflict with Phoenix Bio, Cyteir, Molecular Partners, Torque, Tizona, Janssen, Tolero, TD2 (Translational Drug Development), Moderna Therapeutics, Tanabe Research Laboratories, Beigene and Continuum Clinical.
Funding information
The trial was sponsored by Eli Lilly and Company.
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martin-Liberal, J., Hollebecque, A., Aftimos, P. et al. First-in-human, dose-escalation, phase 1 study of anti-angiopoietin-2 LY3127804 as monotherapy and in combination with ramucirumab in patients with advanced solid tumours. Br J Cancer 123, 1235–1243 (2020). https://doi.org/10.1038/s41416-020-1011-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-1011-7
- Springer Nature Limited
This article is cited by
-
Etiological relationship between lipid metabolism and endometrial carcinoma
Lipids in Health and Disease (2023)
-
Targeting the Angiopoietin/Tie Pathway: Prospects for Treatment of Retinal and Respiratory Disorders
Drugs (2021)